Abstract
Models of optimal primate group size suggest that group formation and growth arise to benefit individual fitness, but that size is limited by costs. The ecological constraints hypothesis posits that group formation and growth is driven by protection from predation or the advantages of group foraging, while an upper limit on group size is constrained by travel costs and intragroup competition for food or other critical resources. Socioecological models also predict that individual reproductive success, hypothesized to decrease with increasing group size, also places an upper limit on the number of individuals in a group. Our analysis of 23 yr of group composition data on mantled howlers (Alouatta palliata) from a single Panamanian study site on Barro Colorado Island not only corroborates the socioecological model but also shows that female reproductive success increased, whereas that of males decreased, with the less female-biased sex ratios in larger groups. We suggest that the conflict of interest between the sexes over adult sex ratio, particularly the male proportion in a group, in combination with ecological factors, is an important determinant of group size and composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behavioral ecologists have studied the costs and benefits of living in groups for many organisms (Alexander 1974; Eisenberg et al. 1972; Krause and Ruxton 2002). Because many primates live in multimale, multifemale groups (Crook and Gartlan 1966; Eisenberg et al. 1972), a major focus of primatological research has been to identify and model factors that predictably explain group formation and size (Dunbar 1996; Treves and Chapman 1996; van Schaik 1996). The ecological constraints hypothesis, as formulated specifically for primates (Chapman and Chapman 2000; Dunbar 1996; Janson 1988; Milton 1984; van Schaik 1996; Wrangham 1980), posits that protection from predation and the advantages of group foraging drive group formation and growth, while travel costs and intragroup competition for food or other critical resources constrain an upper limit on group size (Chapman et al. 1995; Wrangham et al. 1993). The hypothesis has significant empirical support; e.g., reduced predation in larger groups of vervets (Cercopithecus aethiops: Hill and Lee 1998; Isbell 1994; Isbell et al. 1990) and colobines (Chapman and Chapman 2000; Horwich et al. 2001); increased foraging benefits with larger group size in capuchins (Cebus apella: Janson 1988); the combined benefits of lower predation and higher foraging benefits in New and Old World monkeys (Chapman and Chapman 2000; Dunbar 1988, 1992; Hill and Dunbar 1998; Isbell 1994; Janson 1992; Janson and Goldsmith 1995); and the effect of travel costs due to food distribution on feeding group sizes in spider monkeys (Ateles geoffroyi: Chapman et al. 1995) and chimpanzees (Pan troglodytes: Chapman et al. 1995).
Though ecological constraints emphasize the benefits of forming groups to maximize individual survival, the effect of group size on individual reproductive success is also important to overall individual fitness. Steenbeek et al. (1999) suggested group size as an explanatory factor in the folivore paradox, (in which several folivorous primates appear to defy ecological constraints, keeping groups small, despite low or absent resource competition in larger groups (Janson and Goldsmith 1995). Treves and Chapman (1996) proposed that in folivorous langurs (Presbytis spp.), the costs of female harassment and infanticide during male group takeovers in larger groups is an alternative factor limiting group size. Crockett and Janson (2000) also showed that the rate of infanticide in red howlers (Alouatta seniculus) increases with adult female group size, and developed a model of food competition and infanticide risk as limits to adult female group size in primates. Chapman and Pavelka (2005) extended the findings to explain the phenomenon of smaller group sizes in infanticidal black-and-white colobus (Colobus guereza) relative to the more resource-restricted red colobus (Piliocolobus rufomitratus tephrosceles). They drew parallels with the difference in reported group sizes between infanticidal black howlers (Alouatta pigra) and mantled howlers (Alouatta palliata), suggesting that social constraints, instead of resource availability, limit group sizes (cf. Snaith and Chapman 2007).
Socioecological models propose that the reproductive interests of males are limited by access to females, while the reproductive interests of females are limited by access to resources and quality mates (Emlen and Oring 1977; Nunn 1999; Sterck et al. 1997;Wrangham 1980). Thus the sex ratio and age structure of a group likely play important roles in reproductive success, and the optimal male and female reproductive strategies may differ (Altmann 1990; Clutton-Brock 1989; van Schaik 1996; van Schaik and Horstermann 1994). In an interspecific analysis of 26 populations of howlers, combining data from 5 species, Treves (2001) showed that female reproductive success increases with an increased proportion of male (adult and subadult) group members. In addition, Treves suggested that survivorship of younger group members is enhanced with increased male presence, possibly because of increased predator defense and resource acquisition with additional adult males (van Schaik and Horstermann 1994), or if invading extragroup males are infanticidal, a higher number of resident males could effectively exclude them (Crockett and Janson 2000).
