Abstract
Nitrogen pollution of waters has sometimes caused severe eutrophication, leading to the death of fishes and most aquatic life. There is therefore a need for efficient and cost-effective methods to remove nitrogen from ammonium-rich wastewaters. Anaerobic ammonium oxidation (ANAMMOX) is a promising process to remove nitrogen because this process directly oxidizes ammonium (NH4 +) to dinitrogen gas (N2) under anoxic condition. Nonetheless, a challenge of this process is that chemolithoautotrophic Anammox bacteria grow slowly at the beginning, thus resulting in low Anammox biomass and instability of reactors. Such issues can be overcome by granulation of the Anammox sludge. Here, we review the characteristics of the Anammox bacteria, and the formation, structure and flotation of Anammox granules under high hydraulic loadings. We also evaluate the performances of full-scale granular Anammox processes. The major points are: 1) Anammox bacteria secrete a large amount of extracellular polymeric substances (EPS), up to 415 mg g−1 of volatile suspended solids (VSS), containing many hydrophobic functional groups that facilitate biomass granulation. 2) Granulation enhances the sludge settling property and retention time, which contributes to the extremely high nitrogen removal rate of 77 kg m−3 d−1 of Anammox upflow reactors. 3) Flotation of Anammox granules frequently occurs under nitrogen removal rate higher than 10 kg m−3 d−1, which is mainly due to the overproduction of EPS under high hydraulic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution is a key problem for human beings (Wang et al. 2010a, 2015b; Xue et al. 2011; Yin et al. 2012; Chai et al. 2013; Yang et al. 2013; Dai et al. 2015; Deng et al. 2015; Xiao et al. 2015a, b, c; Sultan 2016; Wei et al. 2016). There are a number of industrial wastewaters containing high content of organic matters, heavy metals, fluorides, salts, toxicants, pharmaceutics produced all around the world (Amine-Khodja et al. 2006; Wiszniowski et al. 2006; Chai et al. 2009, 2010; Yang et al. 2010; Jiang et al. 2013, 2014; Zhang et al. 2013; Kanakaraju et al. 2014; Li et al. 2014;Wang et al. 2014; Wu et al. 2014, 2015; Xiao et al. 2014; Li et al. 2015; Cai et al. 2016; Chen et al. 2016a; Tappin et al. 2016; Trubetskaya et al. 2016). Among them, ammonium pollution has become an increasing environmental concern afflicting humans and the ecosystems in the world (Tang et al. 2011a; Shen et al. 2012; Jin et al. 2013; Peng et al. 2017b). The effluent ammonium of wastewaters from monosodium glutamate (MSG) manufacturing, food fermentation, and antibiotics processing industries was detected to be 5000–5500 mg L−1 (Shen et al. 2012; Jin et al. 2013). The discharge during agricultural activities especially fertilizing and livestock farming composed another source for ammonium. As reported, 60% of nitrogen was lost to runoff during fertilizer usage (Maciej 2000), while the swine wastewater contains ammonium concentration as high as 1800 mg L−1 (Qiao et al. 2010). Further, the complicated landfill leachate from municipal refuse becomes an important ammonium release with high content (2000–2500 mg L−1) (Wiszniowski et al. 2006; Li et al. 2011). Such high levels of ammonium released to water bodies lead to fast bloom of algae and ultimately severe eutrophication problem. Addressing this problem calls out both science and technology development to reduce the ammonium pollution with low cost.
