Abstract
Multidimensional gas chromatography (MDGC) has already been established as a method which enables the separation and identification of compounds which co-elute in conventional gas chromatography, as well as obtaining more accurate qualitative and quantitative characterisation of compounds of low abundance. In the first part of this review mini-series, we discussed advances in technical aspects of the MDGC systems for essential oil (EO) analysis (Lebanov et al. in Chromatographia, 2018). This second part critically discusses the application of MDGC to the process of characterisation and authentication of EOs, illustrating the benefits of the increased separation power to achieve the resolution of the full chemical profile of EOs, as well as the identification of chiral compounds and aroma analysis. Papers focused on MDGC in essential oil analysis are reviewed from the perspective of the influence of different variables, such as species, chemotype, plant organ, environmental conditions, plant age, and distillation processes, on the chemical profile of the essential oil. Special attention is placed on qualitative and quantitative aspects of EO analysis by MDGC. One section of this review is dedicated to data analysis and the application of multivariate statistical analysis in the process of essential oil authentication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential oils (EOs) are natural products obtained by hydro-distillation, steam distillation or dry distillation, or in case of citrus oils by cold pressing methods, that contain mostly volatile and semi-volatile compounds. Because of their pleasant odour, and potential positive effects on human health and wellbeing, large quantities of EOs are produced annually to fulfil the needs of the flavour and fragrance, perfumery, cosmetics, and pharmaceutical industries, specifically in the areas of aromatherapy and phytomedicine. The EO profile is unique for each species and chemotype and influenced by environmental factors as well as distillation process in their manufacturing [1, 2].
Compounds present in EOs are products of the secondary metabolism of plants, thus differences in chemical profiles between different EOs come from the fact that each plant species and chemotype has a distinctive secondary metabolism [3, 4]. These metabolites, depending on their purpose in the plant, are produced and accumulated at different rates in different plant organs. Additionally, environmental conditions, such as climate, precipitation, temperature, and time of harvest have a great impact on the secondary metabolism and chemical profile of the EO. Hence, the relative presence of ‘marker’ chemicals, used to establish EO authenticity must be well-defined, establishing the links between the chemical profile of the sample, and the species, chemotype and environmental conditions under which the plant was grown [5, 6].
Due to the nature of the EOs constituents, gas chromatography (GC) is the most widely applied analytical technique for their qualitative and quantitative characterisation [1]. Routine EO analysis is typically performed by GC equipped with a single column. However, the complexity of most EOs, has led to an increasing interest in multidimensional separations. Multidimensional gas chromatography (MDGC) systems offer an increased separation power and peak capacity compared to standard GC systems, which can help in the identification and quantification of compounds present at trace levels that might be co-eluted with others present in higher amounts on a single column [7]. In a typical MDGC system, where a second column with a different stationary phase is connected to the first dimension (1D) column by means of a transfer device, increased peak resolution and peak capacity can be achieved. Analysis can be performed either in heart-cut (GC–GC) or in comprehensive mode (GC × GC) [1, 7]. In GC–GC, selected discrete fractions of the 1D column eluent are sent to the second dimension (2D) column [2], whilst in comprehensive two-dimensional gas chromatography (GC × GC), there is continuous modulation (fractionation) of the eluent, where each fraction is transferred to a short narrow-bore 2D column [3]. Multidimensional GC in its various forms has been reviewed extensively in the past, where such standard configurations are explained in detail [8,9,10]. The latest technological and instrumental developments in MDGC were the subject of part 1 of this review mini-series [11], which specifically complements this current review focused upon applications.
MDGC is increasingly being applied to the characterisation of EOs, due specifically to its greater resolution and peak capacity. This increased capacity can help identify important differences in the EO composition arising from plant source, species, chemotype, plant organ or environmental factors [2]. MDGC can also improve detection of low levels of environmental pollutants in EOs, or compounds produced by oxidative degradation of compounds from prolonged exposure to higher temperatures and/or light, due to poor storage conditions. In addition, some EOs, e.g. tea tree EO, can contain sensitisers which arise from the oxidative formation of peroxides, endoperoxides, and epoxides, and their abundance must also be closely monitored [12].
In this review, the application of MDGC in EO analysis reported over the past decade or so, will be critically discussed. Special attention will be paid to the qualitative and quantitative aspects of EO characterisation by MDGC. The review is focused on studies looking into the increased separation power of MDGC and its application in the characterisation and aroma analysis of EOs. Additional focus will be given to studies reporting on the application of MDGC in the identification and determination of the enantiomeric excess (EE) of chiral compounds and its importance in EO characterisation. The role data analysis has in the process of characterisation of EOs, and the importance of multivariate statistical analysis in the authentication process of complex matrices is included.
Qualitative MDGC Analysis of the Essential Oils
Advantages of MDGC in Essential Oil Analysis
MDGC, with its greater resolving power, provides the opportunity to separate and identify compounds that co-elute in conventional GC, facilitating much more comprehensive chemical profiles [13, 14]. For example, Baharum et al. [15] identified 48 significant compounds from Polygonum minus Huds EO, using GC × GC, compared with only 21 detected utilising one-dimensional (1D) GC/mass spectrometry (MS), while the rest were not identified due to the co-elution with other compounds. Similarly, Ray et al. [16], applying GC × GC-Time of flight (TOF)-MS, managed to identify 53 terpenoids for the first time in the Hedychium coronarium J. Koening EO.
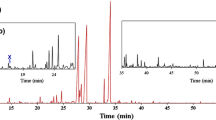
The increase in separation power of the MDGC technique can best be seen in the example of spathulenol, from the EO of Manekia obtusa (Miq.) Arias, Callejas & Bornst., which in 1D GC is observed as one overlapping peak, while in GC × GC can be resolved to six separate peaks (Fig. 1) [17]. A similar situation was observed with limonene, o-cymene, 1,8-cineole and phenylmethyl alcohol in the Kewda flower oil which could only be separated in the 2D [12]. Cai et al. [18] observed that curcumene, α-ionone, and 2,3-dehydro-α-ionone co-elute in the 1D GC analysis of Lonicerae japonicae Thunb. EOs, but can be separated utilising GC × GC with TOF-MS detection. These compounds were separated on a mid-polar 2D column, with (50%) phenyl (50%) dimethylpolysiloxane as the stationary phase [18]. Sandalwood EO was analysed by Sciarrone et al. [19], where the sesquiterpenoids (E,E)-farnesol and (Z)-β-santalol co-eluted on a single apolar column, and (E,E)-farnesol and (Z)-α-santalol co-eluted on a polar column. However, these three compounds were readily separated using GC–GC. Detection and quantification of (Z)-β-santalol is particularly important, being one of the most significant sandalwood EO constituents for its sensorial qualities.
a Spathulenol, overlapping peak in 1D GC; b resolved to six compounds in GC × GC. Reproduced and adapted with permission from [17]
Off-line normal phase liquid chromatography (LC) can be applied as an additional separation step in EO analysis to decrease the complexity of the EO by pre-separating different chemical classes, e.g. more polar oxygenated compounds from mono- and sesquiterpenes. This way, by optimising separation conditions for more chemically homogenous groups of compounds, it is possible to obtain higher resolution, identify new compounds and define differences between different chemotypes, as in the case of different mandarin EOs. For instance, in Mexican mandarin EO, 16 compounds were identified for the first time, while in green manadarin EO, 35 compounds were found, which had not been reported in mandarin EOs previously [19]. Several compounds identified in the green mandarin EO, such as γ-terpinen-7-al, n-dodecanal, karahanenone, trans-α-hydroxy-pinocamphone or ethyl butyl ketone, (Z)-jasmone, umbellenone, and prenol, had only previously been identified in yellow manadarin EO [20].
