Abstract
Bioindicators of wildlife health are useful tools for studying the viability of various organisms and populations, and can include a range of phenotypic variables, such as behavior, body size, and physiological parameters, such as circulating hormones and nutrients. Few studies have investigated the utility of total plasma protein as a predictor of environmental or nutritional variation among birds, as well as variation across different habitats and life-history stages. Here, we examined relationships between plasma protein and season, urbanization, sex, body condition, molt status, and disease state in House Finches (Haemorhous mexicanus). We sampled blood from House Finches across three seasons (winter, summer, and fall 2021) and measured plasma protein levels using a Bradford assay. We also collected data including condition, sex, and poxvirus infection state at capture, as well as fecal samples to assess gut parasitism (coccidiosis). During the fall season, we also estimated molt status, as number of actively growing feathers. We found a significant relationship between circulating protein levels and capture site, as well as novel links to molt state and pox presence, with urban birds, those infected with pox, and those in more intense molt having higher protein levels. Our results support the hypotheses that plasma protein concentration can be indicative of a bird’s body molt and degree of habitat urbanization, although future work is needed to determine why protein levels were higher in virus-infected birds.
Zusammenfassung
Variation des Plasmaproteinspiegels beim Hausgimpel ( Haemorhous mexicanus ): Auswirkungen von Jahreszeit, Krankheitszustand und Urbanisierung
Bioindikatoren für die Gesundheit von Wildtieren sind nützliche Instrumente zur Untersuchung der Überlebensfähigkeit verschiedener Organismen und Populationen und können eine Reihe von phänotypischen Parametern wie Verhalten, Körpergröße und physiologische Parameter, z. B. im Blut zirkulierende Hormone und Nährstoffe, umfassen. Nur wenige Studien haben den Nutzen des Gesamtplasmaproteinspiegels als Indikator für umwelt- und ernährungsbedingte Variation unter Vögeln sowie Unterschiede über verschiedene Lebensräume und -stadien hinweg untersucht. In dieser Studie haben wir die Beziehungen zwischen dem Plasmaproteinspiegel und Jahreszeit, Urbanisierung, Geschlecht, Körperkondition, Mauser- und Krankheitszustand beim Hausgimpel (Haemorhous mexicanus) beleuchtet. Es wurden Blutproben vom Hausgimpel über drei Jahreszeiten (Winter, Sommer, Herbst 2021) entnommen und der Plasmaproteinspiegel mithilfe eines Bradford-Tests gemessen. Weiterhin sammelten wir Daten zu Körperkondition, Geschlecht und Grad der Pockenvirusinfektion beim Fang sowie Kotproben, um den Befall mit Darmparasiten (Kokzidiose) zu beurteilen. Weiterhin schätzten wir während der Herbstsaison den Mauserzustand ein, d. h. die Anzahl an aktiv wachsenden Federn. Wir fanden eine signifikante Beziehung zwischen dem zirkulierenden Proteinspiegel und dem Fangort sowie neue Zusammenhänge zwischen dem Mauserzustand und vorhandenen Pockenviren, wobei Vögel in der Stadt, mit Pockenviren infizierte Vögel und Vögel, die sich in einer intensiveren Mauser befanden, einen höheren Proteinspiegel aufwiesen. Unsere Ergebnisse unterstützen die Hypothese, dass der Plasmaproteinspiegel einen Hinweis auf den Mauserstatus der Vögel und den Grad der Verstädterung ihres Habitats bieten kann, wenn auch noch zukünftige Untersuchungen benötigt werden, um festzustellen, warum der Proteinspiegel bei Vögeln mit einer Virusinfektion höher war.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological indicators of health are useful tools with which to assess the viability and persistence of wildlife, both at the individual and population levels, and can include morphological, metabolic, endocrine, and genetic traits (Cazenave et al. 2009; Koelmel et al. 2019). These bioindicators have proven useful in investigating the effects of anthropogenic activities such as urbanization on various wildlife species (Chaousis et al. 2018; Ortiz-Santaliestra et al. 2019). Major environmental challenges presented by these anthropogenic factors to organisms include novel vegetation and habitats, dietary shifts, traffic, introduced species, and exposure to noise, chemical, and light pollution (Mills et al. 1989; Chace and Walsh 2006; Bichet et al. 2013; Dominoni 2015; Job et al. 2016; Peneaux et al. 2021), and biomarkers such as circulating levels of glutathione and carotenoid coloration have been used to evaluate effects of urban stressors and pollution in Great Tits (Parus major; Isaksson et al. 2005). Additionally, telomere length was shown to reliably reveal impacts of novel urban environments on development and lifespan in Great Tits (Salmón et al. 2016).
