Abstract
Bacterial black node caused by Pseudomonas syringae pv. syringae is the most serious bacterial disease of barley and wheat in Japan. The spatiotemporal distribution of barley and wheat plants infected with bacterial black node in fields in 2016–2017 was analyzed using Taylor’s model and Iwao’s model. In Taylor’s model, the sample variance (s 2) of the total and the newly diseased plants at each observation increased with mean plant density (m). In Iwao’s model, although the mean crowding (m*) of total and newly recognized diseased plants increased with m, Taylor’s model fit the data better than did Iwao’s model. Thus, bacterial black node could be explained as a colony expansion model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial black node (BBN), a disease caused by Pseudomonas syringae pv. syringae (PSS) van Hall 1902 [= P. syringae pv. japonica (Mukoo 1955) Dye et al. 1980], is the most important bacterial disease of barley and wheat in Japan (Bull et al. 2010; Fukuda et al. 1990; Mukoo 1955). Although PSS is known to cause basal kernel blight of barley throughout the world (Braun-Kiewnick et al. 2000), the pathogen is known to cause BBN in Japan (Kawaguchi 2013; Kawaguchi et al. 2017; Mukoo 1955; Oba et al. 1990; Yamashiro et al. 2011). In recent years, BBN has also emerged in western regions of Japan (Kawaguchi 2013; Kawaguchi et al. 2017). Although there are reports that BBN is transmitted via seeds (Goto and Nakanishi 1951; Fukuda et al. 1990), the ecology and epidemiology of barley and wheat BBN in commercial fields are still unclear, making the disease hard to control.
Kawaguchi (2013) previously reported the use of differences in hrpZ sequences determined by polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) to investigate the molecular epidemiology of PSS strains isolated from diseased barley and wheat plants in Okayama Prefecture, Japan. Although many strains isolated from barley and wheat belonged to various PCR-RFLP groups of PSS strains, we did not find any specific PCR-RFLP groups from a specific area or specific cultivars (Kawaguchi 2013). Also, to investigate the molecular typing of PSS strains isolated from barley and wheat plants with BBN symptoms grown in different locations of seed-production districts in Japan, we used repetitive sequence-based (rep)-PCR and intersimple sequence repeat (ISSR)-PCR (Kawaguchi et al. 2017). Eighteen genomic fingerprinting (GF) genotypes were obtained from the combined results of rep- and ISSR-PCR, indicating that the PSS population in Japan has high genetic diversity (Kawaguchi et al. 2017). Logistic regression indicated that the population of one of the specific GF genotypes was significantly related to a seed-producing district and that the epidemic of PSS strains in fields originated mainly from seed infection (Kawaguchi et al. 2017). Currently, we continue investigating the epidemiology of BBN, which has multiple infection routes.
According to our previous report (Kawaguchi et al. 2017), seed infection should play an important role in transfer of BBN epidemics among fields. We assume that the first diseased plant germinated from infected seeds plays a role as a primary inoculum, but soil-borne inocula from diseased plant debris of previous years also likely cause secondary infection with PSS in neighboring healthy plants in commercial fields. In any case, it is unclear what roles the primary and secondary inocula play in the disease cycle in fields. Thus, clarifying the roles of the primary and secondary inocula is very important and could allow farmers to control the disease more efficiently. To estimate the role of the primary and secondary inocula, we investigated the spatial distribution of barley and wheat plants naturally infected with BBN in fields after the first detection of the disease, and compared them using several epidemiological models.
Materials and methods
Field survey
We investigated the spatial patterns of BBN spread within 12 fields in 2016 and 6 fields in 2017 in three prefectures (Kagawa, Hiroshima, and Yamaguchi) in western Japan.
Weather data at the weather stations were collected from the Automated Meteorological Data Acquisition System (AMeDAS). In Kagawa (Takamatsu City), mean temperature and total rainfall were 16.1 °C and 84.5 mm in April and 20.8 °C and 85.5 mm in May in 2016 and 15.7 °C and 76.0 mm in April and 20.9 °C and 69.5 mm in May in 2017, respectively. In Hiroshima (Fukuyama City), mean temperature and total rainfall were 15.0 °C and 139.5 mm in April and 19.5 °C and 79.0 mm in May in 2016 and 14.3 °C and 96.5 mm in April and 19.4 °C and 54.5 mm in May in 2017, respectively. In Yamaguchi (Yamaguchi city), mean temperature and total rainfall were 15.8 °C and 84.5 mm in April and 19.8 °C and 62.0 mm in May in 2016 and 14.9 °C and 220.0 mm in April and 19.9 °C and 56.0 mm in May in 2017, respectively. We avoided rainy days when surveying each field. These conditions would not influence the field survey and data analysis.