While physicochemical approaches such as ammonium stripping, struvite precipitation, and chlorination exhibit high operational cost for nitrogen removal from ammonium-rich wastewaters (van der Star et al. 2007; Peng et al. 2017a), the cost-effective biological method (e.g., nitrification–dentrification process) has been widely used (Kuenen and Robertson 1994; van Loosdrecht and Jetten 1998; Carneiro et al. 2010). In the traditional biological nitrogen removal process, ammonium was first converted to nitrite and then nitrate that was later denitrified to dinitrogen gas (van Loosdrecht and Jetten 1998; Tang et al. 2009b, 2013b; Ali et al. 2016a; Xiang et al. 2016). However, the steps of nitrite oxidation to nitrate and subsequent reduction of nitrate to dinitrogen gas demand excess of oxygen and additional organic carbon as electron donor (van der Star et al. 2007; Tang et al. 2011b). In order to further decrease the operational cost, the complete autotrophic Anammox-based process has been developed as a more economic and sustainable way for nitrogen removal from ammonium-rich wastewater (van der Star et al. 2007; Kartal et al. 2010; Tang et al. 2011b). The anaerobic ammonium-oxidizing bacteria (AAOB) used in Anammox belongs to Planctomycetes (Strous et al. 2006). With 50% of contribution to the total nitrogen cycle (Dalsgaard and Thamdrup 2002), Anammox directly oxidizes ammonium to dinitrogen gas using nitrite as electron acceptor under anoxic condition, thereby reducing the denitrification steps and ultimately the operational cost and sludge production (Strous et al. 1998; Ma et al. 2016). Further, the nitrogen removal rate (NRR) by Anammox process was improved to be as high as 77 kg m−3 d−1 (Tang et al. 2011b), which was considerably higher than other biological nitrogen removal processes. Thus, the reactor volumetric requirement could be significantly reduced. Anammox-based process has attracted more and more attention since its first discovery in 1990s (Wett 2006; van der Star et al. 2007; Lackner et al. 2014).
However, the Anammox, characterized by a strictly chemoautotrophic pathway (Strous et al. 2006), utilizes inorganic CO2 as carbon source (Strous et al. 1998; Jetten et al. 2009), which is considered to be the highest energy-consuming pathway for carbon fixation and cellular synthesis. Thus, the cellular yield and bacterial growth rate are both extremely low with doubling time as long as 11 days (Strous et al. 1998). Likewise, Anammox bacteria are very susceptible to environmental conditions, including temperature, pH, and hydraulic loadings (Tang et al. 2009b, 2011a, 2013b; Chen et al. 2016b). Particularly, the existence of toxicants in wastewater imposed severe inhibition on the Anammox process (Tang et al. 2010b, 2011a, 2013c; Xing et al. 2015; Yu et al. 2016; Zhang et al. 2016). Therefore, it has been a challenge to cultivate a fast startup, stable operation, and quick recovery for the Anammox process.
Formation of granular sludge is considered as an effective means to overcome the negative effects caused by various environmental conditions and diverse inhibitors during the Anammox process (Hulshoff Pol et al. 2004; Liu et al. 2009; Ni et al. 2010; Ali et al. 2013; Xing et al. 2015; Zhang et al. 2016). Anammox bacteria can secrete extracellular polymeric substances (EPS), and tend to aggregate together to form granules even under high hydraulic shear stress (van der Star et al. 2007; Tsushima et al. 2007; Tang et al. 2011b; Speth et al. 2016). The aggregated granular sludge with a larger diameter and higher density was prone to settling inside reactors according to Stokes equation (Lu et al. 2012, 2013; Chai et al. 2014; Yan et al. 2014, 2017). Further, the granular sludge features a stable ecological structure where anaerobic bacteria mainly distributes inside the granules, and aerobic bacteria locate in the outside layer of granules (Hulshoff Pol et al. 2004; Liu et al. 2009; Ni et al. 2010; Tang et al. 2014; Gonzalez-Gil et al. 2015; Speth et al. 2016; Song et al. 2017). For the case of Anammox granules (Vazquez-Padin et al. 2010; Speth et al. 2016), the aerobic ammonium-oxidizing bacteria (AOB) (i.e., nitrifying bacteria) in the outer layer can consume the low level dissolved oxygen in reactor. This shell prevents the inhibitors in aqueous solution from penetrating into the inner part of granules with Anammox bacteria, effectively abating the negative influences caused by oxygen and inhibitors.
