Abstract
Bacterial black node, a disease of barley and wheat, is caused by Pseudomonas syringae pv. syringae (synonym pv. japonica, hereafter Psj). Here a selective medium, serine-potassium tellurite-based Psj-selective agar (SPTPsjA) was developed to isolate and detect Psj. After 5–7 days on SPTPsjA at 25 °C, characteristic black Psj colonies formed with an efficiency equal to that on potato–peptone–glucose agar. Except for P. viridiflava, P. cichorii, and some strains of P. syringae group bacteria, SPTPsjA either inhibited growth of other phytopathogenic bacteria or enabled their discrimination. To facilitate diagnosis of Psj, we prepared three antisera against three strains; in a slide agglutination test, the antisera reacted to all tested strains of Psj and failed to react to P. viridiflava and P. cichorii although some strains of other P. syringae group bacteria were indistinguishable from Psj with SPTPsjA and antisera. When barley and wheat seeds from fields in which bacterial black node had occurred were placed on SPTPsjA in the dark at 25 °C for 7 days, black colonies grew on the medium around the seeds. Among these strains, only those responsive to the above antisera were pathogenic on barley. These results show that the combination of SPTPsjA and antisera can isolate Psj from seeds of barley and wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial black node is a disease of barley (Hordeum vulgare) and wheat (Triticum aestivum) caused by Pseudomonas syringae pv. syringae (synonym pv. japonica, hereafter, Psj). Young (1992) reported that Psj and P. syringae pv. syringae were indistinguishable in their biochemical, nutritional, and pathogenic reactions and therefore designated Psj as a junior synonym of P. syringae pv. syringae. This disease emerged as a new bacterial disease after outbreaks occurred around 1945 in Japan over a wide area that included the Seto Inland Sea coastal zone and the Tokai region (Ikata and Hori 1950; Mukoo and Tsuchiya 1950). The ear burn of barley reported by Goto and Nakanishi (1951) is also now regarded as a symptom of bacterial black node. The disease inflicted major damage on barley in particular. The incidence of bacterial black node subsequently declined, only to recur in Japan in 1976 and later in the prefectures of Fukuoka, Niigata, Nagano, and Fukui (Aoyagi et al. 1980; Shimizu et al. 1981; Takamatsu 1983; Yokoyama 1976), and emerged thereafter in the Kyushu, Kinki, and Hokuriku regions (Matsuzawa 1987; Oba et al. 1990; Senba et al. 1994). In Kagawa Prefecture in Japan, bacterial black node was also found in 1996 in hull-less barley (H. vulgare var. nudum Hook. f., cv. Ichibanboshi), causing ear burn and extensive damage.
Psj is transmitted via seeds (Fukuda et al. 1990; Goto and Nakanishi 1951) and infested straw (Kan 1978), but infected seeds are the most important source of inoculum (Kawaguchi et al. 2017, 2018). Therefore, a method to detect seeds infected with pathogenic bacteria is needed for controlling bacterial black node. One such method is use of a selective medium to isolate the living pathogen. Several such media suitable for selectively detecting Pseudomonas species (including species under former classifications) (Fieldhouse and Sasser 1982; Hasebe et al. 1998; Miyoshi and Tachibana 1994; Sato et al. 1981; Tsushima et al. 1986; Uematsu et al. 1982). In the present study, we devised a method for selective isolation and detection of Psj by combining a selective medium, based on already reported selective media, with the slide agglutination method, which enables simple and speedy diagnosis with antiserum prepared from immunized rabbits. Using this detection method, we investigated the percentage of seeds infected with Psj in barley and wheat. Part of the results of this work was reported by Mori et al. (1999).
Materials and methods
Test strains
Among 68 strains of plant pathogenic bacteria evaluated during selective medium tests were 13 strains of Psj and 26 other P. syringae pathovars (Table 1). Although some of the P. syringae group bacteria have been identified as different species in recent years, in this study all are described as P. syringae. The DNA group of each P. syringae strain used in this study was assessed according to the report of Inoue and Takikawa (2006). The pathogenicity of these isolates was confirmed using barley grown in a greenhouse at 25 °C until the internode elongated. A bacterial suspension of about 108 colony-forming units (cfu)/ml was injected in the vicinity of the node with a 1-ml syringe. Plants were then incubated again in a greenhouse. Five days after inoculation, isolates in which the nodes were blackened were regarded as pathogenic. Test strains that had been cultured on PPGA (Nishiyama 1978) at 25 °C for 24 or 48 h were suspended in sterile water at an optical density of 0.3 at 600 nm or at a transmittance of 55–75% at 610 nm (~ 108 cfu/ml in each case) and diluted appropriately.