Details for each field are outlined in Table 1. Field sizes were diverse (maximum 100.0 × 50.0 m and minimum 50.0 × 9.6 m), but investigation plots were similar (maximum 30.0 × 3.6 m and minimum 27.0 × 2.7 m). In each field of barley or wheat, the rows were spaced about 30 cm apart. The disease incidence in three rows of about 360 plant stems was checked and mapped in contiguous quadrats, which consisted of squares of 0.81 m2 (90 × 90 cm, length × width) (Table 1, Online Resource Fig. S1). Plants with BBN symptoms were counted in each field on one to three different dates after the disease was initially detected. To confirm the identification of PSS, we randomly collected symptomatic stems from the fields, and one bacterial strain from each stem was isolated on potato sucrose agar. The PSS strains were identified by a PCR specific for P. syringae group III, the wheat and barley infection group (Inoue and Takikawa 2006).
Mathematical analysis
The sample variance (s 2) of the number of individuals per quadrat usually increases with increasing sample mean (m). Bliss (1941) used the following equation to describe the relationship between s 2 and m:
where a and b are constants. Equation (1) expressed in linear form on a logarithmic scale is called Taylor’s power law (Taylor 1961):
Equation (2) represents the relationship between s 2, a, b, and m. Equations (3) and (4) express the definitions of m and s 2:
where \(\sum _{i = 1}^{n}x\) i is the total number of diseased stems of plant, n is the number of quadrats, and x i is the number of diseased stems of plants in the ith quadrat (i = 1, …, n). The index b is a measure of the dispersion of individuals in a population: 0 ≤ b < 1 indicates a uniform distribution; b = 1, a random distribution; and b > 1, an aggregated distribution (Taylor 1961). The value of b remains constant for the same organisms in the same environment and can be considered an “index of aggregation” that is characteristic of the species because samples collected from different localities and at different times can be fitted to a single regression line by Taylor’s power law (Taylor 1961; Taylor et al. 1978). Accordingly, sets of data obtained from different locations and years were combined and analyzed.
Bliss (1941) used another equation to describe the relation between s 2 and m:
where c and d are constants. Equation (5) expressed in a linear form is called Iwao’s m*–m regression method (Iwao 1968). It is used to quantify the relationship between the mean crowding index (m*) (Lloyd 1967) and mean density (m) using data from several sets of observations as follows:
where α and β are constants, α is an index of basic contagion and related to the size of clusters of diseased stems of plants and β is a density–contagiousness coefficient and indicates the patchiness of clusters (Iwao 1968). The indices α and β are measures of dispersion of individuals in a population: −1 < α < 0 indicates a uniform distribution; α = 0, a random distribution; and α > 0, an aggregated distribution of diseased plant stems when the mean density of diseased stems is very low (m ≈ 0), while 0 ≤ β < 1 indicates a uniform distribution; β = 1, a random distribution; and β > 1, an aggregated distribution. Samples collected from different localities and at different times can be fit to a single regression line by Iwao’s m*–m regression (Iwao 1968). Accordingly, sets of data obtained from different locations and years were combined and analyzed.
The goodness of fit of Taylor’s model was compared with that of Iwao’s model using a coefficient of determination (R 2) based on methods reported previously (Ali et al. 1998; Kawaguchi and Suenaga-Kanetani 2014; Mollet et al. 1984; Soemargono et al. 2008; Yamamura 2000).
Results
Disease assessment
In 2016 and 2017, BBN first appeared in early April, and diseased stems increased in number from the middle of April to late May in several fields in the western region of Japan (Table 1). Fields with BBS symptom (≥ 0.01% diseased stems per plot) were investigated during April to May (Table 1). In 2016, the highest incidence of BBN was 5.96% of diseased stems of barley in field Q in Yamaguchi Prefecture, and the highest incidence in 2017 was 2.56% of that in field R in Yamaguchi Pref. (Table 1). The disease incidence data from all investigated fields in 2016 and 2017 were used in a mathematical analysis for spatiotemporal distribution. Moreover, all the isolated strains from randomly collected symptomatic stems in the investigated fields were confirmed as PSS by the PCR for P. syringae group III (data now shown).