Plasma protein levels present an interesting opportunity for use as such a bioindicator, given the low amount of blood required and the relative simplicity of running plasma protein assays. Plasma proteins play a variety of important biological roles, such as serving as nutrients, maintaining osmotic pressure, and transporting circulating compounds (Schaller et al. 2008). Both total plasma protein as well as individual proteins such as albumin have been previously used as a bioindicator of health in a variety of vertebrate species, including passerine birds and ungulates (Serrano et al. 2008; Norte et al. 2009). Work done with Gambel’s Quail (Callipepla gambelii) found circulating protein concentration to be positively associated with presence of grass but not more general markers of urbanization, suggesting that building- and concrete-dominated urban environments may affect circulating protein levels through limited dietary availability, alterations of osmotic pressure, or other means (Funk et al. 2020). Work in House Sparrows (Passer domesticus) found that urban birds had more access to dietary protein but only mildly increased levels of circulating albumin, the major component of total protein (Gavett and Wakeley 1986). In Galapagos, Sea Lion (Zalophus wollebaeki) colonies impacted by anthropogenic activities, total protein was found to be negatively correlated with antibody levels (Brock et al. 2013).
Total plasma protein has also been studied in relation to life-history events, such as breeding and molt. In American Kestrels (Falco sparverius) in Canada, for example, there is complex variation in total plasma protein levels between sexes, among phases of mating season, and in relation to body condition and temperature, but with no links to malarial infection (Dawson and Bortolotti 1997). A life-history stage that is of particular interest when studying variation in circulating protein in birds is the process of molt. Molt is a several month-long period for birds, as production of new plumage involves large investment of proteins (Heitmeyer 1988; Murphy and King 1992). Molt also affects protein metabolism and synthesis throughout avian tissues (Dolnik and Gavrilov 1979; Murphy and Taruscio 1995) and affects body composition by altering proportional allocation of proteins. For instance in King Penguins (Aptenodytes patagonicus), pectoral muscle and integument serve as sources for protein needed to synthesize new plumage (Cherel et al. 1994). Despite the strong mechanistic links between molt and protein, the relationship between molt and circulating protein is relatively poorly understood. This relationship has only been investigated in a few species of birds, including American Kestrels, in which no relationship was found between total protein and molt presence or intensity (Dawson and Bortolotti 1997). However, a study on captive Bar-Headed Geese (Anser indicus) found that total plasma protein concentrations were reduced during the molt period (Roman et al. 2009). Circulating protein has also been linked to exoskeletal molt in arthropods such as the Whiteleg Shrimp (Penaeus vannamei), with concentrations of hemolymph protein increasing throughout molt (Chan et al. 1988). Interestingly, anthropogenic effects of urbanization such as light pollution have been found to facilitate irregular patterns of molt in European Blackbirds (Turdus merula; Dominoni et al. 2013), which may link to urban effects on circulating protein levels.
Circulating protein titers may also be directly related to infectious disease. In a study of internal parasites in Sheep (Ovis aries), total plasma protein has been found to negatively correlate to parasite fecal egg count (McEwan et al. 1992). In Chickens (Gallus domesticus), coccidiosis—a disease caused by infection by protozoan parasites has been associated with decreased circulating protein (Mondal et al. 2011), and total plasma protein was found to be reduced throughout the course of disease in Goat kids, but ultimately coccidiosis was found to have no significant effect on total protein (Hashemnia et al. 2014). Along with parasites, viruses also present salient health challenges to wildlife. Elevated plasma protein levels have been found in studies of Humans with some viral diseases, such as hepatitis A as well as HIV (Patil and Raghuwanshi 2009; Balan et al. 2018). However, work with Chickens infected with Newcastle virus disease found a reduction of circulating protein caused by infection (Oladele et al. 2005). Given that increased parasite/pathogen infections are another major challenge that wildlife face related to the acceleration of global urbanization (Bradley and Altizer 2007), measuring circulating protein could prove a useful tool in investigating relationships between disease and urbanization.
House Finches (Haemorhous mexicanus) serve as an ideal model to simultaneously study the effects of urbanization, disease, and life-history traits such as molt on a wildlife species. Urbanization affects many phenotypic traits in this species, including parasite/pathogen burden (e.g. canary poxvirus infection, coccidiosis; Giraudeau et al. 2014), expression of sexually attractive male coloration (Giraudeau et al. 2018), and molt dynamics (Hutton et al. 2021), with urban birds having greater disease burden, less colorful plumage, and beginning molt earlier and finishing molt later than rural birds. House Finches in our area of study in Phoenix, Arizona, USA have a long breeding season that spans from spring through mid-summer (March–July), and undergo their annual pre-basic molt starting in late summer and finishing in fall (July–October).