Here we extend Treves’ (2001) findings in Alouatta and suggest that there is a conflict between male and female mantled howlers over intragroup sex ratio (the proportion of males). The value of the conflict depends on group size if a minimum number of males are required to defend resources and against extragroup male invasion. Thus, we tested an intersexual conflict hypothesis for both group size and adult sex ratio via data from mantled howlers (Alouatta palliata) on Barro Colorado Island (BCI) in Panama. In accordance with Treves (2001), our hypothesis predicts that per capita female reproductive success increases with an increase in the proportion of males within a group. However, the hypothesis also predicts that male reproductive success per capita decreases with an increase in the proportion of males within a group. The first prediction arises because an increase in the proportion of males is likely to enhance a group’s competitive ability in terms of access to high-quality dietary resources (Milton 1980; Treves 2001), predator defense (Anderson 1986; van Schaik and Horstermann 1994), and protection of females and their offspring against possible infanticidal takeovers (DeGusta and Milton 1998). The second prediction arises because a finite number of breeding opportunities are shared by all males in a group, so group males will want to keep the male proportion limited. Dominance hierarchies could be a compounding factor: as the proportion of males in a group increases, subdominant males receive fewer mating opportunities and are subject to increased intragroup competition (Glander 1980). At Guanacaste, Costa Rica, only the α-male in each social group appears to have access to estrous females (Glander 1980) whereas at BCI, all adult males in a given social group have access to estrous females (Wang and Milton 2003).
Howlers (Alouatta) are medium-sized (5–8 kg) arboreal New World primates in Atelidae, along with spider monkeys (Ateles), woolly monkeys (Lagothrix), and woolly spider monkeys (Brachyteles; Fleagle 1999). Primatologists recognize 9–10 species of howlers (Cortes-Ortiz et al. 2003), with an extremely wide geographical range that extends from southern Mexico to northern Argentina. Howlers are folivore-frugivores, and can inhabit a wide range of forest types. Our study species, mantled howlers (Alouatta palliata), live largely in Central America, but their range extends into limited areas of South America. Mantled howlers are the only howler species that consistently lives in large social groups, with ≥10 individuals in the majority of study sites (Chapman and Balcomb 1998), containing 3–7 resident adult male members (Wang and Milton 2003). Within social groups, adult females consistently outnumber adult males (Crockett and Eisenberg 1987; Estrada 1982), and females come into estrus throughout the year, so there is no discrete birth season (Glander 1980; Milton 1982).
Collias and Southwick (1952), Froelich et al. (1981), Jones et al. (2000), Milton (1980, 1982, 1996), and Milton et al. (2005) have studied the ecology and behavior of the resident howlers of BCI. The howling vocalization, emitted by the adult males of each group, helps to space neighboring groups over the day such that intergroup contact is minimized, but home range areas can overlap completely (Milton 1980). Despite shifts in home range area use, on BCI, particular howler groups remain faithful to their general home range area (Milton 1980). At some study sites, both male and female mantled howlers disperse from their natal troop around the age of sexual maturity (Clarke and Glander 2004; Glander 1992). On BCI, some male dispersal occurs; researchers believe that female dispersal, though not well documented, also occurs (K. Milton, pers. comm.).
Intragroup relations between individuals are generally amicable; however, adult males, are often highly intolerant of extragroup male conspecifics and violent fighting may ensue if a strange male attempts to approach a group. An extragroup male approaches a potential new group as an individual and either shadows the group over a long period, attempting to gain entry slowly, or provokes actual fighting with ≥1 group males and, if successful, takes over the α position. In such cases, limited data on BCI suggest the resident males in the group remain and accept the new individual (K. Milton, pers. comm.). On BCI, in 23 yr, no instance of group takeover by male coalitions occurred (K. Milton, pers. comm.). Similar data on female group switching behavior on BCI are lacking, but as Treves (2001) noted, immigration into a group is likely easier for a female mantled howler than for a male, and female immigration into established groups occurred at another site (Glander 1992). Early researchers on BCI howlers reported little or no male aggression and noted that intergroup dispersal appeared to occur only in males (Altmann 1959; Carpenter 1934; Southwick 1963). More recent studies suggest that there may be more male-male conflict, infanticide (Cristobal-Azkarate et al. 2004; DeGusta and Milton 1998), and female natal dispersal (Wang and Milton 2003) on BCI than previously supposed.
Our howler subjects experienced no severe resource fluctuation over the period of time during which we recorded the data (Milton et al. 2005). Instead, Milton (1996) and Milton et al. (2005) suggested that the disease burden induced by bot fly (Alouattamyia baeri) infestation may regulate the population.
We use 23 yr of group composition data on the howler population of BCI to test a basic prediction of the ecological constraints model of optimal group size, then expand on it to examine intersexual conflict over group structure. We first test the consistency of group sizes in the population during the study. We then test the prediction that male reproductive success per capita for males and females correlates negatively with increasing group size. Next, we test the hypothesis that intersexual conflict is a factor determining the group size and composition distribution in the mantled howlers. We predict that whereas female mean reproductive success will increase with a more male-biased sex ratio in the group, male reproductive success will decrease. Because more male-biased sex ratios occur in larger groups, we suggest that the conflict is another factor in the tradeoffs driving groups toward an optimum size.