Therefore, the granulation of Anammox biomass plays a key role in stable and high-rate operation of Anammox process. However, the formation of Anammox granules and other related information and mechanism are still not well addressed. The present review gives an overview on the characteristics of Anammox bacteria and Anammox granules. Particularly, formation and flotation of Anammox granules, together with related mechanisms, are comprehensively addressed. The full-scale application of granular Anammox process is also discussed in this paper.
Bacteria in Anammox
Anammox is discovered as a new biochemical reaction for nitrogen conversion in nature for the past two decades (Strous et al. 1998). As compared to typical nitrification/denitrification processes, Anammox utilizes nitrite instead of oxygen as electron acceptor to oxidize ammonium, whereas the electron donor is replaced by ammonium over organic matter. The discovery of Anammox not only contributes to the knowledge of global nitrogen cycle, but also provides theoretical principles for developing new biological nitrogen removal processes for wastewater treatments.
Types of Anammox bacteria
All identified Anammox bacteria belong to the order Brocadiales, and are affiliated to the phylum Planctomycetales (Strous et al. 2006). Up to date, there are 17 species belonging to 6 genera, including Candidatus Brocadia anammoxidans (Kuenen and Jetten 2001), Candidatus Brocadia fulgid (Kartal et al. 2008), Candidatus Brocadia sinica (Hu et al. 2010), Candidatus Kuenenia stuttgartiensis (Schmid et al. 2000), Candidatus Jettenia asiatica (Quan et al. 2008), Candidatus Anammoxoglobus propionicus (Kartal et al. 2007), Candidatus Anammoxoglobus sulfate (Liu et al. 2008), Candidatus Scalindua brodae, Candidatus Scalindua wagneri (Schmid et al. 2003), Candidatus Scalindua sinooilfield (Li et al. 2010), Candidatus Scalindua zhenghei (Hong et al. 2011), Candidatus Scalindua sorokinii (Kuypers et al. 2003), Candidatus Scalindua arabica (Woebken et al. 2008), Candidatus Scalindua japonica (Mizuto and Okabe 2014), Candidatus Scalindua profunda (van de Vossenberg et al. 2013), Candidatus Scalindua marina (Dang et al. 2010; Brandsma et al. 2011) and Candidatus Scalindua pacifica (Dang et al. 2013). The first five genera are identified from wastewater treatment plants or lab-scale reactors, while the Candidatus Scalindua has often been detected in natural habitats, especially in marine sediments. All the discovered Anammox bacterial species are chemolithoautotrophs that have the ability to oxidize ammonium under anaerobic condition with CO2 as carbon source.
Compartmentalization in Anammox cell
The Anammox bacterial cryosubstituted cell structure is found to be internal compartmentalized (Lindsay et al. 2001; Sinninghe Damste et al. 2002; Fuerst 2005; van Niftrik et al. 2010). As observed under transmission electron microscopy (TEM), the innermost compartment is the anammoxosome, which is filled with material of moderate electron density and granular texture, but devoid of ribosome-like particles (Lindsay et al. 2001; Sinninghe Damste et al. 2002). The anammoxosome, where Anammox reaction occurs, is considered to be the most important part of Anammox bacterial cells (Lindsay et al. 2001; Sinninghe Damste et al. 2002). The anammoxosome is surrounded by a single membrane (MB), which is shown as MB 3 in Fig. 1. A riboplasm compartment containing both ribosomes and a fibrillar nucleoid completely surrounds the anammoxosome. The nucleoid appears to be attached to the anammoxosome membrane. The riboplasm is surrounded by a single intracytoplasmic membrane (MB 2 in Fig. 1) and the paryphoplasm, in this case relatively electron transparent, surrounds the rim (MB 1 in Fig. 1) of the cell. The typical characteristic of inner compartmentalization simply makes the Anammox bacterial cells being identified from other bacterial cells under TEM.