We hypothesized that plasma protein levels may be a salient physiological integrator of these various morphological and health responses to urbanization. In this field study, we drew blood from House Finches captured along an urban gradient and at multiple timepoints throughout the year in order to measure total plasma protein in addition to disease state (presence and severity of both canarypox virus infection and coccidiosis) and test for environmental and life-history predictors of circulating protein. For birds sampled during the molt period, we also recorded molt progress to investigate how molt may additionally relate to circulating plasma protein. Given known urban–rural differences in molt duration and intensity in House Finches (Hutton et al. 2021), we predicted that urban and rural birds would differ most in total plasma protein during peak molt (August–September). Since protein demands can increase when molt is most intense (Heitmeyer 1988), we expected circulating protein concentration to be positively correlated with molt intensity. Additionally, we predicted that plasma protein would be higher in urban compared to rural finches given prior work in house sparrows showing mildly higher levels of albumin in urban than rural birds (Gavett and Wakeley 1986), which may reflect higher dietary protein intake. We also predicted that birds circulating more plasma protein would have less severe coccidiosis and poxvirus infections, if protein is positively associated with health (e.g. coccidiosis has been associated with reduced plasma protein; McEwan et al. 1992). Finally, based on prior studies finding significant sex differences in plasma protein only during the breeding season (Dawson and Bortolotti 1997), which was a season we did not study (see more below), we predicted that there would not be significant sex differences in total plasma protein.
Methods
Field methods
Using basket traps hung around sunflower-seed feeders (as described in Hill 2002), we trapped a total of 214 House Finches (Table 1; each bird appears only once in our study) at an urban (Arizona State University campus in Tempe, AZ; lat 33°25′11.4″ N, long 111°55′59.3″ W), a suburban (residential backyard in Tempe, AZ; lat 33°20′41.1″ N, long 111°55′49.5″ W), and a rural site (South Mountain Regional Park in Phoenix, AZ; lat 33°21′01.9″ N, long 112°04′33.6″ W). These sites differ in population density as well as urban land-use and land cover metrics (see Giraudeau et al. (2014) and McGraw et al. (2020) for additional site and urbanization-scoring details). We trapped during three separate sessions/seasons—winter 2021 (25–30 January), during the postbreeding season, summer 2021 (9–15 July), and during molting season, fall 2021 (14–28 September). At capture, we banded finches with United States Geological Survey numbered metal leg rings for individual identification. We determined sex and age of birds using plumage traits (i.e. red/orange/yellow carotenoid pigmentation of the crown and breast of sexually mature males, presence of buff wing coverts in juveniles during summer and early fall; Pyle 2008); it is worth noting that all birds were designated as after-hatch-year in our January sample because breeding does not commence annually until March (Table 1). We weighed finches with a digital scale (to the nearest 0.1 g) and measured tarsus length using digital calipers (to the nearest 0.01 mm). Mass and tarsus length were used to determine residual mass of birds (from a significant mass–tarsus regression; r2 = 0.16, F1,211 = 38.97, p < 0.001) as our measure of body condition. Finches were inspected for poxvirus lesions and we estimated infection severity on an integer scale of 0–4 (sensu Giraudeau et al. 2014). We collected up to 140 µL of blood from each bird via brachial venipuncture. The blood was collected into heparinized microcapillary tubes, transferred to snap-cap 1.5 mL Eppendorf tubes, and centrifuged at 10,000 RPM for 3 min to save the plasma fraction at − 80 °C indefinitely for later plasma protein analyses (see more below). Afterward, to measure levels of endoparasitic infection (coccidiosis), we housed finches temporarily in individual cages with ad lib sunflower seed and water access inside the certified Department of Animal Care and Technology vivarium on the Arizona State University Tempe campus. At 1600 h, we placed a fresh piece of tray paper into each cage, and at 1700 h returned to the room to collect fresh feces from the tray paper, which we stored in 2.2% potassium dichromate solution at room temperature until later parasite scoring (see more below).
Molt scoring
We scored molt intensity for birds captured in September 2021. For each individual, we counted the total number of actively growing contour feathers, which includes pinfeathers, sheathed feathers, and unsheathed but incompletely grown feathers, in carotenoid-colored feather regions (crown, breast, and rump for males, rump only for females; sensu Hutton et al. 2021). This metric of molt intensity permitted us to examine how protein levels may vary as a function of shifts in putative protein investment into feather production. We statistically analyzed number of feathers actively growing only on the rump (where both sexes have carotenoid color) to permit proper comparison of males and females in the molt model (see more below).