An increase in sensitivity and S/N ratio in GC × GC due to thermal modulator refocusing enables separation and identification of trace levels of compounds, including identification of environmental pollutants and/or degradation products. This is important from the perspective of quality control in EOs. Kirkin et al. [21] managed to separate and identify the compounds 2-caren-10-al and 4-(1-methylethyl)-1,3-cyclohexadiene-1-methanol, and observe increased level of cumin aldehyde, which had arisen in the irradiation process (utilised for sterilisation) of the EO in the presence of air. As each of these compounds co-elute in the 1D with other compounds, they could not be observed and identified with conventional GC. Sciarrone et al. [22] used GC–GC to analyse tea tree EO for traces of ascaridole and 1,2,4-trihydroxymenthane, which usually occurs from oxidation processes, when the EOs are not properly stored. Hyphenating MDGC with MS/MS detection can further increase the method sensitivity using specific precursor and product ions, multiple reaction monitoring (MRM) and selected ion monitoring (SIM) operational modes, facilitating detection of even lower levels of these compounds [23]. This is important in quality assessment of EOs, especially when these oils are labelled as organic and must comply with more strict regulations [22].
Elution Patterns of Chemical Classes in GC × GC
Due to the orthogonality of the GC × GC system, where the compounds are typically separated according to their dispersion interaction on a non-polar column in 1D, and their polarity in the 2D using a polar column, molecular classes are separated and form clusters in two-dimensional (2D) contour plots [17]. Aliphatic alcohols, aldehydes and acetates elute before monoterpene hydrocarbons in the 1D due to their higher polarity [24]. Alkanes are resolved in the 1D, while in the second, they elute first from the column (Fig. 2) [25]. Compared to the sesquiterpenes, monoterpenes elute at lower retention times in the 1D, due to their lower molecular weights [17, 26]. Sesquiterpenes and norsesquiterpenes elute in the 1D as per conventional GC, while in the 2D they are distributed according to the number of double bonds present, with retention time rising with an increase in the number of these bonds, which provide stronger interactions with the polar stationary phase [27]. Monoterpenoids and sesquiterpenoids elute after their hydrocarbon homologues in the 1D and, due to the wider range of polarity, are typically more resolved in the 2D [17, 26]. Monoterpenic alcohols elute at the same time as monoterpenic acetates in the 1D, while in the 2D, monoterpenic acetates exhibit higher retention times [24]. Cluster formation in 2D contour plots is one of the advantages of GC × GC, because it gives a straightforward picture of the chemical classes present in the EO. These observations were used by Tissandie et al. [28] to identify vetiveryl acetates from vetiver EO, which under the thermal conditions of cryogenic modulation eliminate the acetate functional group, producing sesquiterpenes which were aligned with their parent ester peak along the 2D in the 2D contour plots.

Reproduced with permission from [29]
GC × GC total ion current chromatogram contour plots of a distillation fraction collected after 25 min (fraction 1) of distillation of mature ylang–ylang flowers harvested in Mayotte—A, monoterpenes; B, monoterpenoids; C, esters; D, sesquiterpenes; E, sesquiterpenoids, and F, alkanes, respectively.
Identification of Compounds Using GC–GC and GC × GC
Identification of peaks in 1D GC coupled with MS is mainly performed by comparing experimentally obtained mass spectra with appropriate reference libraries, and linear temperature-programmed retention indices (LTPRIs), obtained from retention times using the Van den Dool and Kratz equations for temperature-programmed separation [30,31,32,33]. However, obtaining RIs in GC × GC is more complicated. Several papers have been published attempting to obtain RIs on both columns in GC × GC. The 1D retention times are approximated from the total retention time of the modulated peak with maximum response, after subtraction of the experimental 2D retention time obtained during the GC × GC analysis. LTPRIs for the 1D are calculated using Van den Dool and Kratz equations. Von Mühlen et al. [24] has shown that these are in good agreement with the values obtained from 1D GC separations [34]. Saucier et al. [35] and Brokl et al. [29] applied this method for peak identification in artichoke EO and ylang–ylang EOs, respectively. Savareear et al. [36], used two different columns in the 1D, obtaining two linear retention indices for each compound, which were used as an additional level of identity confirmation. In place of LTPRIs, Namara [6] used retention time locking for peak identification. In this method, the head pressure is set using a regression system so that the retention time of n-pentadecane, the locking compound, is exactly 27.5 min. This enables the use of absolute retention times as an identification tool instead of retention indices, as chemical species have predictable retention times under the same conditions [6].
In GC–GC separation, to determine 2D retention index it is necessary to heart-cut all the alkanes, trap them at the inlet of the 2D column and separate on the 2D column under the same experimental conditions as heart-cut regions of the sample. This way, alkanes and samples are analysed under the same instrumental conditions and the 2D retention index can be calculated. For an isothermal GC × GC separation, the retention index in 2D can be calculated according to Kováts index [10]. Retention times obtained in the 2D of GC × GC temperature-programmed analysis cannot be directly compared with reference literature values, because the retention times of compounds vary according to the exact temperature at the moment of elution. Estimation of 2D retention times in temperature-programmed separations can be performed using the isovolatility curves of an appropriate alkane homologues series, where retention time dependence on temperature must be constructed. To build the isovolatility curve, it is necessary to perform direct injection of alkane mixtures on the 2D column. Due to the isothermal conditions in 2D columns, 2D retention times can be interpolated from isovolatility curves and retention indices calculated by the Kováts equation [10, 33]. However, this process is time consuming, and has not been applied in the analysis of EOs.
Characterisation According to Chemical Markers
Since the chemical profile of the EO depends on the secondary metabolism of the plant, species and chemotype are primary sources of differences between chemical profiles of EOs. Reported studies characterising differences between EOs according to species, chemotype and varieties, carried out over the past decade are summarised in Table 1. Within this body of work, eight different species coming from Citrus family were characterised by Zoccali et al. [37] utilising liquid chromatography as an off-line pre-separation step before the GC × GC analysis of sesquiterpene and sesquiterpenoid fraction. Yan et al. [38] have shown, working with hops, that differences in cultivars lead to unique chemotypic patterns reflected in the abundance of specific terpenes and terpenoids. The highest amount of sesquiterpenes and sesquiterpenoids was found in Helga (66%), with humulene and caryophyllene being major contributors with 22.3% and 14.1% of total peak volume. Monoterpenes and monoterpenoids were big contributors to Cascade with 46.8% of total peak volume, with β-myrcene having 38.4%, while Super Pride was having the highest total amount of esters with 18.3% among all cultivars [38]. Another good example is the case of Ocimum basilicum var. thyrsiflora and Ocimum basilicum L. leaf EOs, two varieties which belong to Ocimum genus, as analysed by MDGC [39]. In O. basilicum var. thyrsiflora leaf EO, methyl chavicol was the dominant compound identified (81.82%), while the main component of O. basilicum leaf oil was linalool at 43.78%. Eight compounds which could be utilised as markers for differentiation of these two types were detected in O. basilicum. The analysis determined that O. basilicum var. thyrsiflora contains a significantly higher number of monoterpenoids, while O. basilicum was richer with sesquiterpenoids [39]. These differences could be readily observed from the full chemical profile of the EO on 2D contour plots, where the monoterpenoids and sesquiterpenoids were well separated [17, 26].