Methods
Study Site
BCI (9°10′N, 79°51′W) is a densely forested 1600 ha nature reserve in the Republic of Panama. The island was isolated from the mainland between 1912 and 1914 during construction of the Panama Canal (Milton 1982), and Gov. Jay J. Morrow declared it a nature preserve in 1923. There are distinct dry (December–April) and wet seasons (May–December). For most nonvolant mammals, including howlers, BCI is a closed system where human interference is limited to scientific research. As Janson (2000) noted, there are very few remaining intact natural settings in the world in which to conduct primate research. Unlike other study sites for howlers (Chiarello and de Melo 2001; Clarke et al. 1986; Estrada et al. 2002; Gonzalez-Solis et al. 2001;), no trees have been felled at BCI since 1920 or earlier, and intact, likely undisturbed, primary forest has covered some parts of the island for ≥500 yr (Hubbell and Foster 1990).
Data Collection
We collected 26 yr of census data between 1974 and 2000 (except 1984) on free-ranging mantled howlers inhabiting BCI (Milton 1982, 1996; Milton et al. 2005). The number of howler groups remained relatively constant over the period: ca. 60 (Milton 1982, 1996; Milton et al. 2005).
We collected composition data on groups comprising a subset of the full island census (Milton 1982). Data included the number of adult males, adult females, juveniles, and infants for each group. It is possible to distinguish among groups because of individually identifiable subjects, including some individuals marked with color-coded earrings and anklets (Wang and Milton 2003), locations of bot fly lesions, and other natural identifying characteristics. We lack information on all particular individuals within groups among years, as would be necessary for formal time-series analyses of reproduction. In addition, we cannot rule out the possibility that we overlooked occasional lone dispersing individuals during the censuses.
BCI has an extensive named and mapped trail system; thus we recorded group locations via trail markers. Per Chapman and Balcomb (1998), we maintained independence of samples by using only identifiable groups sampled within their distinct home ranges. We often conducted censuses in multiple months of the year; therefore, we chose to be conservative in the analysis and to use records only from the monthly census containing the largest number of groups in a given year, and only the ones with n ≥ 5 groups. The resulting census months are randomly distributed across years, and consecutive censuses are always ≥7 mo apart, which reduced the data set from 26 to 23 years (N = 206 group observations; mean ± SD: 8.96 ± 2.80 individual group entries per year). The population reproduces in all months of the year (Milton 1982); thus our treatment of the data allows time for females to enter a group, mate, and conceive [gestation is ca. 186 d (Glander 1980)] and stay, or emigrate before the next census date.
Adult male group tenure on BCI can last 15–20 yr, but only a small proportion of males become resident adults. Howler generation time is ca. 5 yr (K. Milton, pers. comm.). Thus our 23-yr data set contains information on multiple howler generations.
Data Analysis
Group size and structure
We used an ANOVA with Tukey-Kramer’s honest significant difference (HSD) test of means post hoc to test the consistency of group size over the study. We also report simple measures of group structure, reproductive success, and sex ratio over the time period.
Reproductive success
The population has an estimated infant mortality of 39% (Milton 1982), meaning that infants may not be the appropriate unit of reproductive success as a surrogate for fitness. To incorporate a measure of offspring survival, we conducted the analysis using both the number of infants (INF) and infants and juveniles collectively as immatures (IMM; Horwich et al. 2001; Zucker and Clarke 2003). Because IMM is subject to the potential bias of pseudoreplication if the same infants are present as juveniles in the next year, and stay in the natal group until dispersal (3.5–5 yr), we selected the data at 5-yr intervals for tests including IMM, a subset comprising 37 total data points (groups).
To compare across groups, we measured male and female reproductive success as the deviation from a population expectation of the number of infants (or immatures) per group, with respect to the number of males or females. The observed measure is denoted as F INF , F IMM , M INF, and M IMM. We followed the method of Treves (2001; Eqs. 1 and 2):
and similarly for F IMM, M INF, and M IMM.
To test the relationship between reproductive success and the number of females, males, and adults per group, and group size, we regressed reproductive success of males and females on the number of females (F), males (M), and adults (A) in the group. To control for the correlation between group size and the number of infants or immatures, we took the residual, G, of the group size regressed upon INF or IMM. We then regressed reproductive success upon G also.
Since we are essentially comparing 4 factors (F, M, A, G) within an analysis of 4 reproduction measures (F INF, F IMM, M INF, M IMM), we used a Bonferroni correction to assess the significance of slopes at the α = 0.05 level, i.e., p < 0.0125. The results of the univariate regressions are in Table 1.
To test the hypothesis that there is a reproductive success tradeoff for males and females between the size of a group and the sex ratio (proportion of males in that group), we conducted 4 separate multiple regressions of reproductive success (F INF , F IMM , M INF , M IMM) upon residual group size (G) and sex ratio. Simple sex ratio (M/F) can generate spurious statistical results when females appear on both axes of the analysis. Thus we used the residual male proportion (S), regressing adult males upon adult females, corresponding to Treves’ residual sex ratio, S (Treves 2001). The results of the regressions are in Table 2.