Mathematical analysis for spatiotemporal distribution
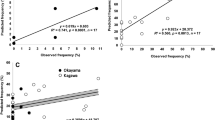
The results of Taylor’s model when applied to the combined data for the total number of recognized diseased stems of the plants observed in each field showed that sample variance (s 2) increased with mean density (m) (Fig. 1a). Taylor’s regression formula was log s 2 = 1.342 log m + 0.490, with coefficients of determination (R 2) = 0.958 (P < 0.001) and b = 1.342 [99% confidence interval (CI) 1.216–1.469]. Thus, b was significantly greater than 1.0 (P < 0.001). Also, the sample variance of newly recognized diseased plants at each observation increased with the mean density of diseased plants (Fig. 1b). Taylor’s regression formula was log s 2 = 1.319 log m + 0.458, with R 2 = 0.967 (P < 0.001) and b = 1.319 (99% CI: 1.208 to 1.431). Thus, b was significantly greater than 1.0 (P < 0.001).
Taylor’s power law regression (Taylor 1961) and Iwao’s m*–m regression (Iwao 1968) for diseased stems of barley and wheat plants naturally infected with bacterial black node (BBN) in fields. a Regression analysis for all diseased stems recognized at each observation by Taylor’s model. b Regression analysis for diseased stems newly recognized at each observation by Taylor’s model. For a, b, the solid line shows the Taylor’s power law regression line; the dotted line shows log s 2 = log m (a = 0, b = 1), indicating a random distribution. c Regression analysis for all diseased stems recognized at each observation by Iwao’s model. d Regression analysis for newly recognized diseased stems at each observation by Iwao’s model. Coefficient of determination (R 2) values of the two different regressions were significant, ***P < 0.001. c, d The solid line is the m*–m regression line. The dotted line shows m* = m (α = 0, β = 1), indicating a random distribution
When applied to the combined data for total number of recognized diseased stems of the plants observed in each field, the results of Iwao’s m*–m regression showed that mean crowding (m*) increased with mean density (m) (Fig. 1c). The m*–m regression formula was m* = 1.511 m + 1.469 with R 2 = 0.863 (P < 0.001), α = 1.469 (95% CI 0.458–2.481), and β = 1.511 (99% CI 1.238–1.784). Thus, α was significantly different from 0 (P < 0.01), and β was significantly greater than 1.0 (P < 0.001). On the other hand, the mean crowding of newly recognized diseased plants at each observation increased with the mean density of diseased plants (Fig. 1d). The m*–m regression formula was m* = 1.670 m + 0.773 with R 2 = 0.863 (P < 0.001), α = 0.773 (95% CI − 0.055 to 1.601), and β = 1.670 (99% CI 1.368–1.972). Thus, α was not significantly different from 0 (P > 0.05), and β was significantly greater than 1.0 (P < 0.001).
Discussion
Taylor’s power law and Iwao’s m*–m regression are two statistical methods commonly used in population research to analyze the distribution of organisms. Taylor’s model is a colony expansion model, and Iwao’s model is a colony increase model (Yamamura 2000; Fig. 2). When Taylor’s model was applied to the total of all recognized diseased stems of plants observed in the combined field data, the index b was significantly greater than 1.0, suggesting that there was an aggregated distribution of diseased stems. When Iwao’s model was applied to the total of all recognized diseased stems of plant observed in the combined field data, α (relating to the size of clusters of diseased stems) was significantly different from 0, suggesting that there was an aggregated distribution of all recognized diseased stems at each observation when the mean density of diseased stems was very low (m ≈ 0). Also, when Iwao’s model was applied, β (indicating the patchiness of clusters) was significantly greater than 1.0, suggesting that there was an aggregated distribution of diseased stems when the mean density of the diseased stems was much greater than 0. For the data of all recognized diseased stems observed in the combined field data, Taylor’s model fit the data of all recognized diseased stems at each observation better than did Iwao’s model because R 2 of Taylor’s model was 0.958 and higher than that of Iwao’s model, 0.863. Therefore, the distribution of all recognized diseased stems with BBN at each observation best fit the colony expansion model (Fig. 3).