Differences in secondary metabolism and accumulation rates of different compounds between different plant organs give rise to different chemical profiles. This can be seen in the example of EOs obtained from the leaves, stems and flowers of Piper regenllii (Miq.) (see Fig. 3). EO obtained from the leaves has the highest content of monoterpenes (35.85%), with prominent compounds eluting before 22.7 min (See Fig. 3a), stems have the highest content of phenylpropanoids (39.23%), while EO from the flowers has the highest relative abundance of sesquiterpenes (31.11%) (Figs. 3c, 4) [42].

Reproduced and adapted with permission from [42]
2D contour plots (GC × GC/TOFMS) of Piper regnelliia leaves, b stems, c flowers.

Reproduced with permission from [42]
Semi-quantitative class distribution of compounds in each Piper regnellii essential oil studied by GC/qMS and GC × GC/TOFMS.
Besides species, chemotype and plant organ, it is well known that geographical origin, soil, and climate, can also affect the expression of the secondary metabolism in the plants, thereby affecting the chemical profile of EOs. Additionally, the time of harvest and distillation process also have a significant impact on the final chemical composition, and thus value of the EO [29]. In recent years, many authors have reported effects of these parameters on the EO chemical profiles (Table 1).
Vetiver EO, obtained by hydro-distillation of the fragrant roots of Chrysopogon zizanioides L., is of high commercial value as a common ingredient within the perfumery industry. Therefore, it is important to determine geographical influence on the chemical profile of vetiver EO, as this impacts the fragrance of the EO. With this in mind, Filippi et al. [27] and Cordero et al. [40] determined the fingerprints of vetiver EOs from Brazil, Java, Haiti (Fig. 5) and Bourbon using GC × GC. It was shown that the Brazilian oil had characteristically high levels of zizanoic acid and khusimol; the Haitian had higher levels of oxygenated compounds, and those from Java had more sesquiterpenes, such as β-vetivenene. Furthermore, Filippi et al. showed, in several samples, significant variations in the levels of different compounds with regard to the international ISO standard 4716:2002. Such discrepancies arise from the fact that the international standard was set utilising 1D GC, which is obviously much lower in resolution compared to the GC × GC method applied by Filippi et al., and Cordero et al. From this example, it is clear then how MDGC has an important role to play in setting improved authenticity and quality standards.
Besides differences according to geographical origin, Kutlular et al. [48] showed that harvest time caused observable differences in EO chemical profiles. Leaves and grains obtained from Origanum onites L. harvested on three different dates, 15th June, 25th June, and 5th July, provided different EO yields, as well as chemical profiles. Samples showed an increase of linalool from 0.14% on 15th June to 5.14% when harvested on 5th July, and similar changes occurred with thymol and β-pinene. They also observed an increase in the concentration of oxygenated compounds in oregano, which contribute more to its fragrance [48]. This observation relates to the scope of climate conditions, number of sunny or rainy days, average temperature, humidity, etc., as well as other environmental factors influencing secondary metabolism, which helps map optimal conditions necessary to obtain the best EO quality.
It is important to take into account the distillation process used to produce the EO, when assessing its chemical profile. For example, the steam distillation of Chrysopogon zizanioides, producing vetiver EO, gives a more distinctive chemical profile, containing α-cubebene and α-ylangene (floral scents), with several other compounds only identified in the distilled product, such as 9,10-dehydro-2-norzizaene, β-copaene and trans-calamenene [54]. Besides the extraction technique, distillation time also has a great influence on the composition of the EOs. Brokl et al. [29] showed with ylang–ylang that EO collected after 25 min of distillation is much richer in more volatile compounds compared to the those collected after 8 h, which have higher abundance of compounds with higher boiling points, such as sesquiterpenes. The lowest grade ylang–ylang EOs, with short distillation times, are mostly utilised in the perfume industries due to the high content of very volatile compounds.
Comparison of the chemical composition of EOs obtained from Aniba rosaeodora Ducke (rosewood) leaves and small branches of 4-, 10- and 20-year-old trees using conventional GC, showed greater similarity in identified compounds between oils from the 10- and 20-year-old plants, identifying 22 compounds, compared to 31 identified in the 4-year-old ones. However, analysis of the same oils by GC × GC showed fewer dissimilarities, whilst separating and identifying over 90 compounds in each of these three EOs [46]. This shows that GC × GC, by resolving otherwise co-eluting compounds, provided much more accurate and reliable chemical profiles. Subsequently, it was concluded that rosewood EO from younger plants and older trees are essentially similar, and can be used equally in the perfume industry, with the fragrance obtained from the young plant oil very similar to the older one [30]. This has obvious economic impact on rosewood EO production.
Any industrial process performed on the plant organ before distillation can influence the chemical profile of the EO. Polidor et al. [53] compared chemical profiles of fresh leaves obtained from Ilex paraguariensis A. St. Hil. (popularly called “mate”) with leaves obtained after industry processing through blanching and drying, where these harsh processes lead to a change in chemical composition. Analysis of the samples showed an increase in the number of compounds in the processed mate, probably due to chemical degradation of certain compounds. For instance, some norisoprenoids observed in the green (or processed) mate EO, such as (E)-β-damascenone (3.03%), and (E)-β-ionone (0.81%), might come from the degradation of carotenoids determined in fresh leaves. It was also observed an increase of monoterpenoids (to 41.46% in green mate) and furans, as well as a decrease of linalool (from 17.61% in fresh leaves to 5.5% in green mate), all come as a result of industrial processing. These compounds proved to be excellent chemical markers for differentiation of processed and non-processed leaves [53].
Enantioselective MDGC
EOs are complex mixtures containing many chiral compounds which may be present in enantiomeric or diastereomeric forms. Enantioselective GC is commonly adopted for the assessment of an EOs authenticity, as the enantiomeric excess (EE) of chiral compounds is often peculiar for each plant species, chemotype, geographical origin, or in some instances the technological treatment the EO has undergone. Many chiral compounds, such as limonene, β-phellandrene, γ-muurolene and germacrene D tend to co-elute in 1D GC. In an attempt to separate them and obtain a more reliable EE measurement, Lo Presti et al. [55] applied enantioselective GC–GC to the determination of 17 enantiomeric pairs in Pistacia lentiscus L. twig oil, obtaining enantiomeric ratios for each pair, while Bonaccorsi et al. [56] applied enantioselective GC–GC to bergamot petitgrain EO, determining ten pairs of enantiomers.