Structure of compartmentalized Anammox bacteria cell based on the TEM observation. The innermost compartment is the anammoxosome, which is filled with material of moderate electron density and granular texture, but devoid of ribosome-like particles. The anammoxosome is surrounded by a single membrane (MB), which is shown as MB 3. A riboplasm compartment containing both ribosomes and a fibrillar nucleoid completely surrounds the anammoxosome. The nucleoid appears to be attached to the anammoxosome membrane. The riboplasm is surrounded by a single intracytoplasmic membrane (MB 2) and the paryphoplasm, in this case relatively electron transparent, surrounds the rim (MB 1) of the cell Lindsay et al. (2001)
Distribution of Anammox bacteria
Anammox bacteria were frequently discovered in fresh water and marine sediments (Quan et al. 2008; Li et al. 2010; Hong et al. 2011; Dang et al. 2013; Wang et al. 2015a; Li and Gu 2016), featured by a diversely geographical distribution (Hu et al. 2010; Dang et al. 2013; van de Vossenberg et al. 2013): nitrogen gas production was reported during the anaerobic digestion tests using the sediments from Lake Mendota, USA, to Lake Kizakiko, Japan. Particularly, Anammox bacteria are broadly distributed in ocean and contribute higher than 50% to the global nitrogen cycle (Dalsgaard and Thamdrup 2002). The preconditions for Anammox reaction occurrence were the coexistence of ammonium and nitrite and absence of oxygen. In natural water bodies, the oxidation of ammonium to nitrite occurs due to low oxygen penetration; in the oxic/anoxic interfaces of sediments in lakes, rivers, and ocean, nitrite coexist with ammonium, which are the suitable habitat for Anammox bacteria. In addition, wastewater treatment systems are also the suitable places for Anammox bacterial growth where oxygen supply is usually not sufficient and the oxidation of ammonium to nitrite tends to occur (Wang et al. 2010b). For example, Tsushima et al. (2007) detected that the abundance of Anammox bacteria in denitrifying sludge was about 1.1 × 107–1.8 × 108 copies mg−1 VSS (VSS: volatile suspended solids), while it reduced to 1.7 × 106–1.9 × 107 copies mg−1 VSS in nitrifying sludge and anaerobic digestion sludge (Table 1). Yang et al. (2007) discovered that the methanogenic sludge treating brewery wastewater also contained Anammox bacteria. Pynaert et al. (2004) found that the Anammox bacterial content in anaerobic granular sludge was about 2.5 × 104 copies ng−1 (total DNA). These studies showed that Anammox bacteria are broadly distributed in natural ecosystems and wastewater treatment plants. Thus, all these sludges and sediments could serve as the inocula to enrich Anammox biomass.
Anammox granules
Formation of Anammox granules
Effect of different inocula
Anammox granulation that occurs in upflow reactors under high loading rate was ascribed to the nature of the Anammox bacteria. Inocula are an important factor that affects the fast granulation of Anammox biomass leading to different startup performance of Anammox reactors (Tang et al. 2008). As tabulated in Table 2, the startup performance seeded with different inocula experienced four phases (i.e., cell lysis, lag, activity elevation, and stationary phase) (Tang et al. 2009b, 2013a). The startup time, especially the cell lysis duration time, was significantly reduced by seeding flocculent nitrifying sludge and denitrifying sludge as compared to the anaerobic granular sludge (Tang et al. 2013a). However, the nitrogen removal performance for reactor seeded with anaerobic granular sludge was 2–3 times higher than flocculent sludges (see Table 2). This higher nitrogen removal rate was ascribed to the easier formation of Anammox granules from anaerobic granular sludge. As reported by Tang et al. (2010c), granular sludge was a good carrier for Anammox bacterial growth providing nuclei for granulation. Thus, the Anammox bacteria grow on the surface of the anaerobic granular sludge resulting in the direct transition from anaerobic granular sludge to Anammox granules (Tang et al. 2011b, 2013a). The Anammox granules were gradually enriched inside the upflow reactors and finally fully filled the whole reactor (as shown in Fig. 2). Due to the challenging and long startup period for Anammox granulation from the flocculent denitrifying sludge and nitrifying sludge (Tang et al. 2013a), researchers have selected the granular sludge (anaerobic or aerobic) as inoculum to seed Anammox reactors for improved nitrogen removal performance (Ni et al. 2009; Tang et al. 2010a, c, 2011b; Xiong et al. 2013; Wang et al. 2016).