Composition of selective medium
Based on the report of Sato et al. (1981) and Tsushima et al. (1986), we chose the following composition for the basal medium: 1.3 g KH2PO4, 1.2 g Na2HPO4·12H2O, 5.0 g (NH4)2SO4, 0.25 g MgSO4·7H2O, 24 mg Na2MoO4·2H2O, 15 g agar, 1 l distilled water.
For testing utilization of carbon sources (Table 2) by the isolates, 10 g of each test substance was added to a liter of the basal medium. This test solution was then autoclaved at 115 °C for 15 min. After that about 10 ml of the solution was dispensed into 90-mm Petri dishes to prepare the agar plates. To test the selectivity of each medium, 100 µl of approximately 103 cfu/ml of the bacterial suspension was spread on the plates, using two plates per strain. After incubating at 26 °C in the dark for 4 days, the number of colonies formed on the medium was counted, and colony diameters were measured. The bacterial growth on each medium was compared with that on King’s B medium (Eiken Chemical, Tokyo, Japan) because the composition is simple, and the nutrients are commonly used (peptone and glycerin).
Growth-inhibiting substances such as antibiotics were added to PPGA and their effectiveness evaluated (Table 2). Test substances were selected based on the report of Wakimoto and Uematsu (1993). The amount of each substance used per 1 l is shown in Table 2. Boric acid, sodium taurocholate, sodium dodecyl sulfate, and lithium chloride were added to PPGA before sterilization of the medium at 115 °C for 15 min. Kabicidin, an amount equal to one bottle (100 mg titer) was ground into a fine powder in a mortar, triturated with 10 ml of ethyl alcohol, and added to PPGA before sterilization. The other growth-inhibiting test substances were sterilized by filtration through a filter (pore size: 0.45 µm) and were added to PPGA after it had been autoclaved and cooled in a water bath to 55 °C. Methyl violet was dissolved (10 mg in 2 ml of ethanol), and then 8 ml of sterilized distilled water was added; 1 ml of this solution was added to the medium to achieve a concentration of 1 mg/l. Phenol red was dissolved (0.2 g in 10 ml of 1/20 N NaOH), and 1 ml of the resulting solution was added to the medium to achieve a concentration of 20 mg/l. Cycloheximide and rifampicin were dissolved in ethanol, and the others were dissolved in distilled water and then filtered. About 10 ml of the medium dispensed into 90-mm Petri dishes to prepare the agar plates. To test the selectivity of each medium, 100 µl of approximately 103 cfu/ml of the bacterial suspension were spread on the plates, which were incubated at 26 °C in the dark for 3 and 7 days. Colonies were then counted and compared with counts on PPGA medium.
Selectivity of the developed medium
To investigate the growth of a given bacterium on the selective medium we developed, 10 µl of the bacterial suspension (approximately 108 cfu/ml) was spotted onto three places on the medium. After 2 weeks, bacterial growth and colony color were evaluated. We made dilution plates of Psj and of strains that formed colonies similar to Psj in the above test. Test bacteria were suspended in sterilized water and then diluted with 1 mM HEPES buffer (pH 7.0) to approximately 103 cfu/ml. We spread 100 µl of each bacterial suspension on the plate, using three plates per strain. After incubating at 25 °C in the dark for 7 days, the colonies were counted, and the average of the three plates was calculated and compared with that on King’s B and PPGA. This test was repeated twice.