Many enantiomers present in citrus EOs, such as (−)-camphene and (−)-α-terpineol, partially or completely co-elute with other compounds in 1D GC, which makes their full characterisation impossible. Application of enantioselective GC–GC/MS techniques carried out by Bonaccorsi et al. [43] and Schipilliti et al. [41] managed to separate ten pairs of enantiomers obtaining the EE for each of them and setting quality markers for both citrus EOs and petitgrain lemon and mandarin EOs, as well as markers for differentiation between different lime species. Both research groups characterised the same eight enantiomeric pairs. Besides these enantiomeric pairs, Bonaccorsi et al. also identified and characterised α- and β-phellandrene, while Schipilliti et al. identified linalyl acetate and α-pinene. Separation of nine enantiomeric pairs from petitgrain lemon EO on 2D chiral column of GC-GC/MS system is presented in the Fig. 6, while EE of α-pinene was determined from the 1D enantioselective GC/ flame ionisation detector (FID). In addition, Bonaccorsi and Schipilliti observed certain differences in EE among the characterised chiral components. Harvey et al. [57] and Barba et al. [58] performed enantioselective GC–GC analysis of Mentha spicata L. and Mentha × piperita L. EOs, respectively, finding differences in the two chiral profiles which reflect the difference in the plant material used to prepare the EOs. Mentha × piperita was distilled as a mixture of M. spicata and M. aquatica, chemotype menthone, compared to the pure species M. spicata used by Harvey.

Reproduced with permission from [41]
GC–GC separation of chiral compounds in petitgrain lemon EO. 2 (+)-α-thujene, 2′ (−)-α-thujene; 5 (−) camphene, 5′ (+)-camphene; 6 (+)-sabinene, 6′ (-)-sabinene; 7 (+)-β-pinene, 7′ (−)-β-pinene; 16 (−)-limonene, 16′ (+)-limonene; 26 (−)-linalool, 26′ (+)-linalool; 39 (+)-terpinen-4-ol, 39′ (−)-terpinen-4-ol; 41 (−)-α-terpineol, 41′ (+)-α-terpineol; 49 (−)-linalyl acetate, 49′ (+)-linalyl acetate.
Krupcik et al. [59] applied enantioselective flow-modulated GC × GC with a FID and quadruple mass spectrometry (qMS) to the determination of the enantiomers in lavender EO. However, due to the complexity of the matrix and variety of chiral compounds, two different sets of columns were used, differing in the 1D cyclodextrin column (Rt-βDEXse and β-DEX 120), while having the same polyethylene glycol stationary phase column as in the 2D. The EE obtained for α-pinene, α-phellandrene and α-terpineol from both column sets were in good agreement. Discrepancies were observed for β-pinene, limonene and camphene, which might have arisen from co-elution with other compounds in either one of the column systems [59]. Sgorbini et al. [12] also showed good agreement of EE of authenticity markers for lavender EOs with the standards for this EO set by European Pharmacopeia [60].
As most MDGC separations are rather time consuming, Wong et al. [45] proposed a method for fast enantioselective GC–GC and GC × GC for authentication of tea tree EOs coming from Australia. They separated three main chiral monoterpenes: limonene, terpinen-4-ol and α-terpineol, in under 25 min with an enantioselective column in 2D, and in under 20 min when an enantioselective column was used in 1D. The fast separation was achieved by optimising the column configuration and oven temperature ramp rates. This method was applied to determine the EE of these compounds in the tea tree EOs coming from different geographical locations and can be used in the authentication of this EO. Utilising GC–GC, Greule et al. [61] used different oven temperature ramp rates in both dimensions for the characterisation of different chiral compounds in oregano, thyme, savory, and fennel EOs.
The application of MDGC in combination with chiral analysis is a powerful tool for the characterisation of EOs from different geographical origins [4, 5, 22, 62, 63]. Krupcik et al. [31] characterised chiral compounds present in EOs obtained from Rosa damascene Miller from Bulgaria and Turkey. Several quality markers, such as α-pinene and β-pinene, can be used as authenticity markers for rose oil, while R-limonene, R-linalool and α-terpineol can be used to distinguish the Bulgarian rose EO from the Turkish variant. Dugo et al. [47] showed that there are seasonal variations in the EE of chiral compounds present in mandarin EO from Citrus deliciosa Tenore (cultivars ‘Avana’ and ‘Tardivo di Ciaculli’) between September, when the fruit is still green (unripe), and March (late season). These EE ratios can be used as standards when the quality of these EOs is assessed. Finally, Schmarr et al. [64] showed that the EE of (S)-linalool in cacao oil obtained by simultaneous steam distillation–extraction, does not change after industrial processing and can be used for authentication of the cacao.
Aroma Analysis
Gas chromatography hyphenated with olfactometry (GC–O) enables the detection of odour active compounds using sniffing ports [5, 65, 66]. GC × GC is not an ideal technique for olfactometric analysis because the human breathing cycle is too slow for this type of MDGC, where several compounds can exit the 2D column in a few seconds. However, in GC–GC only a selected portion of the effluent is sent to the 2D column, using its maximum resolving power to separate a limited number of analytes, and can therefore be successfully used to resolve selected odour-active regions [65]. Eyres et al. [65] applied this method for the analysis of the co-eluting floral odour region in coriander. The region of interest was heart-cut from the first column and cryotrapped before being separated on the second column of the GC–GC–O system. It was determined that E-2-dodecenal was the only compound contributing to the floral odour of coriander (Fig. 7a). The same method was later applied to hops, where the authors wanted to determine the compound responsible for its woody odour, which was achieved by GC–O (Fig. 7b). GC × GC/FID analysis of the hops woody active region, separated 6 compounds, formally co-eluting in GC-O analysis, which might contribute to the odour (Fig. 7c) [65]. To capture and determine the aromas of rosemary EO, oleoresin, and dried leaves, Van der Wat et al. [67] applied headspace analysis using multi-channel polydimethylsiloxane traps, olfactometry and GC × GC/TOFMS characterisation. It was observed that the dried rosemary had a grassier, more farmyard-like, and woodier aroma, while the EO was dominated by a menthol aroma, with prominent eucalyptus and camphor notes mostly coming from terpenes and terpenoids, and high concentrations of eucalyptol, pinene, camphor, and limonene. In addition, it was observed that the dried herb had evident loss of limonene and phellandrene, which was responsible for peppery and citrus fruit aromas [67].

Reproduced with permission from [65]
a GC–GC–O separation of co-eluting cluster in coriander leaf on 2D column, where (E)-2-docenenal was found to contribute to the floral odour; b shaded region that was perceived as woody during GC–O analysis of hop; c outlined segment corresponding to the woody perceived region of hop, resolved into six different compounds using GC × GC/FID.
Quantitative Analysis
Co-elution in the conventional 1D GC analysis affects not just qualitative, but also quantitative analysis. Applying GC–GC/MS to tea tree EO, Sciarrone et al. [22] completely separated and quantified p-cymene, limonene, and 1,8-cineole, which are known to co-elute in 1D GC, obtaining 5% and 10% higher values for p-cymene and 1,8-cineole, and a 4 times smaller value for limonene, compared to that determined using 1D GC. Sesquiterpenes, especially those present in lower concentration, are also affected by co-elution in conventional GC. For instance, the quantity of δ-cadinene, co-eluting in the conventional GC analysis of tea tree oil with trans-calamenene, was overestimated by 30% [22]. Thus, application of MDGC can be necessary to obtain more accurate quantitative information on compounds present in EOs.