We illustrate the results of the multiple regressions with rescaled partial regression leverage plots (PRLPs; Sall 1990; Velleman and Welsch 1981) to demonstrate the separate effects of group size and sex ratio for both male and female reproductive success. They plot the role of each factor (G, S) in each multiple regression model while one controls for the other factor. A line fit to the points in each plot has a slope equal to the parameter estimate (coefficient) in the full model (Sall 1990).
As we simultaneously tested the influence of S and G, we report the associated variance inflation factors (VIFs) to assess whether collinearity among G and S would interfere with model interpretation (Belsey et al. 1980; Hsieh et al. 2003). Variance inflation factors are a scaled version of the multiple correlation coefficient between variable i and the rest of the independent variables:
We performed all statistical analyses via JMP 5 (SAS 1989–2002).
Results
General Characteristics
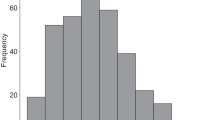
The mean (± SD) group size during the 23 yr is 19.0 ± 6.0 (Fig. 1). An ANOVA with post hoc Tukey-Kramer’s honest significant difference (HSD) test of means showed that there is no significant difference in the mean across the 23 yr (DF = 22, F = 1.10, p = 0.35). Mean group composition is 11.6 ± 3.6 adults, 2.6 ± 1.5 juveniles, and 4.9 ± 2.6 infants.
There was a mean (± SD) per capita female reproductive success of 0.92 ± 0.33 immatures/female (of which 0.59 ± 0.24 are infants/female), and one of 2.57 ± 1.13 immatures/male (1.67 ± 0.86 infants/male). On average, there were fewer males than females within a group, and the group adult sex ratio (M:F) is 0.40 ± 0.16.
Univariate Regressions
Male and female reproductive success decreased with group size (G) in all cases (Table 1). Reproductive success via INF is significant in 2 cases: the negative relationship between male reproductive success and the number of males (R 2 = 0.22), and the number of females (R 2 = 0.04). Via IMM, the largest slope (–1.65) and highest R 2 (0.50) are again between male reproductive success and the number of males (Table 1). However, the negative relationship between F IMM and the number of females also captured 23% of the variance (R 2 = 0.23; Table 1).
The Intersexual Conflict Hypothesis
The multiple regressions of reproduction on S and G are significant for both females and males (Table 2). The partial regression leverage plots demonstrate the negative relationship between reproduction and group size (G) for both sexes, a positive relationship with sex ratio (S) for females, and a relationship for males (Fig. 2a,b). The variance inflation factors (VIFs) of G and S in the models are small (1.10), which means our interpretation is not affected, as the literature cautions that the effects of collinearity will affect parameter estimates at inflation factors of ≥10 (Belsey et al. 1980).
Partial regression leverage plots showing the effects of residual group size (G; open circles or diamonds) and residual sex ratio (S; filled circles or diamonds) on (a) F INF (circles) and M INF (diamonds) and (b) F IMM (circles) and M IMM (diamonds). Ssee also Table 2. The group size variable, G, in the plots is the number in the group greater or less than expected for the number of infants (or immatures) per group in the population. Similarly, the sex ratio variable, S, is the number of males greater or less than expected given the number of females in the group, relative to the population expectation. The reproductive success measurements (F INF, M INF, F IMM, and M IMM) represent relative measures: the number of infants (or immatures) in a group deviating from the population expectation with respect to the number of males or females in the group.
Discussion
The results of our analysis are consistent with previous findings in primates that suggest living in large groups has an adverse effect on reproductive success of each member (Treves 2001; van Schaik 1983). The observed reproductive success of adult members of mantled howler groups decreased with group size, for both males and females, which held true using both infants and immatures as a metric of reproductive success (Table 1). Treves’ (2001) comparative study across 26 populations of howlers indicated no relationship between female reproductive success and the number of males, females, or adults within the group. Within one population, there are negative relationships between female reproduction and the number of females, but not with the number of adults or males (Table 1). We suggest that Treves was averaging across multiple social systems and grouping patterns in the different species of howlers, losing some of the information in the specific data (Treves, Fig. 2, p. 67).
We also found a negative relationship between male reproductive success and the number of males in a group, but no relationship with the number of adults or females (Table 1). This suggests that both sexes benefit from fewer members in a group, conforming to prior socioecological models, but it may be due to each sex competing with same-sex adult members for reproductive and offspring survival opportunities, not simply the cost of an increase in overall number in the group.
Exploring this further, we found that the role of the proportion of males in the group differed between the sexes in the context of reproductive success. We used the regression coefficient as a surrogate for the strength of selection. Lande and Arnold (1983) demonstrated that the coefficients in a multivariate regression of individual fitness traits on behavioral traits, such as reproductive success on group size here, provide a measure and direction of selection upon that trait. Though we cannot compare between the analyses (INF and IMM), within analyses, the variables are normalized on the same residual scale. Our male and female reproductive measures represent essentially a stake in the same pool of infants or immatures, so they are in a selection tradeoff that one can directly compare. The strongest selective cost was for males when the proportion of males (S) in the group increased. In the first analysis, comprising infants only (INF), the cost of S was almost 3 times greater for males than for females (–1.37/0.47 = –2.92) and comprising all immatures (IMM), nearly twice (1.97) as much. The comparison is obvious in the PRLP plots in Fig. 2a and b, wherein the opposing slopes of male and female reproductive success as a function of sex ratio, while controlling for group size. In addition, as a result of a positive correlation between sex ratio and overall group size (Pearson’s r = 0.33, p < 0.001), we predict the fitness cost to males for additional males is highest in the largest groups.