Antiserum preparation and applicability
Antisera were prepared using the method of Maeda and Tsuchiya (1993). Psj strains Psj-5, 76A-7, and Kurofushi ①-1-1-1 were used as immunization sources. After culturing on a King’s B plate at 25 °C for 2 days, each strain was suspended in 0.85% (w/v) physiological saline to create solutions of about 108 cfu/ml, which were washed twice with saline, centrifuged for 10 min at 5000 rpm, and then frozen and stored at − 20 °C. We immunized rabbits sequentially at 7-day intervals with thawed bacterial suspension at doses of 0.5, 1, 2, 3, 4, and 5 ml, and then collected blood 7 days after the last immunization into a 50 ml round bottom centrifuge tube. The centrifuge tube was capped and kept at 37 °C for 2 h while removing the clot from the wall with a glass rod. After overnight incubation at 4 °C, the supernatant was recovered as antiserum. The prepared antisera were used for the slide agglutination reaction. To carry out this reaction, 30 µl of antiserum diluted 1:30 with 0.85% (w/v) physiological saline were placed onto hemagglutination plates. Bacteria grown on the nutrient medium to be tested were then scraped off with a platinum loop and mixed well into the serum in sufficient quantity to make the serum solution slightly cloudy. After mixing, the presence or absence of agglutination was determined by observing the plates by eye and with a stereomicroscope while tilting the plates back and forth for about 1 min. This test was repeated three times.
Isolation and identification of Psj from seeds of barley and wheat
Barley seeds (cv. Ichibanboshi) were collected in 1996, 1997, and 2014 from fields in which bacterial black node had occurred. Wheat seeds (cv. Sanukinoyume) were also collected in 2014. The seeds were placed on plates containing the selective medium and the plates kept in the dark at 25 °C for 7 days. Any bacteria that grew on the surface of the medium around the seeds were then subjected to the slide agglutination reaction with 1:30-diluted Psj-5 antiserum solution. Isolated bacteria were tested for pathogenicity to barley as described above.
Results
Selective medium composition
In tests of Psj growth on media with one of 30 types of sugars, glycosides, alcohols, hydroxy acids, or amino acids (Table 2), media containing glucose, galactose, or d-xylose as a monosaccharide; sucrose or maltose as a disaccharide; glycerin, mannitol, or sorbitol as an alcohol; quinic acid as a hydroxy acid; or l-serine, l-proline, or l-alanine as an amino acid yielded colony numbers much the same as those obtained on King’s B. However, colony diameters were smaller on media containing d-xylose, maltose, glycerin, or l-alanine. Among the tested substances, l-serine, l-proline, quinic acid, and sorbitol supported little growth of phytopathogenic bacteria species of the Pectobacterium, Clavibacter, Xanthomonas, and Acidovorax. On media containing l-serine, Rhizobium and Ralstonia species either did not grow or formed notably smaller colonies (supplementary Table S1).
As for antibiotics and other additives, the growth of Psj was clearly suppressed on media containing novobiocin, penicillin G, polymyxin B, rifampicin, boric acid, sodium dodecyl sulfate, crystal violet, or lithium chloride. Bacitracin, 2,3,5-triphenyl tetrazolium chloride, phenol red, and cetyltrimethylammonium bromide suppressed the growth of Psj and all other tested phytopathogenic bacteria. Cycloheximide and kabicidin are antifungal agents and do not affect the growth of Psj. Ampicillin sodium, phenethicillin potassium, vancomycin, potassium tellurite, sodium taurocholate, cadmium chloride, and methyl violet allowed Psj growth equivalent to that of the control medium while suppressing the growth of several other phytopathogenic bacteria (supplementary Table S2).
On the basis of the above results, we created a selective medium for isolating Psj and named it serine-potassium tellurite-based Psj-selective agar (SPTPsjA; Table 3).
Selectivity on SPTPsjA
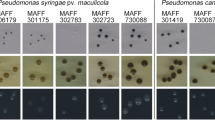
All of the Psj strains tested grew on SPTPsjA, with characteristic black colonies on the after 5–7 days (Fig. 1a, b). In addition to Psj, the phytopathogenic bacteria P. viridiflava, P. cichorii, Rhizobium radiobacter, and Burkholderia gladioli pv. gladioli also grew. The colonies formed by R. radiobacter and B. gladioli pv. gladioli were brown (Fig. 1c) and thus easy to distinguish from the black Psj colonies; however, the black colonies of P. viridiflava and P. cichorii were difficult to differentiate. To investigate the selectivity of the medium, we also tested 147 bacterial isolates (which grew on PPGA medium at 28 °C and were nonpathogenic to barley) from nonsterilized barley seed and four isolates from barley plants. Except for one aberrant strain isolated from a plant (which was considered to Pseudomonas sp.), the other strains did not grow on the medium (data not shown). The colony-forming efficiency of Psj strains on SPTPsjA varied from 56.2 to 121.7% of that on PPGA medium (Table 4). The colony-forming efficiency of strains of P. syringae pathovars syringae, alisalensis, aptata, coronafaciens, lachrymans, mellea, pisi, phaseolicola, sesami, solidagae, and theae was similar to that of the Psj strains. The colonies of P. syringae pv. phaseolicola and sesami were very small (< 0.1 mm diameter). Other strains of P. syringae either did not grow or only grew slightly on SPTPsjA.