Normalised percentage abundance is the most common method for quantitative characterisation of EOs. This way of comparing the abundances is only recommended between the samples coming from the same family or chemotype. Otherwise, absolute concentration of compounds can be determined using internal and external standards. Since EOs are often complex mixtures containing hundreds of compounds, pure standards are not always commercially available, and it is impossible to use reference standards for the quantification of each compound in an EO. Thus, it is necessary to compromise and use compounds belonging to the same chemical class as reference standards [3, 68]. Filippi et al. [27] applied this principle to determine relative response factors (RRFs) for the experimental FID quantification of one compound as a representative of a whole chemical class [27].
Another way to overcome the lack of reference compounds in quantification is to adopt a predicted relative response factor (PRRF). Calculation of PRRFs is based on combustion enthalpies and structures of compounds, taking into account the elemental composition of each constituent, and also specific molecular features, such as aromatic rings. This method was introduced by de Saint Laumer et al. [69] and was validated by Tissot et al. [70]. Filippi et al. [27] compared RRFs obtained experimentally, with PRRFs for compound quantification and obtained very good agreement. For terpenic alcohols and terpenes, PRRF values were consistent with their corresponding RRFs, while terpenic ketones and aldehydes showed significantly higher PRRF values than those obtained experimentally. Cordero et al. [40] and Sgorbini et al. [12] applied PRRF for quantification of compounds within lavender and mint EOs. The values obtained were mostly in good agreement with external reference standards but a larger deviation, exceeding 20% of relative error, was observed for menthone and carvone in mint and 1,8-cineole and (S)-borneol in the lavender EO. In a work performed by Cordero, PRRF values were also in good agreement with quantitative data obtained by external calibration and the relative error did not exceed +/− 32%. Notar-Francesko et al. [51] applied PRRFs as a quantification method for the constituents of Haitian vetiver oil which was altered using a lipase–catalysed acylation reaction.
Data Analysis in EO Characterisation and Authentication
By applying modern instrumental techniques such as GC × GC, it is possible to separate and identify a very high number of the compounds present in EOs. Therefore, data analysis, using statistical and mathematical principles, is becoming an inseparable part of MDGC, especially in EO characterisation. Multivariate statistical techniques, such as principal component analysis (PCA) [26, 49, 50] and hierarchical cluster analysis (HCA) [44] can be used to cluster samples according to their chemical profiles, emphasising variances between the samples, and leading to grouping of samples according to the similarity of their chemical profiles and the possibility of building fitting models. Partial least squares discriminant analysis (PLS-DA) and variable importance in the projection (VIP) analysis, can be applied to maximise variation between the groups and to detect variables which have the greatest influence on classification, identifying variables responsible for classification [49]. Applying PCA, HCA and orthogonal projections to latent structure discrimination analysis (OPLS-DA), Nsuala et al. [44], managed to classify EOs obtained from several species of Leonotis leonurus (L.) R. Br, into two classes—inland and coastal regions (Fig. 8)—identifying chemical markers for this differentiation, as well as chemical markers for the identification of EOs obtained from the species L. leonurus.

Reproduced with permission from [44]
PCA and OPLS-DA scatter plots showing the distribution of L. leonurus essential oils into two major groups; inland (green upward triangle Mpumalanga and KwaZulu-Natal) and coastal (blue upward triangle Western Cape).
An interesting application of GC × GC together with PCA and HCA is found within the field of forensics and criminology, where knowledge of chemical profiles of EOs, including possible impurities, can provide important information to help identify suppliers and pathways for drug trafficking. One of the EOs of interest is sassafras obtained from the species Sassafras albidum (Nutt.) Nees (native of North America), which is an important ingredient within the illicit drug ecstasy. Schaffer et al. [71] combined GC × GC/TOF with PCA and HCA techniques to determine similarities between sassafras EOs. HCA was applied and a dendrogram formed which could unambiguously show the chemical similarity between the samples, and identify those coming from the same batch, and plants coming from the same geographical location. In the analysis of sassafras EOs, ketones and aldehydes are of special interest because they can be found in the final product, either unaltered or converted to amines. This can be a direct link between the final product and the starting raw material. In addition, PCA has shown that the main variation in seized sassafras EOs comes from the differences in the distillation procedure, which can connect a certain product with its producer [71].
Multivariate curve resolution analysis (MCR) is a chemometric technique which deals with the background signals arising from the GC instrumentation, such as baseline drift, spectral background, noise, low S/N ratio, changes in peak shape and co-elution [72]. In combination with alternating least squares (ALS), it can be a powerful technique to resolve and provide relative determination of compounds in complex mixtures and matrices [73]. De Godoy et al. [74] applied a MCR-ALS algorithm to build a multivariate quantitative model using data obtained from GC × GC/FID to identify and quantify rosemary EO in complex samples, with interferences such as perfumes present. He et al. [75] applied several chemometric techniques, namely heuristic evolving latent projections (HELP), selective ion analysis (SIA), alternative moving window factor analysis (AMWFA) and alternating trilinear decomposition (ATLD), which enabled mathematical peak separation and identification of 216 terpenoids and phthalides in a herbal Chinese mixture, Chaihu Shugan San, which contains seven individual herbs. Based on the obtained data, some potential quality markers (Q-markers) were identified for the quality of the products. They also managed to build a “connectivity map”, revealing that the main compounds, terpenes and phthalides, have different pharmacological effects [75].
Concluding Remarks and Future Work
The development of MDGC has had a significant impact on the way modern laboratories characterise and identify EOs. With the increase in separation power and resolution, the number of compounds identified has significantly increased, solving numerous co-elution issues, which caused problems for the qualitative and quantitative characterisation of many EO volatiles. It is now possible to obtain more accurate chemical profiles and to detect subtle differences among samples, which may come from the geographical origin, time of harvest, distillation and fractionation procedures. Increased separation power is also of significant importance in the detection of trace levels of harmful compounds such as sensitisers, or other degradation products that may arise due to poor storage conditions. In the future, MDGC will continue to play a crucial role in the authentication and quality assurance of EOs and EO based products. In addition, the application of multivariate statistical analysis on chemical profiles obtained by MDGC can be a powerful tool in the authentication process, where even small differences in certain parameters can be detected. Thus, the development of authentication procedures depends not only on the development of instrumental techniques, but also on the application of appropriate mathematical and statistical techniques.