The finding of an intersexual conflict is not surprising given that male fitness is predicted to be limited by access to females, and female fitness by access to high-quality resources and good genes, i.e., high-quality males. If males are better at defending resources than females are (Milton 1980), a higher proportion of males within a group will ensure better resource defense and access than a smaller proportion. Accordingly, females should choose groups that contain a high proportion of males. However, males pay a cost for belonging to a group containing a high proportion of males because each male will, on average, have reduced access to sexually receptive females. Thus, males should choose and maintain groups with as low a ratio of males to females as possible, subject to the constraints of troop viability, i.e., being able to defend against extragroup males and to complete with other groups for resources.
It therefore appears that divergent male and female interests may be simultaneously placing upper and lower limits on group size. The relatively symmetric distribution of group sizes and the consistency of average group size over time suggest that, within our study population, the opposing processes are reasonably balanced in their effects (Fig. 1).
Male Dominance Hierarchy
For adult males, the addition of noncompeting, nonreproductive group members may confer fitness benefits by ensuring offspring survival via vigilance, male-alliance formation, and defense against infanticidal takeovers. However, the cost of adding adult males to a group (here, measured as an increased proportion of males) is obviously quite high. This is not surprising because α-males tend to monopolize breeding in many primates, including Alouatta. Several mantled howler studies report dominance hierarchies combined with monopoly of estrous females by the dominant male (Glander 1980). Wang and Milton (2003) observed subordinate intragroup male mating behavior in this population of mantled howlers on BCI, but perhaps only when females are not at peak estrus (Carpenter 1964). Genetic data is not yet available for paternity testing, but behavioral evidence from other populations suggests that the dominant male may enjoy a majority of the mating success of the group. Though our measure of reproductive success (M INF, M IMM) may not reflect the true structure of paternity, the divergence of interest from females in a higher proportion of males is accentuated only in a dominance structure that confers unequal mating opportunities for males. The cost of adding males, even as subordinates, will shape group size and structure: α-males are at risk of takeovers and aggression, while established subordinates may fall further in the hierarchy and suffer lowered reproductive opportunities. Even if the direct fitness cost of being a subordinate male is offset by a high level of relatedness among the male cohort, including the α-male, adding new unrelated males to the group will induce a cost. The cost is demonstrated clearly by the selective cost to males in groups with more males as compared to the female benefit. In the population, males may be behaviorally more influential than females in determining group size because they aggressively exclude nongroup males from group membership (Milton 1980).
Female Aggression
If the cost to females of other females in the group has a negative impact on reproduction, as we suggest, there must also be mechanisms to mitigate the impacts. Researchers at other study sites have suggested that adult females may oust other adult females from howler groups because infanticide rates increase with increasing female group size (Janson and Goldsmith 1995; Ostro et al. 2001). Though the emphasis in infanticidal takeovers is on male-male aggression in mantled howlers, intense female-female aggression has also occurred in howlers at various study sites, both intragroup and during emigration events (Cristobal-Azkarate et al. 2004; Crockett 1984). On BCI Collias and Southwick (1952) noted female aggression over an infant, despite their comment that aggression among howlers is rare.
Dispersal
Owing to active exclusion by resident males, the fitness cost to a howler male for attempting to join a large group may be prohibitive. In such cases, one would predict that any immigrating males would attempt to join a smaller group. Similarly, as the number of reproductive adults in a group increases, it may become beneficial for a male or a subset of males to leave a group with a few females. Clarke and Glander (1984) and Zucker and Clarke (2003) observed bisexual dispersal for Alouatta palliata in Costa Rica as Pope (1998, 2000) also observed for A. seniculus in Venezuela. Collias and Southwick (1952) suggested group fission in the population, but no researcher has observed it on BCI. Clearly it would benefit the emigrating males but could present a cost to the accompanying dispersing females. The cost would be significantly lessened if the females were daughters of the dominant male(s) and were thus avoiding inbreeding by leaving the group.
Conclusion
Our results confirm, within a single well-studied population, the essential findings of Treves (2001): an increased male proportion in howler troops corresponds to increased female reproductive success. Using a similar surrogate for male reproductive success, we find that this may create a conflict between the sexes over group composition and size, given the reproductive cost to males of an increased proportion of males within groups. The conflict of interest is likely to act in concert with ecological factors to place upper and lower limits on group size. Though ecological constraints limit the minimum and maximum group sizes, our data indicate that one may explain observed group size and sex ratio as a balance between male and female reproductive interests. The applicability of our findings to other primate populations and other species of group-living organisms are uncertain until tested via similar long-term demographic data on other populations.
References
Alexander, R. D. (1974). The Evolution of Social Behavior. Annual Review of Ecology and Systematics, 5, 325–383.