Applicability of antisera
The antiserum for Psj-5, 76A-7, and Kurofushi ①-1-1-1 at 1:30 dilution yielded positive slide agglutination reactions (supplementary Fig. S1) with all tested Psj isolates and negative reactions with P. viridiflava and P. cichorii (Table 4). Of the 26 isolates of P. syringae pathovars, 17 isolates had positive reactions, and nearly all reacted with two or all three of the antisera—albeit with slight differences in the strength of the positive reactions. The use of SPTPsjA with the slide agglutination reaction differentiated Psj except for a positive result also for P. syringae pathovars syringae, alisalensis, aptata, pisi, and solidagae. We classified all these pathovars except pv. alisalensis into group III by genetic grouping (Table 4). In tests of the agglutination reaction of the other strains with the Psj-5 antiserum, the 1:30 dilution did not react with any of the phytopathogens other than P. syringae (Table 1) or with the nonpathogenic bacteria isolated from barley seeds (data not shown).
Detection of Psj in seeds of barley and wheat
Barley seeds were collected from two fields in 1996; when these seeds were placed on SPTPsjA plates, bacteria grew around 38 of 40 seeds from one field and 54 of 60 seeds from the other. Among the bacteria isolated from the seeds, 19 and 38 isolates from the respective fields reacted to the antiserum of Psj-5. Twenty-five of these isolates were then used in a pathogenicity test; 15 reacted to the antiserum and were pathogenic to barley, and the other 10 did not react with the antiserum and were not pathogenic. From 100 barley seeds collected from each of three fields in 1997, bacteria grew on SPTPsjA from 52, 57, and 57 seeds, respectively, and 12, 16, and 6 of these isolates reacted to the Psj-5 antiserum. In 2014, bacteria grew from 93 to 92 seeds of 100 seeds each from two fields, and 86 and 92 of these isolates reacted to the Psj-5 antiserum. For 100 wheat seeds collected in 2014 from each of four fields, 17, 13, 65, and 58 seeds yielded bacteria on the selective medium, and 15, 9, 54, and 49 of the respective isolates reacted to the Psj-5 antiserum.
Discussion
Tsao (1970) stated that a selective medium can have three effects to select for a target bacterium: selective growth promotion of the target bacterium by an amendment that is utilized specifically by the bacterium, selective growth inhibition of the nontarget bacteria, and selective identification by adding a substance only the target bacterium uses to produce a characteristic indicator pigment. Although Hasebe et al. (1998) reported a selective medium for Psj, they suggested that it be refined to improve its specificity. They attributed that medium’s lack of specificity to the use of peptones (a mixture of various organic compounds), and the use of glycerin, a substance used by many different bacteria as a carbon source. To overcome these problems, we prepared a completely synthetic medium, and the single carbon source, l-serine, increased selectivity. Also, of various substances we tested for their selective inhibition potential, we chose ampicillin sodium and methyl violet—both have been used in media selective for Pseudomonas spp. and Burkholderia glumae (Fieldhouse and Sasser 1982; Hasebe et al. 1998; Miyoshi and Tachibana 1994; Sato et al. 1981; Tsushima et al. 1986; Uematsu et al. 1982). These substances did not inhibit Psj multiplication but did inhibit the multiplication of other phytopathogenic bacteria and nonpathogenic bacteria isolated from barley. The substance with the greatest selective inhibition of bacteria other than Psj was potassium tellurite, used in a selective medium for P. cichorii created by Uematsu et al. (1982), but allowed the growth of black Psj colonies, thereby aiding selective identification as well as selective inhibition. To complete the medium, we also added cycloheximide and kabicidin, which suppress filamentous fungi; kabicidin, an antibiotic extracted from Streptomyces gougerotii culture liquid, only suppresses the growth of true fungi.