Schematic illustrations of the assumptions of the models. Individual circles indicate individuals (diseased plants, in this study), and aggregated circles indicate colonies. In Taylor’s model (Taylor 1961), the population increase is described by the development of each colony, while the number of major colonies is fixed (colony expansion model, upper). In Iwao’s model (Iwao 1968), by contrast, the population increase is described by an increase in the number of colonies, while the colony size is fixed (colony increase model, lower)
Schematic illustrations of the assumptions of the model of BBN based on this study. Circles indicate individuals (diseased plants, in this study), and aggregated circles indicate colonies. The population increase is mainly described by the development of each colony, while the number of major colonies is fixed (colony expansion), and new independent foci form and expand during outbreaks
Similarly, when applying Taylor’s model to newly recognized diseased stems at each observation, Taylor’s model fit the data of newly recognized diseased plants at each observation better than did Iwao’s model because R 2 of Taylor’s model and Iwao’s model were 0.967 and 0.863, respectively. Therefore, the distribution of newly recognized diseased stems with BBN at each observation also best fit the colony expansion model (Fig. 2). The newly recognized diseased plants at each observation showed an aggregated distribution, suggesting that newly diseased plants formed new independent foci during outbreaks. Thus, this is the colony expansion model, in which new independent foci form and expand during outbreaks (Fig. 3).
The R 2 values of the Taylor model and Iwao model were significant (P < 0.001) although their R 2 values differed (Fig. 2). These results showed that either colony (diseased stems with BBN symptom) expansion or increase might occur in commercial fields. However, colony expansion phenomenon might have occurred previously in fields because Taylor model fit the data better than the Iwao model did (Fig. 3).
In this study, our statistical analyses showed that the populations of the PSS pathogen formed new independent clusters and that each cluster expanded during outbreaks, leading us to hypothesize a 3-step disease cycle: (1) diseased stems develop from infected seeds in a field and serve as primary inoculum, (2) PSS physically spreads from diseased stems as a result of wind or rain or both and initiate new infection, (3) each new infection initiates other new infections around it, and then each cluster expands. Steps (1), (2), and (3) repeat during outbreaks. To clarify the hypothesis directly, we could label PSS strains by inserting antibiotic or fluorescence genes and investigate the BBN outbreak in more detail, but that is difficult in open-air commercial fields. Thus, a statistical analysis of the spatiotemporal distribution is essential for us to plan efficient management of this disease as soon as possible. According to our results, farmers should not only disinfect seeds before planting but also spray a bactericide in fields to control secondary infection and avoid outbreaks of BBN. Under Japanese agricultural chemical regulations, we can use a wettable copper powder to control BBN. Spraying barley and wheat plants with wettable copper powder is especially necessary in breeder’s seed-producing stock fields.
So far, there have been no reports on the pattern of spread of BBN infection in fields. In general, the spread of disease in plants in fields tends to follow an aggregated distribution pattern (Madden and Hughes 1995), but the pattern of spread of plants with bacterial disease symptoms such as BBN caused by different infection pathways is unknown. In a recent report on the pattern of spread of a plant disease in greenhouses, Kawaguchi et al. (2013) investigated the spatiotemporal distribution pattern of tomato bacterial canker caused by Clavibacter michiganensis subsp. michiganensis (CMM) within a greenhouse. Their results indicated that CMM from the primary inoculum can cause new infections over long periods and that tomato bacterial canker fits a colony increase model. Thus, our current study provides fundamental information on the pattern of spread of diseased plants infected with bacteria in fields.
In conclusion, PSS appears to initiate new infections from the primary inoculum at the onset of an outbreak, then each new infection leads to another round of new infections, the area of diseased plants increases, and each cluster of diseased stems expands in the disease cycle in commercial fields. This study shows that statistical analysis of the spatiotemporal distribution provides clues to infer the source and the mode of spread of the bacteria causing BBN.