Altmann, S. (1959). Field observations on a howling monkey society. Journal of Mammalogy, 40, 317–331.
Altmann, J. (1990). Primate males go where the females are. Animal Behaviour, 39, 193–195.
Anderson, C. (1986). Predation and primate evolution. Primates, 27, 15–39.
Belsey, D. A., Kuh, E., & Welsch, R. E. (1980). Regression diagnostics, identifying influential data and sources of collinearity. New York: John Wiley & Sons.
Carpenter, C. R. (1934). A field study of behavior and social relations of howling monkeys Alouatta palliata. Comparative Psychology Monographs, 10, 1–168.
Carpenter, C. R. (1964). Grouping behavior of howling monkeys. In C. R. Carpenter (Ed.) Naturalistic Behavior of Nonhuman Primates (pp. 386–406). University Park, PA : Pennsylvania State University Press.
Chapman, C. A., & Balcomb, S. R. (1998). Population characteristics of howlers: Ecological conditions or group history. International Journal of Primatology, 19, 385–403.
Chapman, C. A., & Chapman, L. J. (2000). Interdemic variation in mixed-species association patterns: common diurnal primates of Kibale National Park, Uganda. Behavioral Ecology and Sociobiology, 47, 129–139.
Chapman, C. A., & Pavelka, M. S. M. (2005). Group size in folivorous primates: Ecological constraints and the possible influence of social factors. Primates, 46, 1–9.
Chapman, C. A., Wrangham, R. W., & Chapman, L. J. (1995). Ecological constraints on group-size—An analysis of spider monkey and chimpanzee subgroups. Behavioral Ecology and Sociobiology, 36, 59–70.
Chiarello, A. G., & de Melo, F. R. (2001). Primate population densities and sizes in Atlantic forest remnants of northern Espirito Santo, Brazil. International Journal of Primatology, 22, 379–396.
Clarke, M. R., & Glander, K. E. (1984). Female reproductive success in a group of free-ranging howling monkeys (Alouatta palliata) in Costa Rica. In M. Small (Ed.) Female primates: Studies by women primatologists (pp. 111–126). New York : Alan R. Liss.
Clarke, M. R., & Glander, K. E. (2004). Adult migration patterns of the mantled howlers of La Pacifica. American Journal of Primatology, 62, 87.
Clarke, M. R., Zucker, E. L., & Scott, N. J. J. (1986). Population trends of the mantled howler groups of La Pacifica, Guanacaste, Costa Rica. American Journal of Primatology, 11, 79–88.
Clutton-Brock, T. H. (1989). Mammalian mating systems. Proceedings of the Royal Society of London Series B–Biological Sciences, 236, 339–372.
Collias, N., & Southwick, C. (1952). Field study of population density and social organization in howling monkeys. Proceedings of the American Philosophical Society, 90, 143–155.
Cortes-Ortiz, L., Bermingham, E., Rico, C., Rodriguez-Luna, E., Sampaio, I., & Ruiz-Garcia, M. (2003). Molecular systematics and biogeography of the Neotropical monkey genus, Alouatta. Molecular Phylogenetics and Evolution, 26, 64–81.
Cristobal-Azkarate, J., Dias, P. A. D., & Vea, J. J. (2004). Causes of intraspecific aggression in Alouatta palliata mexicana: Evidence from injuries, demography, and habitat. International Journal of Primatology, 25, 939–953.
Crockett, C. M. (1984). Emigration by female red howler monkeys and the case for female competition. In M. F. Small (Ed.) Female primates: Studies by women primatologists (pp. 159–173). New York: Alan R. Liss.
Crockett, C. M., & Eisenberg, J. F. (1987). Howlers: Variations in group size and demography. In B. B. Smutts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham, & T. T. Struhsaker (Eds.) Primate Societies (pp. 54–68). Chicago, IL: University of Chicago Press.
Crockett, C. M., & Janson, C. H. (2000). Infanticide in red howlers: Female group size, male membership, and a possible link to folivory. In C. P. van Schaik, & C. H. Janson (Eds.) Infanticide by males and its implications (pp. 75–98). Cambridge, U.K.: Cambridge University Press.
Crook, J. H., & Gartlan, J. S. (1966). Evolution of primate societies. Nature, 210, 1200–1203.
DeGusta, D., & Milton, K. (1998). Skeletal pathologies in a population of Alouatta palliata: Behavioral, ecological, and evolutionary implications. International Journal of Primatology, 19, 615–650.
Dunbar, R. I. M. (1988). Primate social systems. London: Chapman & Hall.
Dunbar, R. I. M. (1992). Time—A hidden constraint on the behavioral ecology of baboons. Behavioral Ecology and Sociobiology, 31, 35–49.
Dunbar, R. I. M. (1996). Determinants of group size in primates: A general model. In W. G. Runciman, J. Maynard-Smith, & R. I. M. Dunbar (Eds.) Evolution of social behavior patterns in primates and man (pp. 33–58). Oxford: Oxford University Press.