Measuring plasma protein
Total plasma protein levels were determined using the Bradford method (Sigma Aldrich TP0100 kit and Supelco Bradford reagent). Samples were prepared by mixing 2.5 mL Bradford assay solution with 1 µL finch plasma and 49 µL 0.85% saline solution. Negative controls were prepared by mixing 2.5 mL Bradford assay solution with 50 µL 0.85% saline solution and positive controls were prepared by mixing 2.5 mL Bradford assay solution with 50 µL protein standard (0.3 mg/mL human albumin). Negative controls (saline only) were used to calibrate the spectrophotometer, and absorbance for all samples was measured at 595 nm. Absorbance was used to calculate the total protein concentration according to the bulletin of the Sigma TP0100 kit. To confirm repeatability of the procedure (see Lessells and Boag 1987), we ran 204 samples in duplicate and 10 samples in quadruplet and found moderately high repeatability for these measurements (r2 = 0.71, F1,223 = 556.79, p < 0.001); averages of multiple samples/measurements per bird were then used in statistical analyses.
Scoring coccidiosis
Presence and severity of coccidiosis infection was assessed using the fecal floatation method (McGraw and Hill 2000). Fecal samples in potassium dichromate solution were combined with Sheather's sugar solution to fill an 8 mL glass culture tube and centrifuged at 2100 RPM for 7 min with a microscope cover slip on top. Cover slips were removed and placed atop microscope slides, which were analyzed under a compound light microscope and scored for the estimated number of oocysts present (sensu McGraw and Hill 2000) using a logarithmic scale of 0–5 (0 corresponding to 0 oocysts present, 1: 1–10 oocysts, 2: 11–100, 3: 101–1000, 4: 1001–10,000, and 5: > 10,000). All slides were scored independently by two individuals and, when scores differed, were rescored until the observers agreed on a score; fractional scores were used in just two cases where a score could not be agreed upon.
Statistics
Data were analyzed using JMP 16.2.0 (SAS Institute Inc., Cary, NC, USA). Because not all variables were measured in each season (e.g. molt scored only during fall 2021 and coccidiosis was not scored in winter 2021 due to logistical reasons), we ran two separate standard least-squares regression models to determine the factors that best predicted plasma protein levels. Model 1 contained data for all seasons except winter 2021 (n = 120 birds, because we excluded one bird that lacked a tarsus measurement and one bird that lacked coccidiosis data) and contained season, site, pox presence, coccidia score, age, and residual mass as predictors. Model 2 contained data just for fall 2021 (n = 54 birds, because we excluded one bird that lacked coccidiosis data) and included all variables except season and age (as all birds caught in fall were hatch-years) as predictors—site, sex, molt intensity, molt proportion, pox presence, coccidiosis score, and residual mass. All regressions were run initially as a global model (i.e. with all relevant predictors) and from these a best-fit model was determined based on the lowest Akaike’s Information Criterion (AICc) value (Bozdogan 1987), through running all possible combinations excluding interactions of variables and comparing the AICc values of these models.
Results
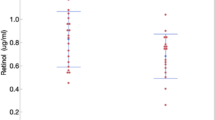
In Model 1, which included summer- and fall-caught birds (i.e. those for which we had both coccidiosis and poxvirus data), season was the only statistically significantly predictor of plasma protein concentration, although the effects of pox presence and coccidia score approached significance (Table 2). We found total protein levels to be greater in birds sampled during summer than in fall, although total protein levels were greatest in winter (Fig. 1). In Model 2, which included finches caught in fall 2021 (when we scored molt intensity), we found in the best-fit model that capture site, pox presence, and rump molt intensity score were the remaining (and statistically significant) predictors of plasma protein concentration (Table 3). Urban finches had significantly higher total plasma protein than suburban or rural birds (Fig. 2). Birds with higher molt intensity circulated a higher concentration of plasma protein overall (Fig. 3), although when analyzed by site, birds with higher molt intensity had higher levels of plasma protein in urban and suburban birds, but higher molt intensity was associated with lower levels of circulating protein in rural birds (Fig. 4). Birds infected with poxvirus circulated a higher concentration of plasma protein (Fig. 5).