Because we were unable to completely suppress growth of P. viridiflava and nonpathogenic bacteria, we also prepared antisera by using Psj strains as antigens and used these antisera to test isolates that grew on the selective medium by the slide agglutination method. The antiserum reacted to some of the P. syringae strains, including all Psj strains. By combining the slide agglutination method with the selective medium, we could specifically detect Psj in barley and wheat seeds. On the other hand, six strains of P. syringae pathovars syringae, alisalensis, aptata, pisi, and solidagae grew on SPTPsjA and reacted with the antisera in the slide agglutination reaction test just as Psj did (Table 4). Except for one strain (pv. alisalensis), these strains are in the same genetic group (group III) and thus cannot be identified by molecular techniques such as PCR. It is important to easily isolate living pathogenic bacteria using selective medium because identification of Psj depends on confirming pathogenicity to wheat or barley by inoculation test. A PCR method is effective for rapid and high-sensitivity detection of the pathogen without isolation (Yoshioka et al. 2014). These various methods can be useful depending on the intended purpose. Although a PCR method can be used to identify bacteria grown on SPTPsjA medium, we used the slide agglutination reaction here because it produces results in a few minutes and does not require equipment such as a thermal cycler.
Because seeds infected with Psj are the most important source of inoculum for bacterial black node (Kawaguchi et al. 2017), control of the disease depends largely on determining whether seeds are infected. Our study revealed that the proportion of seeds infected with Psj varies widely from year to year in barley and, depending on the field, even in the same year in wheat. Unfortunately, all seeds collected from fields were infected with Psj. In contrast, barley and wheat seeds from plants cultivated in the greenhouse are rarely infected with Psj (data not shown). Fukuda et al. (1990) confirmed that Psj cells enter through pores in the epidermis of the lemma and multiply in the intercellular spaces in parenchymal tissue. The weather (rain and wind) during the time when Psj can infect seeds (such as at flowering stage) can strongly influence bacterial colonization in seed. In the future, we need to study the interrelationship among weather factors, seed infections, and the occurrence of bacterial black node disease.
References
Aoyagi K, Sakai T, Iwata K (1980) Occurrence and damage of the barley ear-burn in Niigata Prefecture (in Japanese). Proc Assoc Plant Prot Hokuriku 28:84–86
Fieldhouse DJ, Sasser M (1982) A medium highly selective for Pseudomonas syringae. pv. glycinea. Phytopathology 72:706
Fukuda T, Azegami K, Tabei H (1990) Histological studies on bacterial black node of barley and wheat caused by Pseudomonas syringae pv. japonica (in Japanese with English summary). Ann Phytopathol Soc Jpn 56:252–256
Goto K, Nakanishi I (1951) Ear burn, a new bacterial disease of barley (in Japanese with English summary). Ann Phytopathol Soc Jpn 15:117–120
Hasebe M, Hino K, Kondo A, Tsuchiya K (1998) Examination of selective isolation medium of bacterial black node of barley and wheat caused by Pseudomonas syringae pv. japonica. Ann Rept Kansai Plant Prot 40:87–88
Ikata S, Hori M (1950) Showa 24 nendo shukaku mugi ni gekihatsu shita shin saikinbyo ni tsuite (in Japanese). Ann Phytopathol Soc Jpn 15:32–33
Inoue Y, Takikawa Y (2006) The hrpZ and hrpA genes are variable, and useful for grouping Pseudomonas syringae bacteria. J Gen Plant Pathol 72:26–33
Kan M (1978) Mugirui kurofushibyo no hassei seitai to shuryo heno eikyo (in Japanese). Kongetsuno Nouyaku 22:14–18
Kawaguchi A, Tanina K, Takehara T (2017) Molecular epidemiology of Pseudomonas syringae pv. syringae strains isolated from barley and wheat infected with bacterial black node. J Gen Plant Pathol 83:162–168