Eisenberg, J., Rudran, R., & Muckenhin, N. (1972). Relation between ecology and social structure in primates. Science, 176, 863.
Emlen, S. T., & Oring, L. W. (1977). Ecology, sexual selection, and the evolution of mating systems. Science, 197, 215–223.
Estrada, A. (1982). Survey and census of howler monkeys (Alouatta palliata) in the rain-forest of Los Tuxtlas, Veracruz, Mexico. American Journal of Primatology, 2, 363–372.
Estrada, A., Mendoza, A., Castellanos, L., Pacheco, R., Van Belle, S., Garcia, Y., et al. (2002). Population of the black howler monkey (Alouatta pigra) in a fragmented landscape in Palenque, Chiapas, Mexico. American Journal of Primatology, 58, 45–55.
Fleagle, J. G. (1999). Primate adaptation and evolution (2nd ed.). New York: Academic Press.
Froelich, J. W., Thorington, R. W., & Otis, J. S. (1981). The demography of howler monkeys (Alouatta palliata) on Barro Colorado Island, Panama. International Journal of Primatology, 2, 207–236.
Glander, K. E. (1980). Reproduction and population growth in free-ranging mantled howling monkeys. American Journal of Physical Anthropology, 53, 25–36.
Glander, K. E. (1992). Dispersal patterns in Costa Rican mantled howling monkeys. International Journal of Primatology, 13, 415–436.
Gonzalez-Solis, J., Guix, J. C., Mateos, E., & Llorens, L. (2001). Population density of primates in a large fragment of the Brazilian Atlantic rainforest. Biodiversity and Conservation, 10, 1267–1282.
Hill, R. A., & Dunbar, R. I. M. (1998). An evaluation of the roles of predation rate and predation risk as selective pressures on primate grouping behaviour. Behaviour, 135, 411–430.
Hill, R. A., & Lee, P. C. (1998). Predation risk as an influence on group size in cercopithecoid primates: Implications for social structure. Journal of Zoology, 245, 447–456.
Horwich, R. H., Brockett, R. C., James, R. A., & Jones, C. B. (2001). Population structure and group productivity of the Belizean black howling monkey (Alouatta pigra). Primate Report, 61, 47–65.
Hsieh, F. Y., Lavori, P. W., Cohen, H., & Feussner, J. R. (2003). An overview of variance inflation factors for sample-size calculation. Evaluation & the Health Professions, 26, 239–257.
Hubbell, S. P., & Foster, R. B. (1990). Structure, dynamics and equilibrium status of old-growth forest on Barro Colorado Island. In A. Gentry (Ed.) Four neotropical rainforests (pp. 522–541). New Haven: Yale University Press.
Isbell, L. A. (1994). Predation on primates: Ecological patterns and evolutionary consequences. Evolutionary Anthropology, 3(2), 61–71.
Isbell, L. A., Cheney, D. L., & Seyfarth, R. M. (1990). Costs and benefits of home range shifts among vervet monkeys (Cercopithecus aethiops) in Amboseli National Park, Kenya. Behavioral Ecology and Sociobiology, 27, 351–358.
Janson, C. H. (1988). Food competition in brown capuchin monkeys (Cebus apella)— Quantitative effects of group-size and tree productivity. Behaviour, 105, 53–76.
Janson, C. H. (1992). Evolutionary ecology of primate social structure. In E. A. Smith, & B. Winterhalder (Eds.) Evolutionary ecology and human behavior (pp. 95–130). New York: de Gruyter.
Janson, C. H. (2000). Primate socio-ecology: The end of a golden age. Evolutionary Anthropology, 9, 73–86.
Janson, C. H., & Goldsmith, M. L. (1995). Predicting group-size in primates—Foraging costs and predation risks. Behavioral Ecology, 6, 326–336.
Jones, A. L., DeGusta, D., Turner, S. P., Campbell, C. J., & Milton, K. (2000). Craniometric variation in a population of mantled howler monkeys (Alouatta palliata): Evidence of size selection in females and growth in dentally mature males. American Journal of Physical Anthropology, 113, 411–434.
Krause, J., & Ruxton, G. D. (2002). Living in groups. Oxford: Oxford University Press.
Lande, R., & Arnold, S. J. (1983). The measurement of selection on correlated characters. Evolution, 37, 1210–1226.
Milton, K. (1980). The foraging strategy of howler monkeys: A study in primate economics. New York: Columbia University Press.
Milton, K. (1982). Dietary quality and demographic regulation in a howler monkey population. In E. G. Leigh, E. S. Rand, & D. M. Windsor (Eds.) The ecology of a tropical rain forest: Seasonal rhythms and long-term changes (pp. 273–289). Washington, DC: Smithsonian Institution Press.
Milton, K. (1984). Habitat, diet, and activity patterns of free-ranging woolly spider monkeys (Brachyteles arachnoides E: Geoffroy 1806). International Journal of Primatology, 5, 491–514.
Milton, K. (1996). Effects of bot fly (Alouattamyia baeri) parasitism on a free-ranging howler monkey (Alouatta palliata) population in Panama. Journal of Zoology, 239, 39–63.