Discussion
We examined habitat-type and life-history predictors of circulating protein levels in House Finches across multiple seasons and found that degree of urbanization proved to be a significant predictor of plasma protein levels in our fall-only model during molt, with urban birds having significantly higher plasma protein concentrations than suburban or rural birds. Habitat type proved to have a relatively large relative effect on total protein levels in this model. The average marginal mean of plasma protein in rural birds, in which total protein levels were lowest, was 15.8 mg/mL, as compared to urban finches, which had a marginal mean of plasma protein of 18.1 mg/mL. Prior work in House Sparrows found non-significant elevations in albumin concentration in urban birds relative to rural ones as well as elevations in blood urea nitrogen, suggesting higher dietary protein intake (Gavett and Wakeley 1986). It is possible that, during fall molt, urban areas may offer more dietary protein to House Finches, as monsoon rains in late summer may bring on an abundance of plant life (finches are herbivores) even more so in cities, with their rich sources (e.g. yards, gardens, parks) of ornamental vegetation, compared to the desert (Hope et al. 2006). Alternatively, or additionally, the fact that circulating protein concentration was higher in urban birds during fall could be explained by the fact that finches molt (at this time of year) differently in urban v. rural areas (Hutton et al. 2021). Work in Mallards (Anas platyrhynchos) found dietary protein requirements to be significantly elevated during molt above baseline (Heitmeyer 1988), and because urban House Finches start molting earlier but take longer to molt, it is possible that their protein needs are elevated and levels stay higher as they complete this more protracted period of feather replacement. Work in European Blackbirds demonstrates a similar pattern, with urban birds beginning molt earlier and finishing molt later than rural birds, and suggests that chronic light exposure may contribute to earlier onset of molt (Dominoni et al. 2013), and its possible that factors such as light exposure may contribute to differences in molt between these habitats. The time point at which we sampled birds in fall was during the later portion of molt (molt in these populations occurs from July to the first part of October), and had we examined protein levels throughout the fall/molt season we may have uncovered a closer link between circulating protein and molt progress in urban, suburban, and rural birds.
Still, to more explicitly examine the potential link between protein levels and the molt process, we scored molt intensity of sampled birds and, in our fall-only statistical model, found that birds with more actively growing feathers had higher levels of circulating protein, with a modestly sized relative effect (means of approximately 15.5 mg/mL in birds with the lowest molt scores, and approximately 16.5 mg/mL in birds with the highest molt scores). This pattern is similar to that in Mallards, where protein requirements are highest for birds in peak feather molt (Heitmeyer 1988). Of note when each site was assessed separately, the positive correlation between molt intensity and total plasma protein was only found in urban and suburban birds, as molt intensity was negatively correlated with total protein in rural birds. This trend may reflect differences in molt dynamics driven by habitat urbanization. However, this negative trend in rural birds should be interpreted cautiously, given that we caught significantly less rural birds in fall. Interestingly, some studies have examined levels of particular proteins during molt, and found that some types rise (e.g. prealbumin), while others fall (e.g. albumin) when molt is at its peak (e.g. in Bar-Headed Geese, Anser indicus; Roman et al. 2009). Future experimental work aimed at more intricate assessments of intake (through supplemental feeding or restriction studies) and utilization (through isotope tracing) of specific proteins will yield powerful insights into the roles of proteins during the molt process in finches.
We also found that, during the molt season, canary poxvirus presence was a significant predictor of circulating protein, such that finches infected with pox had higher levels of total protein than those without pox. This pattern was counter to our prediction that healthier birds would circulate more protein. To our knowledge, this is the first study to document increased levels of total protein being correlated with viral infection in a wild bird species, and thus further work should be done to investigate the mechanism underlying this pattern. The relative effect of poxvirus infection in Model 3 was fairly moderate, with birds without pox having a marginal mean of total protein of 15.8 mg/mL, and birds with pox having a marginal mean of 17.7 mg/mL. Increases in other circulating nutrients such as glucose have been demonstrated in poxvirus-infected House Finches (McGraw et al. 2020), and given that elevated blood glucose and plasma protein both have links to dehydration (Ashraf and Rea 2017), the effects of poxvirus on protein could be explained by dehydration. Poxvirus infection is associated with production of immunoglobins, cytokines, and other proteins by host cells, as well as virokines by poxviruses (Saghazadeh and Rezaei 2022), and these proteins may contribute to the elevation in total protein titers that we observed in poxvirus-infected birds. In addition, various viral diseases such as hepatitis A and HIV have also been associated with increases of total plasma protein and globulins specifically in Human subjects (Patil and Raghuwanshi 2009; Balan et al. 2018), and thus there likely may be links in the pathologies of these diseases to circulating proteins.