Transition of Anammox granules from anaerobic granular sludge a Day 10; b Day 150; and c Day 400. The red granules indicate Anammox granules, while the gray or light black granules are the inoculum of anaerobic granules. After about 400 days’ operation, the red Anammox granules are enriched but some of the black anaerobic granules still exist in the upflow reactor (Tang et al. 2011b)
Formation process of Anammox granules
The structure of Anammox granules was divided into four levels from micro-scale to macro-scale: single cell, microbial cell cluster, subunit, and granule (Lu et al. 2012). The single cell under TEM observation has been illustrated above, whereas the microbial cell cluster was among 200 μm in diameter (Fig. 3). Inside the microbial cell clusters, microbial cells were assumed to be packed together by extracellular polymeric substances (EPS). The neighboring microbial cell clusters were connected by the filamentous bacteria-EPS bonds (Lu et al. 2012). From the scanning electron microscopy (SEM) picture, we can identify that considerable cocci aggregate together (the white arrow), showing the microbial cells cluster (Fig. 3d). The subunit was visually observable. Figure 3a shows that an Anammox granule is consisted of several subunits, as indicated by the white arrows. The subunits ranged about 1 mm, which could be separated from the granule by external pressure. The force cohering these subunits to form a large scale (2–5 mm) granule might also be from EPS bonding (Lu et al. 2012).
Structure of Anammox granule. a a single granule (white arrows indicate subunits); b subunits separated from a granule; c SEM picture of a granule (white rectangle indicates a subunit); and d the microbial cell clusters (white arrows) in a subunit Lu et al. (2012)
In conclusion, the Anammox cells first gather together to form a microbial cell cluster, followed by the clusters aggregation through the filamentous bacteria-EPS bonds to form a subunit. Then, several subunits coheres each other under the extensive mixing condition in high hydraulically loaded upflow reactors, where collision among the subunits easily occurs by the aiding of EPS. Finally, granules are formed inside the upflow reactors.
Physical, chemical, and microbial properties of Anammox granules
Size and density of Anammox granules
The red Anammox granules are obtained under high hydraulic loading rates up to 200 L L−1 d−1 with superficial liquid upflow rate of 7 m3 m−2 h−1 (Tang et al. 2011b). The size distribution of the Anammox granules ranged from 1.5 to 4 mm (Tang et al. 2011b; Lu et al. 2012, 2013). The average density of Anammox granules reached 1.03 g mL−1 and the specific density reached 91–120 g VSS L−1 (granules), which is indicative of a high settling property. The large size and high density of Anammox granules result in high settling velocity of 73–88 m h−1 (Chen et al. 2010; Tang et al. 2011b; Lu et al. 2013). The 5 min sludge volume index (SVI5), an important parameter representing for settling performance, ranges 24–25 mL g−1 VSS with a thickening process verified by an SVI5/SVI30 ratio of 1 (Tang et al. 2011b; Lu et al. 2013). The compact Anammox granules with a high settling property lead to high biomass retention in bioreactors with Anammox biomass concentrations of 42–57 g L−1 (Tang et al. 2011b). Therefore, the nitrogen removal performance of Anammox upflow reactors with carmine granules was elevated to a considerably high value of 77 kg m−3 d−1 as compared the conventional biological nitrogen removal process (typically lower than 0.5 kg m−3 d−1) (Tang et al. 2009b).