Milton, K., Giacalone, J., Wright, S. J., & Stockmayer, G. (2005). Do population fluctuations of neotropical frugivores reflect fruit production estimates? Evidence from Barro Colorado Island. In J. L. Dew, & J. P. Boubli (Eds.) Tropical fruits and frugivores: The search for strong interactors (pp. 5–35). The Netherlands: Springer.
Nunn, C. L. (1999). The number of males in primate social groups: A comparative test of the socioecological model. Behavioral Ecology and Sociobiology, 46, 1–13.
Ostro, L. E. T., Silver, S. C., Koontz, F. W., Horwich, R. H., & Brockett, R. (2001). Shifts in social structure of black howler (Alouatta pigra) groups associated with natural and experimental variation in population density. International Journal of Primatology, 22, 733–748.
Pope, T. R. (1998). Effects of demographic change on group kin structure and gene dynamics of populations of red howling monkeys. Journal of Mammalogy, 79, 692–712.
Pope, T. R. (2000). Reproductive success increases with degree of kinship in cooperative coalitions of female red howler monkeys (Alouatta seniculus). Behavioral Ecology and Sociobiology, 48, 253–267.
Sall, J. (1990). Leverage plots for general linear hypotheses. American Statistician, 44, 308–315.
SAS (1989–2002) JMP Version 5 (Academic). In SAS Institute Inc., Cary, NC.
Snaith, T. V., & Chapman, C. A. (2007). Primate group size and interpreting socioecological models: Do folivores really play by different rules. Evolutionary Anthropology, 16, 94–106.
Southwick, C. H. (1963). Challenging aspects of the behavioral ecology of howling monkeys. In C. H. Southwick (Ed.) Primate social behavior (pp. 185–191). Princeton: D. Van Nostrand.
Steenbeek, R., Piek, R. C., van Buul, M., & van Hooff, J. (1999). Vigilance in wild Thomas’s langurs (Presbytis thomasi): The importance of infanticide risk. Behavioral Ecology and Sociobiology, 45, 137–150.
Sterck, E. H. M., Watts, D. P., & van Schaik, C. P. (1997). The evolution of female social relationships in nonhuman primates. Behavioral Ecology and Sociobiology, 41, 291–309.
Treves, A. (2001). Reproductive consequences of variation in the composition of howler monkey (Alouatta spp.) groups. Behavioral Ecology and Sociobiology, 50, 61–71.
Treves, A., & Chapman, C. A. (1996). Conspecific threat, predation avoidance, and resource defense: Implications for grouping in langurs. Behavioral Ecology and Sociobiology, 39, 43–53.
van Schaik, C. P. (1983). Why are diurnal primates living in groups. Behaviour, 87, 120–144.
van Schaik, C. P. (1996). Social evolution in primates: the role of ecological factors and male behaviour. In W. G. Runciman, J. Maynard-Smith, & R. I. M. Dunbar (Eds.) Evolution of social behavior patterns in primates and man (pp. 9–31). Oxford: Oxford University Press.
van Schaik, C. P., & Horstermann, M. (1994). Predation risk and the number of adult males in a primate group—A comparative test. Behavioral Ecology and Sociobiology, 35, 261–272.
Velleman, P. F., & Welsch, R. E. (1981). Efficient computing of regression diagnostics. American Statistician, 35, 234–242.
Wang, E., & Milton, K. (2003). Intragroup social relationships of male Alouatta palliata on Barro Colorado Island, Republic of Panama. International Journal of Primatology, 24, 1227–1243.
Wrangham, R. W. (1980). An ecological model of female-bonded primate groups. Behaviour, 75, 262–300.
Wrangham, R. W., Gittleman, J. L., & Chapman, C. A. (1993). Constraints on group size in primates and carnivores population density and day range as assays of exploitation competition. Behavioral Ecology and Sociobiology, 32, 199–209.
Zucker, E. L., & Clarke, M. R. (2003). Longitudinal assessment of immature-to-adult ratios in two groups of Costa Rican Alouatta palliata. International Journal of Primatology, 24, 87–101.
Acknowledgments
A Miller Fellowship from the Miller Institute for Basic Research in Science supported P. T. Starks and an EPA-STAR fellowship (FP-916382) and an NSF Bioinformatics post-doctoral fellowship (0630709) supported S. J. Ryan, with additional support under an NSF/NIH EID Grant DEB-0090323 and James S. McDonnell Foundation 21st Century Science Initiative Award to W. M. Getz. We thank C. A. Blackie, M. E. Hauber, C. L. Nunn, J. V. Redfern, and J. M. Reed for comments on early versions of the manuscript. We also thank C. A. Chapman for constructive comments and edits on later versions. We gathered all data provided in this article observationally. The authors do not have any financial, personal, or professional interests that could be construed to have influenced this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryan, S.J., Starks, P.T., Milton, K. et al. Intersexual Conflict and Group Size in Alouatta palliata: A 23-year Evaluation. Int J Primatol 29, 405–420 (2008). https://doi.org/10.1007/s10764-007-9172-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-007-9172-2