Of note, we found no significant sex differences in total plasma protein concentration in our molt season model, which matched our expectations. However, we did not measure plasma protein in the breeding season of finches (spring), during which sex differences in protein may manifest, given the protein demands of egg production in females (Dawson and Bortolotti 1997). Contrary to our expectations, coccidiosis did not significantly predict plasma protein titers in Model 1 or Model 2, although it did approach significance in Model 1 (p = 0.051). However, there is precedent for this observation, as prior studies have found negative or no relationships between coccidiosis and total protein in various species (Mondal et al. 2011; Hashemnia et al. 2014). One potential driver of these different patterns and our lack of a significant correlation is the study of different parasite species, as a study of Chickens found that the effect of coccidiosis on total protein was significant for one of two parasite species (Ruff and Augustine 1982). We lacked fecal samples in the winter season to survey for coccidiosis, and poxvirus was absent in our study population during winter, which prevented us from including disease state in our analyses of all seasons. In addition, due to season and age being confounded in our samples of birds, we were unable to analyze the effects of season on circulating protein across our entire dataset. We lacked any after-hatch-year birds in our fall sampling period, which matches patterns from prior work with House Finches in this area (Giraudeau et al. 2014).
Taken together, our results support the hypotheses that circulating protein levels are associated with molt status, disease burden, and habitat of origin of House Finches. However, it will be important now to follow-up these results in wild birds with refined experimental studies, including a common-garden approach to determine if urban–rural differences in plasma protein during fall disappear if birds from different habitat types are housed under standard conditions in the lab, or if there is a strong genetic or developmental component to levels of circulating protein in House Finches, at least during autumn. Experimental procedures, including medical treatment for poxvirus infections, might also reveal if there is a causal effect of the virus on plasma protein levels, or reciprocally if plasma protein titers directly impact the likelihood of acquiring a poxvirus infection. In addition, further work evaluating poxvirus infection and hydration state via markers such as hematrocrit might allow for a more thorough understanding of poxvirus infection’s effects of these birds.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Ashraf M, Rea R (2017) Effect of dehydration on blood tests. Pract Diabetes 34:169–171. https://doi.org/10.1002/pdi.2111
Balan DG, Sianu DP, Stanescu II, Ionescu D, Stroescu Balcangiu AE, Raducu L, Stoicescu SM, Ceau AM, Tiliscan C, Nimigean VR, Tarmure V, Croitoru AG (2018) A comparative evaluation of serum and salivary total proteins and immunoglobulins in patients with hepatitis A and healthy subjects. Rev Chim 69:1125–1128. https://doi.org/10.37358/RC.18.5.6273
Bichet C, Scheifler R, Cœurdassier M, Julliard R, Sorci G, Loiseau C (2013) Urbanization, trace metal pollution, and malaria prevalence in the house sparrow. PLoS ONE. https://doi.org/10.1371/journal.pone.0053866
Bozdogan H (1987) Model selection and Akaike’s information criterion (AIC): the general theory and its analytical extensions. Psychometrika 52:345–370
Bradley CA, Altizer S (2007) Urbanization and the ecology of wildlife diseases. Trends Ecol Evol 22:95–102. https://doi.org/10.1016/j.tree.2006.11.001
Brock PA, Hall AJ, Goodman SJ, Cruz M, Acevedo-Whitehouse K (2013) Immune activity, body condition and human-associated environmental impacts in a wild marine mammal. PLoS ONE. https://doi.org/10.1371/journal.pone.0067132
Cazenave J, Bacchetta C, Parma MJ, Scarabotti PA, Wunderlin DA (2009) Multiple biomarkers responses in Prochilodus lineatus allowed assessing changes in the water quality of Salado River basin (Santa Fe, Argentina). Environ Pollut 157:3025–3033. https://doi.org/10.1016/j.envpol.2009.05.055
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landsc Urban Plan 74:46–69. https://doi.org/10.1016/j.landurbplan.2004.08.007
Chan SM, Rankin SM, Keeley LL (1988) Characterization of the molt stages in Penaeus vannamei: setogenesis and hemolymph levels of total protein, ecdysteroids, and glucose. Biol Bull 175:185–192