References
Rubiolo P, Sgorbini B, Liberto E, Cordero C, Bicchi C (2010) Essential oils and volatiles: sample preparation and analysis. A review. Flavour Fragr J 25:282–290. https://doi.org/10.1002/ffj.1984
Do TKT, Hadji-Minaglou F, Antoniotti S, Fernandez X (2015) Authenticity of essential oils. TrAC. Trends Anal Chem 66:146–157. https://doi.org/10.1016/j.trac.2014.10.007
Bicchi C, Liberto E, Matteodo M, Sgorbini B, Mondello L, Zellner BdA, Costa R, Rubiolo P (2008) Quantitative analysis of essential oils: a complex task. Flavour Fragr J 23:382–391. https://doi.org/10.1002/ffj.1905
Bicchi C, Cagliero C, Rubiolo P (2011) New trends in the analysis of the volatile fraction of matrices of vegetable origin: a short overview. A review. Flavour Fragr J 26:321–325. https://doi.org/10.1002/ffj.2059
Marriott P, Shellie R, Cornwell C (2001) Gas chromatographic technologies for the analysis of essential oils. J Chromatogr A 936:1–22. https://doi.org/10.1016/S0021-9673(01)01314-0
Namara KM, Howell J, Huang Y, Robbat A Jr (2007) Analysis of gin essential oil mixtures by multidimensional and one-dimensional gas chromatography/mass spectrometry with spectral deconvolution. J Chromatogr A 1164:281–290. https://doi.org/10.1016/j.chroma.2007.07.042
Seeley JV (2012) Recent advances in flow-controlled multidimensional gas chromatography. J Chromatogr A 1255:24–37. https://doi.org/10.1016/j.chroma.2012.01.027
Tranchida PQ, Franchina FA, Dugo P, Mondello L (2016) Comprehensive two-dimensional gas chromatography-mass spectrometry: recent evolution and current trends. Mass Spectrom Rev 35:524–534. https://doi.org/10.1002/mas.21443
Tranchida PQ, Franchina FA, Mondello L (2017) Analysis of essential oils through comprehensive two-dimensional gas chromatography: general utility. Flavour Fragr J 32:218–227. https://doi.org/10.1002/ffj.3383
Nolvachai Y, Kulsing C, Marriott PJ (2017) Multidimensional gas chromatography in food analysis. TrAC. Trends Anal Chem 96:124–137. https://doi.org/10.1016/j.trac.2017.05.001
Lebanov L, Tedone L, Kaykhaii M, Linford MR, Paull B (2018) Multidimensional gas chromatography in essential oil analysis. Part 1 technical developments. Chromatographia (submitted)
Sgorbini B, Cagliero C, Boggia L, Liberto E, Reichenbach SE, Rubiolo P, Cordero C, Bicchi C (2015) Parallel dual secondary-column-dual detection comprehensive two-dimensional gas chromatography: a flexible and reliable analytical tool for essential oils quantitative profiling. Flavour Fragr J 30:366–380. https://doi.org/10.1002/ffj.3255
Wang K, Zhu R-z, Qu R-f, Li Z-y (2012) Comprehensive two-dimensional gas chromatography—time-of-flight mass spectrometry for the analysis of volatile components in Neroli essential oil. Mendeleev Commun 22:45–46. https://doi.org/10.1016/j.mencom.2012.01.018
Cao G, Cai H, Cong X, Liu X, Ma X, Lou Y, Qin K, Cai B (2012) Global detection and analysis of volatile components from sun-dried and sulfur-fumigated herbal medicine by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Analyst 137:3828–3835. https://doi.org/10.1039/c2an35543d
Baharum SN, Bunawan H, Ghani MA, Mustapha WA, Noor NM (2010) Analysis of the chemical composition of the essential oil of Polygonum minus Huds. using two-dimensional gas chromatography-time-of-flight mass spectrometry (GC-TOF MS). Molecules 15:7006–7015. https://doi.org/10.3390/molecules15107006
Ray A, Dash B, Sahoo A, Nasim N, Panda PC, Patnaik J, Ghosh B, Nayak S, Kar B (2017) Assessment of the terpenic composition of Hedychium coronarium oil from Eastern India. Ind Crops Prod 97:49–55. https://doi.org/10.1016/j.indcrop.2016.11.063
Santos TG, Fukuda K, Kato MJ, Sartorato A, Duarte MCT, Ruiz ALTG, de Carvalho JE, Augusto F, Marques FA, Sales Maia BHLN (2014) Characterization of the essential oils of two species of Piperaceae by one- and two-dimensional chromatographic techniques with quadrupole mass spectrometric detection. Microchem J 115:113–120. https://doi.org/10.1016/j.microc.2014.02.014
Cai H, Cao G, Li L, Liu X, Ma XQ, Tu SC, Lou YJ, Qin KM, Li SL, Cai BC (2013) Profiling and characterization of volatile components from non-fumigated and sulfur-fumigated Flos Lonicerae japonicae using comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry coupled with chemical group separation. Molecules 18:1368–1382. https://doi.org/10.3390/molecules18021368
Sciarrone D, Costa R, Ragonese C, Tranchida PQ, Tedone L, Santi L, Dugo P, Dugo G, Mondello L (2011) Application of a multidimensional gas chromatography system with simultaneous mass spectrometric and flame ionization detection to the analysis of sandalwood oil. J Chromatogr A 1218:137–142. https://doi.org/10.1016/j.chroma.2010.10.117
Zoccali M, Tranchida PQ, Bonaccorsi IL, Dugo P, Mondello L, Dugo G (2016) Detailed profiling of the volatile oxygenated fraction of mandarin essential oils using the off-line combination of high-performance liquid chromatography and comprehensive two-dimensional gas chromatography-mass spectrometry. Food Anal Method 10:1106–1116. https://doi.org/10.1007/s12161-016-0668-2
Kirkin C, Mitrevski B, Gunes G, Marriott PJ (2014) Essential-oil analysis of irradiated spices using comprehensive two-dimensional gas chromatography. ChemPlusChem 79:798–803. https://doi.org/10.1002/cplu.201300430
Sciarrone D, Ragonese C, Carnovale C, Piperno A, Dugo P, Dugo G, Mondello L (2010) Evaluation of tea tree oil quality and ascaridole: a deep study by means of chiral and multi heart-cuts multidimensional gas chromatography system coupled to mass spectrometry detection. J Chromatogr A 1217:6422–6427. https://doi.org/10.1016/j.chroma.2010.08.019
Tranchida PQ, Franchina FA, Zoccali M, Panto S, Sciarrone D, Dugo P, Mondello L (2013) Untargeted and targeted comprehensive two-dimensional GC analysis using a novel unified high-speed triple quadrupole mass spectrometer. J Chromatogr A 1278:153–159. https://doi.org/10.1016/j.chroma.2012.12.066
von Muhlen C, Zini CA, Caramao EB, Marriott PJ (2008) Comparative study of Eucalyptus dunnii volatile oil composition using retention indices and comprehensive two-dimensional gas chromatography coupled to time-of-flight and quadrupole mass spectrometry. J Chromatogr A 1200:34–42. https://doi.org/10.1016/j.chroma.2008.05.070
Ma C, Wang H, Lu X, Li H, Liu B, Xu G (2007) Analysis of Artemisia annua L. volatile oil by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J Chromatogr A 1150:50–53. https://doi.org/10.1016/j.chroma.2006.08.080
Qiu Y, Lu X, Pang T, Zhu S, Kong H, Xu G (2007) Study of traditional Chinese medicine volatile oils from different geographical origins by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GC × GC–TOFMS) in combination with multivariate analysis. J Pharm Biomed Anal 43:1721–1727. https://doi.org/10.1016/j.jpba.2007.01.013