Morphology and ecological structure of Anammox granules
The red-colored mature Anammox granule under SEM was characterized by a cauliflower-like shape (Arrojo et al. 2006; Tang et al. 2011b). The granules surface mainly consisted of spherical and elliptical bacteria; few or even no bacilli and filamentous bacteria were observed on the surface, suggesting the dominance of Anammox bacteria (Tang et al. 2011b). Interestingly, the shapes of dominating cocci in different Anammox granules under different operational conditions were different. As reported, the shapes of cocci showed a shrunken ball without effluent recirculation, while they exhibited a gaseous ball shape when recirculation was applied (Tang et al. 2011b). This difference might be correlated to two factors. One is the different substrate feeding strategies caused by effluent recirculation dilution, although the main operational conditions such as pH and hydraulic retention time (HRT) were relatively stable. As reported, the genus of Anammox bacteria was different under different substrate concentrations. For example, Candidatus Kunenia dominated at high substrate concentration while Candidatus Brocadia prevailed at low substrate concentration (Tang et al. 2010a). However, there is no further and direct evidence to confirm whether these different morphologies of bacteria belong to Brocadia or Kunenia. The other factor is correlated to certain known or unknown substances contained in the effluent (Tang et al. 2011b). Some soluble microbial products (SMP) were released from the metabolism process of bacteria (Xie et al. 2013). After long-term operating with effluent recirculation, these soluble microbial products could also contribute to the morphological differences for Anammox bacteria.
Figure 4 shows an example of the ecological structure of Anammox granules (Vazquez-Padin et al. 2010). In the outermost 200 μm layer of the granule, the biomass are dominated by ammonium-oxidizing bacteria (AOB) (Nitrosomonas spp.), the amount of which decreases at depths of 200–600 μm. Anammox bacteria are mainly located between 400 and 1000 μm in depth inside the granule where dissolved oxygen could not penetrate. In the depth interval among 400–600 μm, AOB and Anammox bacteria coexisted. In addition, it should be pointed out that the total number of bacteria decreased with the increase in depth inside the granule. Thus, the activity was mainly located within the 1000 μm of the granule. Furthermore, the substrate penetration becomes more difficult for larger Anammox granules (Ni et al. 2009). Therefore, a suitable diameter (i.e., less than 2 mm) for Anammox granules is suggested (Ni et al. 2009; Lu et al. 2012).
Ecological structure of Anammox granule. a Depth distribution of aerobic ammonium-oxidizing bacteria (AOB) populations, anaerobic ammonium-oxidizing bacteria (AAOB) and all bacteria inside the granule. The value of depth equal to 0 mm corresponds to the granule surface. b Image of a cryosectioned slice of a granule with a triple hybridization of FISH (fluorescence in situ hybridization) probes targeting AOB (light green), AAOB (pink) and all bacteria (blue) Vazquez-Padin et al. (2010)
Extracellular polymeric substances
Extracellular polymeric substances (EPS) are considered to be a key reason for biomass granulation (Hulshoff Pol et al. 2004; Liu et al. 2009). They are secreted by bacteria as sticky materials constituting proteins, polysaccharides, humic acids, and lipids that assisted cell adhesion. Therefore, EPS should be helpful to initiate the aerobic as well as anaerobic granulation process (Hulshoff Pol et al. 2004; Liu et al. 2009; Ni et al. 2010, 2015; Tang et al. 2011b; Hou et al. 2015). Accumulation of the secreted EPS improves the biological adhesion and microbial aggregation, since EPS could bridge bacterial cells and other particles into an aggregate (Liu and Tay 2002; Adav et al. 2008; Hori and Matsumoto 2010; Ismail et al. 2010; Hou et al. 2015; Ni et al. 2015; Schluter et al. 2015). High polysaccharide content was supposed to facilitate bacterial adhesion and strengthen the microbial structure through a polymeric matrix (Liu and Tay 2002; Adav et al. 2008).