Chaousis S, Leusch FD, van de Merwe JP (2018) Charting a path towards non-destructive biomarkers in threatened wildlife: a systematic quantitative literature review. Environ Pollut 234:59–70. https://doi.org/10.1016/j.envpol.2017.11.044
Cherel Y, Charrassin JB, Challet E (1994) Energy and protein requirements for molt in the king penguin Aptenodytes patagonicus. Am J Physiol Regul Integr Comp Physiol 266:1182–1188. https://doi.org/10.1152/ajpregu.1994.266.4.R1182
Dawson RD, Bortolotti GR (1997) Total plasma protein level as an indicator of condition in wild American kestrels (Falco sparverius). Can J Zool 75:680–686. https://doi.org/10.1139/z97-088
Dolnik VR, Gavrilov VM (1979) Bioenergetics of molt in the chaffinch (Fringilla coelebs). Auk 96:253–264
Dominoni DM (2015) The effects of light pollution on biological rhythms of birds: an integrated, mechanistic perspective. J Ornithol 156:409–418. https://doi.org/10.1007/s10336-015-1196-3
Dominoni DM, Quetting M, Partecke J (2013) Long-term effects of chronic light pollution on seasonal functions of European blackbirds (Turdus merula). PLoS ONE. https://doi.org/10.1371/journal.pone.0085069
Funk A, Hutton P, Earl S, Deviche P, Sweazea K (2020) Levels of land use and land cover in Phoenix, Arizona are associated with elevated plasma triglycerides in the Gambel’s Quail, Callipepla gambelii. Comp Biochem Physiol Part A Mol Integr Physiol 247:110730. https://doi.org/10.1016/j.cbpa.2020.110730
Gavett AP, Wakeley JS (1986) Blood constituents and their relation to diet in urban and rural house sparrows. Condor 88:279–284. https://doi.org/10.2307/1368873
Giraudeau M, Mousel M, Earl S, McGraw K (2014) Parasites in the city: degree of urbanization predicts poxvirus and coccidian infections in house finches (Haemorhous mexicanus). PLoS ONE. https://doi.org/10.1371/journal.pone.0086747
Giraudeau M, Toomey MB, Hutton P, McGraw KJ (2018) Expression of and choice for condition-dependent carotenoid-based color in an urbanizing context. Behav Ecol 29:1307–1315. https://doi.org/10.1093/beheco/ary093
Hashemnia M, Khodakaram-Tafti A, Razavi SM, Nazifi S (2014) Hematological and serum biochemical analyses in experimental caprine coccidiosis. J Parasit Dis 38:116–123. https://doi.org/10.1007/s12639-012-0205-1
Heitmeyer ME (1988) Protein costs of the prebasic molt of female mallards. Condor 90:263–266. https://doi.org/10.2307/1368465
Hill GE (2002) A red bird in a brown bag: the function and evolution of colorful plumage in the house finch. Oxford University Press, New York
Hope D, Gries C, Casagrande D, Redman CL, Grimm NB, Martin C (2006) Drivers of spatial variation in plant diversity across the Central Arizona-Phoenix ecosystem. Soc Nat Resour 19:101–116
Hutton P, McKenna J, McGraw KJ (2021) Urban links to molt schedule, body condition and carotenoid-based coloration in the house finch Haemorhous mexicanus. J Avian Biol. https://doi.org/10.1111/jav.02761
Isaksson C, Örnborg J, Stephensen E, Andersson S (2005) Plasma glutathione and carotenoid coloration as potential biomarkers of environmental stress in great tits. EcoHealth 2:138–146. https://doi.org/10.1007/s10393-005-3869-5
Job JR, Kohler SL, Gill SA (2016) Song adjustments by an open habitat bird to anthropogenic noise, urban structure, and vegetation. Behav Ecol 27:1734–1744. https://doi.org/10.1093/beheco/arw105
Koelmel JP, Ulmer CZ, Fogelson S, Jones CM, Botha H, Bangma JT, Guillette TC, Luus-Powell WJ, Sara JR, Smit WJ, Albert K, Miller HA, Guillette MP, Olsen BC, Cochran JA, Garrett TJ, Yost RA, Bowden JA (2019) Lipidomics for wildlife disease etiology and biomarker discovery: a case study of pansteatitis outbreak in South Africa. Metabolomics 15(3):1–11. https://doi.org/10.1007/s11306-019-1490-9
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121. https://doi.org/10.2307/4087240
McEwan JC, Mason P, Baker RL, Clarke JN, Hickey SM, Turner K (1992) Effect of selection for productive traits on internal parasite resistance in sheep. Proc NZ Soc Anim Prod 52:53–56
McGraw KJ, Hill GE (2000) Differential effects of endoparasitism on the expression of carotenoid- and melanin-based ornamental coloration. Proc Roy Soc B 267:1525–1531. https://doi.org/10.1098/rspb.2000.1174
McGraw KJ, Chou K, Bridge A, McGraw HC, McGraw PR, Simpson RK (2020) Body condition and poxvirus infection predict circulating glucose levels in a colorful songbird that inhabits urban and rural environments. J Exp Zool A Ecol Integr Physiol 333:561–568. https://doi.org/10.1002/jez.2391
Mills GS, Dunning JB, Bates JM (1989) Effects of urbanization on breeding bird community structure in southwestern desert habitats. Condor 91:416–428. https://doi.org/10.2307/1368320