References
Ali A, Gu WD, Lobinske RJ (1998) Spatial distribution of chironomid larvae (Diptera: Chironomidae) in two central Florida lakes. Environ Entomol 27:941–948
Bliss CI (1941) Statistical problems in estimating populations of Japanese beetle larvae. J Econ Entomol 34:221–232
Braun-Kiewnick A, Jacobsen BJ, Sands DC (2000) Biological control of Pseudomonas syringae pv. syringae, the causal agent of basal kernel blight of barley, by antagonistic Pantoea agglomerans. Phytopathology 90:368–375
Bull CT, De Boer SH, Denny TP, Firrao G, Ficher-Le Saux M, Saddler GS, Scortichini M, Stead DE, Takikawa Y (2010) Comprehensive list of names of plant pathogenic bacteria, 1980–2007. J Plant Pathol 92:551–592
Fukuda T, Azegami K, Tabei H (1990) Studies on bacterial black node of barley and wheat caused by Pseudomonas syringae pv. japonica (Japanese with English summary). Ann Phytopath Soc Jpn 56:252–256
Goto K, Nakanishi I (1951) Ear burn, a new bacterial disease of barley (in Japanese with English summary). Ann Phytopath Soc Jpn 15:117–120
Inoue Y, Takikawa Y (2006) The hrpZ and hrpA genes are variable, and useful for grouping Pseudomonas syringae bacteria. J Gen Plant Pathol 72:26–33
Iwao S (1968) A new regression method for analyzing the aggregation pattern of animal populations. Res Popul Ecol 10:1–20
Kawaguchi A (2013) PCR-RFLP identifies differences in hrpZ sequences to distinguish two genetic groups of Pseudomonas syringae pv. syringae strains from barley and wheat with bacterial black node. J Gen Plant Pathol 79:51–55
Kawaguchi A, Suenaga-Kanetani H (2014) Spatiotemporal distribution of tomato plants naturally infected with leaf mold in commercial greenhouses. J Gen Plant Pathol 80:430–434
Kawaguchi A, Tanina K, Inoue K (2013) Spatiotemporal distribution of tomato plants naturally infected with bacterial canker in greenhouses. J Gen Plant Pathol 79:46–50
Kawaguchi A, Tanina K, Takehara T (2017) Molecular epidemiology of Pseudomonas syringae pv. syringae strains isolated from barley and wheat infected with bacterial black node. J Gen Plant Pathol 83:162–168
Lloyd M (1967) Mean crowding. J Anim Ecol 36:1–30
Madden LV, Hughes G (1995) Plant disease incidence: distributions, heterogeneity, and temporal analysis. Annu Rev Phytopathol 33:529–564
Mollet JA, Trumble JT, Sevacherian V (1984) Comparison of dispersion and regression indices for Tetranychus cinnabarinus (Boisduval) (Acari: Tetranychidae) populations in cotton. Environ Entomol 13:1511–1514
Mukoo H (1955) On the bacterial black node of barley and wheat and its causal bacteria. Publication Society of Jubilee Publication in Commemoration of the Sixtieth Birthdays of Prof. Yoshihiko Tochinai and Prof. Teikichi Fukushi (Japanese), Sapporo, pp 153–157
Oba S, Saito S, Sato M, Ishigaki H, Tanaka T, Uzuki T (1990) On the occurrence of bacterial black node of wheat in Shonai Region of Yamagata Prefecture (Japanese). Ann Rept Plant Prot North Japan 41:50–52
Soemargono A, Ibrahim Y, Ibrahim R, Osman MS (2008) Spatial distribution of the Asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae) on citrus and orange jasmine. J Biosci 19:9–19
Taylor LR (1961) Aggregation, variance and the mean. Nature 189:732–735
Taylor LR, Woiwod IP, Perry JN (1978) The density-dependence of spatial behavior and the rarity of randomness. J Anim Ecol 47:383–406
Yamamura K (2000) Colony expansion model for describing the spatial distribution of populations. Popul Ecol 42:161–169
Yamashiro M, Waki T, Morishima M, Fukuda T (2011) Occurrence of barley bacterial black node (Pseudomonas syringae pv. japonica) in Tochigi Prefecture and its control by barley seed disinfection treatments (Japanese). Ann Rep Kanto-Tosan Plant Prot Soc 58:9–12
Acknowledgements
The authors are grateful to Mr. K. Tanina (Okayama Prefectural Technology Center for Agriculture, Forestry and Fisheries, Okayama, Japan) who helped us survey fields.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10327_2017_757_MOESM1_ESM.pptx
Map of plot in field B in 2016. A square represents a quadrat. Numbers in a quadrat indicate the number of diseased barley stems with bacterial black node. a All diseased barley stems recognized at each survey date. b Diseased barley stems newly recognized at each survey date. (PPTX 64 KB)
Rights and permissions
About this article
Cite this article
Kawaguchi, A., Yoshioka, R., Mori, M. et al. Spatiotemporal distribution of barley and wheat plants naturally infected with bacterial black node in fields in western Japan. J Gen Plant Pathol 84, 35–43 (2018). https://doi.org/10.1007/s10327-017-0757-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-017-0757-0