Di Iaconi et al. (2006) reported that hydrodynamic shear stress did not affect the EPS content and compositions, but compact the granules. On the contrary, many researches supported that the increased shear stress, including hydraulic shear stress, gas-induced shear stress as well as mechanic shear stress, could stimulate the EPS secretion and contribute to the formation of granules (Liu and Tay 2002; Arrojo et al. 2006; Tang et al. 2011b; Song et al. 2017). Particularly, Tang et al. (2011b) pointed out that the EPS content of Anammox granules are significantly increased during long-term operation under increasing loading rate with liquid and gas induced shear stresses. However, the increase for polysaccharides was slower as compared to proteins. The polysaccharide contents of Anammox granules ranged from 60 to 115 mg g−1 VSS at nitrogen removal rate higher than 70 kg m−3 d−1, whereby the extracellular protein contents increased sharply to 300 mg g−1 VSS (Tang et al. 2011b).
The comparison of EPS content in different biological granules is listed in Table 3. The autotrophic Anammox granules contained a high EPS content as compared to the heterotrophic granules (Tang et al. 2011b; Ni et al. 2015), which is beyond our previous expectation. As reported, the autotrophic bacteria usually secreted low EPS content because they were using inorganic carbon as carbon source instead of organic compounds (Tsuneda et al. 2003; Vlaeminck et al. 2010; Tang et al. 2011b). However, the Anammox bacteria secreted a large quantity of EPS under high hydraulic loadings (Tang et al. 2011b; Ni et al. 2015). Therefore, extensive granulation of Anammox biomass inside upflow reactors occurs in a short hydraulic retention time.
Still, the roles between polysaccharide and protein in biomass granulation are controversial. Liu et al. (2004) argued that the protein content was positively correlated with surface hydrophobicity of bacterial cells. Hou et al. (2015) also concluded that protein contained high content of hydrophobic amino acids and loose structure to fully expose inner hydrophobic groups, which promote Anammox sludge aggregation. In contrast, several researchers showed that polysaccharide plays a more significant role in granulation (Chen et al. 2007; Adav et al. 2008; Ni et al. 2010). The polysaccharide possesses a number of negatively charged functional groups such as carboxyl and hydroxyl which could function as a bridging interaction to easily form granules (Adav et al. 2008). Chen et al. (2007) proposed that β-polysaccharide could serve as the skeleton of aerobic granular sludge. Furthermore, Ni et al. (2010) considered that carbohydrates, rather than proteins, might play a more important role in the formation of Anammox granules because the proteins/carbohydrates ratio of Anammox granules was just 0.51, considerably lower than the average of methanogenic granules. These findings indicated that the electrostatic attraction and bridging force caused by polysaccharide should be taken into consideration for Anammox granulation, although protein has shown to play an essential role in the granulation process (Hou et al. 2015).
Functional groups on granular surface
The functional groups on granular surface include negatively charged carboxyl and hydroxyl groups in polysaccharide (Hou et al. 2015). Further identification of functional groups on Anammox granule surface is being under investigation. It was previously estimated that the functional groups might be less on autotrophic Anammox biomass (Tsuneda et al. 2003; Vlaeminck et al. 2010; Tang et al. 2011b; Chai et al. 2015). Hou et al. (2015) systematically analyzed the functional groups on Anammox biomass by taking conventional activated sludge, nitrifying sludge and denitrifying sludge as control. They observed that the types of functional groups are similar for different sludges since the position and numbers of Fourier transform infrared (FTIR) peaks were quite close. With a closer inspection of the intensity of these peaks, fewer hydrophilic functional groups were found in EPS of Anammox sludge compared to other sludges, such as acylamino and carboxyl groups that have high polarity (Yuan et al. 2010; Hou et al. 2015). Consistent with previous results for EPS contents, the Anammox sludge surface characterized with less hydrophilic functional groups helps sludge granulation in upflow reactors.