Mondal D, Chattopadhyay S, Batabyal S, Bera A, Bhattacharya D (2011) Plasma biochemical indices at various stages of infection with a field isolate of Eimeria tenella in broiler chicken. Vet World 4:404–409. https://doi.org/10.5455/vetworld.2011.404-409
Murphy ME, King JR (1992) Energy and nutrient use during moult by white-crowned sparrows Zonotrichia leucophrys gambelii. Ornis Scand 23:304–313. https://doi.org/10.2307/3676654
Murphy ME, Taruscio TG (1995) Sparrows increase their rates of tissue and whole-body protein synthesis during the annual molt. Comp Biochem Physiol Part A Physiol 111:385–396. https://doi.org/10.1016/0300-9629(95)00039-A
Norte AC, Ramos JA, Sousa JP, Sheldon BC (2009) Variation of adult great tit Parus major body condition and blood parameters in relation to sex, age, year and season. J Ornithol. https://doi.org/10.1007/s10336-009-0387-1
Oladele SB, Nok AJ, Esievo KAN, Abdu P, Useh NM (2005) Haemagglutination inhibition antibodies, rectal temperature and total protein of chickens infected with a local nigerian isolate of velogenic Newcastle disease virus. Vet Res Commun 29:171–179. https://doi.org/10.1023/B:VERC.0000047495.03341.2b
Ortiz-Santaliestra ME, Tauler-Ametller H, Lacorte S, Hernández-Matías A, Real J, Mateo R (2019) Accumulation of pollutants in nestlings of an endangered avian scavenger related to territory urbanization and physiological biomarkers. Environ Pollut B 252:1801–1809. https://doi.org/10.1016/j.envpol.2019.06.101
Patil R, Raghuwanshi U (2009) Serum protein, albumin, globulin levels, and A/G ratio in HIV positive patients. Biomed Pharmacol J 2:321–325
Peneaux C, Grainger R, Lermite F, Machovsky-Capuska GE, Gaston T, Griffin AS (2021) Detrimental effects of urbanization on the diet, health, and signal coloration of an ecologically successful alien bird. Sci Total Environ 796:148828. https://doi.org/10.1016/j.scitotenv.2021.148828
Pyle P (2008) Identification guide to North American Birds. Slate Creek Press, Point Reyes Station
Roman Y, Bomsel-Demontoy MC, Levrier J, Ordonneau D, Chaste-Duvernoy D, Saint Jalme M (2009) Influence of molt on plasma protein electrophoretic patterns in bar-headed geese (Anser indicus). J Wildl Dis 45:661–671. https://doi.org/10.7589/0090-3558-45.3.661
Ruff MD, Augustine PC (1982) Effects of coccidiosis on the electrophoretic pattern of serum proteins in chickens. J Parasitol Res 68:107–111. https://doi.org/10.2307/3281331
Saghazadeh A, Rezaei N (2022) Poxviruses and the immune system: implications for Monkeypox virus. Int Immunopharmacol 113:109364. https://doi.org/10.1016/j.intimp.2022.109364
Salmón P, Nilsson JF, Nord A, Bensch S, Isaksson C (2016) Urban environment shortens telomere length in nestling great tits, Parus major. Biol Lett 12:254–260. https://doi.org/10.1098/rsbl.2016.0155
Schaller J, Gerber S, Kaempfer U, Lejon S, Trachsel C (2008) Human blood plasma proteins: structure and function. Wiley, Hoboken
Serrano E, González FJ, Granados JE, Moço G, Fandos P, Soriguer RC, Pérez JM (2008) The use of total serum proteins and triglycerides for monitoring body condition in the Iberian wild goat (Capra pyrenaica). J Zoo Wildl Med 39:646–649. https://doi.org/10.1638/2007-0088.1
Acknowledgements
This work was supported by the National Science Foundation DEB-1832016, Central Arizona-Phoenix Long-Term Ecological Research Program (CAP LTER), and Barrett, The Honors College. We thank Dr. Karen Sweazea and Danny Jackson for input on the manuscript. We would also like to thank the South Mountain Environmental Education Center for graciously allowing us to conduct this research at their facilities. Finally, we would like to thank all of the undergraduate students who helped in this effort, Kathryn DePinto, Ian Sheedy, George Amacher, Elise Crawford-Paz Soldán, Preston Moskal, C. J. Writer, Jake Mitrius, Danielle Pais, Amanda Wrona, Cassie Rueda, Madison Hatcher, and Lauren West, as well as graduate students Victor Penha and Jamie Casseus. The experiments conducted comply with the current law of the United States of America, in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors are not aware of any competing interests related to this work.
Additional information
Communicated by I. Moore.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Drake, D.J., McGraw, K.J. Variation in plasma protein levels in House Finches (Haemorhous mexicanus): effects of season, disease state, and urbanization. J Ornithol 164, 629–638 (2023). https://doi.org/10.1007/s10336-023-02062-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02062-y