Filippi JJ, Belhassen E, Baldovini N, Brevard H, Meierhenrich UJ (2013) Qualitative and quantitative analysis of vetiver essential oils by comprehensive two-dimensional gas chromatography and comprehensive two-dimensional gas chromatography/mass spectrometry. J Chromatogr A 1288:127–148. https://doi.org/10.1016/j.chroma.2013.03.002
Tissandie L, Brevard H, Belhassen E, Alberola M, Meierhenrich U, Filippi JJ (2018) Integrated comprehensive two-dimensional gas-chromatographic and spectroscopic characterization of vetiveryl acetates: Molecular identifications, quantification of constituents, regulatory and olfactory considerations. J Chromatogr A. https://doi.org/10.1016/j.chroma.2018.08.050
Brokl M, Fauconnier ML, Benini C, Lognay G, du Jardin P, Focant JF (2013) Improvement of ylang-ylang essential oil characterization by GC × GC-TOFMS. Molecules 18:1783–1797. https://doi.org/10.3390/molecules18021783
Fidelis CHV, Augusto F, Sampaio PTB, Krainovic PM, Barata LES (2012) Chemical characterization of rosewood (Aniba rosaeodora Ducke) leaf essential oil by comprehensive two-dimensional gas chromatography coupled with quadrupole mass spectrometry. J Essent Oil Res 24:245–251. https://doi.org/10.1080/10412905.2012.676770
Krupcik J, Gorovenko R, Spanik I, Sandra P, Armstrong DW (2015) Enantioselective comprehensive two-dimensional gas chromatography. A route to elucidate the authenticity and origin of Rosa damascena Miller essential oils. J Sep Sci 38:3397–3403. https://doi.org/10.1002/jssc.201500744
Western RJ, Marriott PJ (2002) Retention correlation maps in comprehensive two-dimensional gas chromatography. J Sep Sci 25:831–838. https://doi.org/10.1016/j.chroma.2003.09.006
Bieri S, Marriott PJ (2006) Generating multiple independent retention index data in dual-secondary column comprehensive two-dimensional gas chromatography. Anal Chem 78:8089–8097. https://doi.org/10.1021/ac060869l
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. Allured Publishing Corporation, Carol Stream
Saucier C, Polidoro AdS, dos Santos AL, Schneider JK, Caramão EB, Jacques RA (2014) Comprehensive two-dimensional gas chromatography with mass spectrometry applied to the analysis of volatiles in artichoke (Cynara scolymus L.) leaves. Ind Crops Prod 62:507–514. https://doi.org/10.1016/j.indcrop.2014.09.023
Savareear B, Jacobs MR, Shellie RA (2014) Multiplexed dual first-dimension comprehensive two-dimensional gas chromatography-mass spectrometry with contra-directional thermal modulation. J Chromatogr A 1365:183–190. https://doi.org/10.1016/j.chroma.2014.09.014
Zoccali M, Bonaccorsi IL, Tranchida PQ, Dugo P, Mondello L, Dugo G (2015) Analysis of the sesquiterpene fraction of citrus essential oils using the off-line combination of high performance liquid chromatography and gas chromatography-based methods: a comparative study. Flavour Fragr J 30:411–422. https://doi.org/10.1002/ffj.3262
Yan D, Wong YF, Tedone L, Shellie RA, Marriott PJ, Whittock SP, Koutoulis A (2018) Chemotyping of new hop (Humulus lupulus L.) genotypes using comprehensive two-dimensional gas chromatography with quadrupole accurate mass time-of-flight mass spectrometry. J Chromatogr A 1536:110–121. https://doi.org/10.1016/j.chroma.2017.08.020
Pripdeevech P, Chumpolsri W, Suttiarporn P, Wongpornchai S (2010) The chemical composition and antioxidant activities of basil from Thailand using retention indices and comprehensive two-dimensional gas chromatography. J Serb Chem Soc 75:1503–1513. https://doi.org/10.2298/jsc100203125p
Cordero C, Rubiolo P, Cobelli L, Stani G, Miliazza A, Giardina M, Firor R, Bicchi C (2015) Potential of the reversed-inject differential flow modulator for comprehensive two-dimensional gas chromatography in the quantitative profiling and fingerprinting of essential oils of different complexity. J Chromatogr A 1417:79–95. https://doi.org/10.1016/j.chroma.2015.09.027
Schipilliti L, Bonaccorsi I, Sciarrone D, Dugo L, Mondello L, Dugo G (2013) Determination of petitgrain oils landmark parameters using gas chromatography-combustion-isotope ratio mass spectrometry and enantioselective multidimensional gas chromatography. Anal Bioanal Chem 405:679–690. https://doi.org/10.1007/s00216-012-6031-6
dos Santos AL, Polidoro AdS, Schneider JK, da Cunha ME, Saucier C, Jacques RA, Cardoso CAL, Mota JS, Caramão EB (2015) Comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GC × GC/TOFMS) for the analysis of volatile compounds in Piper regnellii (Miq.) C. DC. essential oils. Microchem J 118:242–251. https://doi.org/10.1016/j.microc.2014.07.007
Bonaccorsi I, Sciarrone D, Schipilliti L, Dugo P, Mondello L, Dugo G (2012) Multidimensional enantio gas chromtography/mass spectrometry and gas chromatography-combustion-isotopic ratio mass spectrometry for the authenticity assessment of lime essential oils (C. aurantifolia Swingle and C. latifolia Tanaka). J Chromatogr A 1226:87–95. https://doi.org/10.1016/j.chroma.2011.10.038
Nsuala BN, Kamatou GP, Sandasi M, Enslin G, Viljoen A (2017) Variation in essential oil composition of Leonotis leonurus, an important medicinal plant in South Africa. Biochem Syst Ecol 70:155–161. https://doi.org/10.1016/j.bse.2016.11.009
Wong YF, West RN, Chin ST, Marriott PJ (2015) Evaluation of fast enantioselective multidimensional gas chromatography methods for monoterpenic compounds: authenticity control of Australian tea tree oil. J Chromatogr A 1406:307–315. https://doi.org/10.1016/j.chroma.2015.06.036
Fidelis CHV, Sampaio PTB, Krainovic PM, Augusto F, Barata LES (2013) Correlation between maturity of tree and GC × GC-qMS chemical profiles of essential oil from leaves of Aniba rosaeodora Ducke. Microchem J 109:73–77. https://doi.org/10.1016/j.microc.2012.03.034
Dugo P, Bonaccorsi I, Ragonese C, Russo M, Donato P, Santi L, Mondello L (2011) Analytical characterization of mandarin (Citrus deliciosa Ten.) essential oil. Flavour Fragr J 26:34–46. https://doi.org/10.1002/ffj.2014
Kutlular Ö, Özel MZ (2009) Analysis of essential oils of Origanum onites by superheated water extraction using GC × GC–TOF/MS. J Essent Oil-Bear Plants 12:462–470. https://doi.org/10.1080/0972060x.2009.10643745
Qiu Y, Lu X, Pang T, Ma C, Li X, Xu G (2008) Determination of radix ginseng volatile oils at different ages by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. J Sep Sci 31:3451–3457. https://doi.org/10.1002/jssc.200800253
Cao G, Cai H, Jiang J, Yao L, Tu S, Wang L, Ma X, Cai B (2014) Chemical differentiation of volatile compounds in crude and processed Atractylodis Macrocephalae Rhizoma using comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with multivariate data analysis. J Sep Sci 37:1194–1198. https://doi.org/10.1002/jssc.201301376