Kawaguchi A, Yoshioka R, Mori M, Nishimura F, Kawata K, Tomioka K, Takehara T (2018) Spatiotemporal distribution of barley and wheat plants naturally infected with bacterial black node in fields in western Japan. J Gen Plant Pathol 84:35–43
Maeda T, Tsuchiya K (1993) Kokessei sakuseiho (in Japanese). In: Wakimoto S (ed) Laboratory guide for plant pathology and microbiology. Soft Science Publications, Tokyo, pp 249–263
Matsuzawa K (1987) Occurrence of bacterial black node of barley in Toyama Prefecture in 1987 (in Japanese). Proc Assoc Plant Prot Hokuriku 35:57–60
Miyoshi T, Tachibana Y (1994) A selective medium for isolation of Pseudomonas syringae, the pathogen of bacterial blossom blight of kiwifruit (in Japanese with English summary). Ann Phytopathol Soc Jpn 60:729–734
Mori M, Sogou K, Kanegae Y (1999) Detection of Pseudomonas syringae pv. japonica from barley seeds by selective medium and antiserum (in Japanese). Ann Phytopathol Soc Japan 65:362–363
Mukoo H, Tsuchiya Y (1950) Mugirui no saikinbyo ni tsuite (in Japanese). Ann Phytopathol Soc Jpn 15:44–45
Nishiyama K (1978) Shokubutsu byogen saikin kan-i doteiho no shian (in Japanese). Plant Prot 32:283–288
Oba S, Saito S, Sato M, Ishigaki H, Tanaka T, Uzuki T (1990) On the occurrence of bacterial black node of wheat in Shonai region of Yamagata Prefecture (in Japanese). Ann Rept Plant Prot North Jpn 41:50–52
Sato M, Takahashi K, Uematsu T, Ohata K (1981) Selective media for isolation of Pseudomonas syringae pv. mori, the pathogen of bacterial blight of mulberry (in Japanese with English summary). J Seric Sci Jpn 50:409–414
Senba T, Kaneko M, Kondo A, Takashi S (1994) Occurrence and its yield loss, control effects of some methods of bacterial black node of wheat and tow-lowed barley (in Japanese). Ann Rept Kansai Plant Prot 36:63–64
Shimizu S, Yanagihara Y, Asaka S (1981) Occurrence of barley bacterial node in Nagano Prefecture (in Japanese). Ann Rep Kanto-Tosan Plant Prot Soc 28:23–24
Takamatsu S (1983) New occurrence of bacterial black node of barley in Fukui Prefecture, in 1979 (in Japanese). Proc Assoc Plant Prot Hokuriku 31:77–78
Tsao PH (1970) Selective media for isolation of pathogenic fungi. Ann Rev Phytopathol 8:157–186
Tsushima S, Wakimoto S, Mogi S (1986) Selective medium for detecting Pseudomonas glumae Kurita et Tabei, the causal bacterium of grain rot of rice (in Japanese with English summary). Ann Phytopathol Soc Jpn 52:253–259
Uematsu T, Takatsu A, Ohata K (1982) A medium for the selective isolation of Pseudomonas cichorii. Ann Phytopathol Soc Japan 48:425–432
Wakimoto S, Uematsu T (1993) Bunri-baiyoho (in Japanese). In: Wakimoto S (ed) Laboratory guide for plant pathology and microbiology. Soft Science Publications, Tokyo, pp 51–77
Yokoyama S (1976) Fukuoka-ken ni toppatsu shita mugirui kurofushibyo (in Japanese). Plant Prot 30:7–10
Yoshioka R, Uematsu H, Takikawa Y, Kajihara H, Inoue Y (2014) Development of PCR primer sets for detection of Pseudomonas syringae. pv. syringae, the causal agent of bacterial black node (in Japanese). Jpn J Phytopathol 80:322–323
Young JM (1992) Pseudomonas syringae pv. japonica (Mukoo 1955) Dye et al. 1980 is a junior synonym of Ps. syringae pv. syringae van Hall 1902. Lett Appl Microbiol 15:129–130
Acknowledgements
We are indebted to Dr. Kenichi Tsuchiya (professor emeritus at Kyushu University) and Dr. Jun-Ichirou Yamaguchi for providing valuable strains, and to Drs. Koji Nishiyama and Takanori Miyoshi for helpful advice. This work was supported by a Grant-in-Aid for Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry from the Ministry of Agriculture, Forestry and Fisheries, Japan (Grant number 25063C).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Kazuhiro Sogou: Retired.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mori, M., Sogou, K. & Inoue, Y. Development of a selective medium and antisera for Pseudomonas syringae pv. syringae from seeds of barley and wheat. J Gen Plant Pathol 85, 211–220 (2019). https://doi.org/10.1007/s10327-019-00838-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-019-00838-w