Notar Francesco I, Filippi J-J, Antoniotti S (2017) Sustainable manufacture of a valuable fragrance ingredient: Lipase-catalyzed acylation of vetiver essential oil and chemoselectivity between sesquiterpene alcohols. ChemPlusChem 82:407–415. https://doi.org/10.1002/cplu.201600617
Omar J, Alonso I, Olivares M, Vallejo A, Etxebarria N (2012) Optimization of comprehensive two-dimensional gas-chromatography (GC × GC) mass spectrometry for the determination of essential oils. Talanta 88:145–151. https://doi.org/10.1016/j.talanta.2011.10.023
Polidoro AdS, Scapin E, Malmann M, do Carmo JU, Machado ME, Caramão EB, Jacques RA (2016) Characterization of volatile fractions in green mate and mate leaves (Ilex paraguariensis A. St. Hil.) by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC × GC/TOFMS). Microchem J 128:118–127. https://doi.org/10.1016/j.microc.2016.03.019
Pripdeevech P, Wongpornchai S, Marriott PJ (2010) Comprehensive two-dimensional gas chromatography-mass spectrometry analysis of volatile constituents in Thai vetiver root oils obtained using different extraction methods. Phytochem Anal 21:163–173. https://doi.org/10.1002/pca.1173
Lo Presti M, Sciarrone D, Crupi ML, Costa R, Ragusa S, Dugo G, Mondello L (2008) Evaluation of the volatile and chiral composition in Pistacia lentiscus L. essential oil. Flavour Fragr J 23:249–257. https://doi.org/10.1002/ffj.1878
Bonaccorsi I, Trozzi A, Cotroneo A, Dugo G (2013) Composition of industrial bergamot petitgrain produced in Calabria. J Essent Oil Res 25:359–363. https://doi.org/10.1080/10412905.2013.809324
Harvey PM, Poynter SDH, Shellie RA (2011) GC × GC with fluidic modulation for enantioselective essential oil analysis. LC GC Eur 24:548–555
Barba C, Toledano RM, Santa-Maria G, Herraiz M, Martinez RM (2013) Enantiomeric analysis of limonene and carvone by direct introduction of aromatic plants into multidimensional gas chromatography. Talanta 106:97–103. https://doi.org/10.1016/j.talanta.2012.11.050
Krupcik J, Gorovenko R, Spanik I, Armstrong DW, Sandra P (2016) Enantioselective comprehensive two-dimensional gas chromatography of lavender essential oil. J Sep Sci 39:4765–4772. https://doi.org/10.1002/jssc.201600986
European Pharmacopeia (2014) VIII edn. Council of Europe: European directorate for the quality of medicines (EDQM), Strasbourg
Greule M, Hänsel C, Bauermann U, Mosandl A (2007) Feed additives: authenticity assessment using multicomponent-/multielement-isotope ratio mass spectrometry. Eur Food Res Technol 227:767–776. https://doi.org/10.1007/s00217-007-0785-7
König W, Hochmuth D (2004) Enantioselective gas chromatography in flavor and fragrance analysis: Strategies for the identification of known and unknown plant volatiles. J Chromatogr Sci 42:423–439. https://doi.org/10.1093/chromsci/42.8.423
Mateus E, Barata RC, Zrostlikova J, Gomes da Silva MD, Paiva MR (2010) Characterization of the volatile fraction emitted by Pinus spp. by one- and two-dimensional chromatographic techniques with mass spectrometric detection. J Chromatogr A 1217:1845–1855. https://doi.org/10.1016/j.chroma.2010.01.045
Schmarr H-G, Engel K-H (2012) Analysis and stereodifferentiation of linalool in Theobroma cacao and cocoa products using enantioselective multidimensional gas chromatography. Eur Food Res Technol 235:827–834. https://doi.org/10.1007/s00217-012-1812-x
Eyres G, Marriott PJ, Dufour JP (2007) The combination of gas chromatography-olfactometry and multidimensional gas chromatography for the characterisation of essential oils. J Chromatogr A 1150:70–77. https://doi.org/10.1016/j.chroma.2006.07.019
Chin S-T, Marriott PJ (2015) Review of the role and methodology of high resolution approaches in aroma analysis. Anal Chim Acta 854:1–12. https://doi.org/10.1016/j.aca.2014.06.029
Van der Wat L, Dovey M, Naudé Y, Forbes P (2013) Investigation into the aroma of rosemary using multi-channel silicone rubber traps, off-line olfactometry and comprehensive two-dimensional gas chromatography-mass spectrometry. S Afr J Chem 66:21–26
Costa R, d’Acampora Zellner B, Crupi ML, Fina MRD, Valentino MR, Dugo P, Dugo G, Mondello L (2008) GC–MS, GC–O and enantio–GC investigation of the essential oil of Tarchonanthus camphoratus L. Flavour Fragr J 23:40–48. https://doi.org/10.1002/ffj.1854
de Saint Laumer JY, Ciccheti E, Merle P, Egger J, Chaintreau A (2010) Quantification in gas chromatography: prediction of flame ionization detector response factors from combustion enthalpies and molecular structures. Anal Chem 82:6457–6462. https://doi.org/10.1021/ac1006574
Tissot E, Rochat S, Debonneville C, Chaintreau A (2012) Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour Fragr J 27:290–296. https://doi.org/10.1002/ffj.3098
Schaffer M, Groger T, Putz M, Zimmermann R (2013) Forensic profiling of sassafras oils based on comprehensive two-dimensional gas chromatography. Forensic Sci Int 229:108–115. https://doi.org/10.1016/j.forsciint.2013.03.046
Jalali-Heravi M, Parastar H (2011) Recent trends in application of multivariate curve resolution approaches for improving gas chromatography-mass spectrometry analysis of essential oils. Talanta 85:835–849. https://doi.org/10.1016/j.talanta.2011.05.045
de Oliveira RR, de Lima KMG, Tauler R, de Juan A (2014) Application of correlation constrained multivariate curve resolution alternating least-squares methods for determination of compounds of interest in biodiesel blends using NIR and UV–visible spectroscopic data. Talanta 125:233–241. https://doi.org/10.1016/j.talanta.2014.02.073
de Godoy LA, Hantao LW, Pedroso MP, Poppi RJ, Augusto F (2011) Quantitative analysis of essential oils in perfume using multivariate curve resolution combined with comprehensive two-dimensional gas chromatography. Anal Chim Acta 699:120–125. https://doi.org/10.1016/j.aca.2011.05.003
He M, Yang ZY, Yang TB, Ye Y, Nie J, Hu Y, Yan P (2017) Chemometrics-enhanced one-dimensional/comprehensive two-dimensional gas chromatographic analysis for bioactive terpenoids and phthalides in Chaihu Shugan San essential oils. J Chromatogr B 1052:158–168. https://doi.org/10.1016/j.jchromb.2017.03.029
Acknowledgements
Authors would like to thank the University of Tasmania, Australia and Plant Therapy Inc., Twin Falls, Idaho, USA, for their generous provision of co-funded PhD scholarship and project support for Leo Lebanov.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Published in the topical collection Rising Stars in Separation Science, as part of Chromatographia’s 50th Anniversary Commemorative Issue.
Rights and permissions
About this article
Cite this article
Lebanov, L., Tedone, L., Kaykhaii, M. et al. Multidimensional Gas Chromatography in Essential Oil Analysis. Part 2: Application to Characterisation and Identification. Chromatographia 82, 399–414 (2019). https://doi.org/10.1007/s10337-018-3651-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3651-9