Hemochrome content
Anammox granules possess a unique red color (Fig. 5) which is quite different from conventional biomass used in wastewater treatments (Tang et al. 2010a, 2011b). Generally, the color of aerobic granules and nitrifying granules is yellow; the color of heterotrophic denitrifying granules, hydrogen-producing granules as well as anaerobic methanogenic granules is black mainly due to the formation of metal sulfides (Hulshoff Pol et al. 2004; Franco et al. 2006; Liu et al. 2009; Tang et al. 2009b, 2010c, 2011b, 2013a). As reported, the Anammox bacteria contains enzymes such as hydroxylamine oxidoreductase (HAO) and hydrazine oxidoreductase (HZO) which are two key enzymes of Anammox reaction pathways (Strous et al. 2006; Schmid et al. 2008). These enzymes are rich in hemochrome (Klotz et al. 2008; Schmid et al. 2008). For example, the HAO and HZO contain 14–26 and 8–16 hemochromes, respectively (Schalk et al. 2000; Cirpus et al. 2005, 2006; Shimamura et al. 2007, 2008). The high content of hemochrome is presumed to form the carmine color of Anammox sludge (Tang et al. 2011b).
Naturally, higher activity of Anammox bacteria indicates higher conversion rate that needs higher content of enzymes including HAO and HZO, to catalyze the Anammox reaction. Therefore, we can simply correlate the biomass activity directly to the degree of red color. Tang et al. (2011b) demonstrated that the heme c content of Anammox granules significantly increased with the improvement of nitrogen removal rate. As reported, the heme c content finally reached 10 μmol g−1 VSS at nitrogen removal rate higher than 70 kg m−3 d−1 (Tang et al. 2011b). Correspondingly, the activity of the high-rate Anammox granules finally reached at 5.6 kg kg−1 VSS d−1, which was much higher than the light red Anammox biomass (lower than 1 kg kg−1 VSS d−1, Tang et al. 2010a, b; Yu et al. 2016; Song et al. 2017). However, a higher hemochrome content did not mean an increasing activity. In our recent study, we found that the floated Anammox granules gradually changed to black color with low activity, but the hemochrome content within the granules was still high (Song et al. 2017) due to the slow degradation of hemochrome (Strous et al. 2006).
Operation of Anammox upflow reactor
Granular packing
The high performance and stability of Anammox reactors largely depend on the quantity of granular sludge (Chen et al. 2010; Tang et al. 2011b). Therefore, previous researchers focused on developing enhanced strategies to improve Anammox granular sludge concentration inside reactors (Tsushima et al. 2007; Ma et al. 2011; Tang et al. 2011b). However, extremely high sludge concentration also increased biomass dead zones, decreased pore volume and shortened actual hydraulic retention time (Tang et al. 2010a, 2011b). The packing pattern of Anammox granules directly determined the pore volume and sludge concentration inside upflow reactors (Tang et al. 2010a). Therefore, it affects the reactor performance significantly, especially when the sludge concentrations inside Anammox reactors was as high as 40–50 g L−1 (Tang et al. 2011b).
Tang et al. (2010a) developed a mathematical model (Eq. 1) to simulate the relationship between granular packing patterns and nitrogen removal performance for a granule-based Anammox upflow anaerobic sludge blanket (UASB) reactor following the principles of crystal lattice packing.
where R is the conversion rate, kg m−3 d−1; C X is the sludge concentration, mg L−1; q is the specific activity of sludge, kg kg−1 VSS d−1; ρ granule is the density of granular sludge, g cm−3; SV is the sludge volume, %; c is the substrate loading, kg m−3 d−1.
Simulation results suggested that the simple cubic packing pattern was the favorable model for Anammox granule packing, with optimal sludge concentrations of 46–49 g VSS L−1 and packing density of 52–55%. The simple cubic packing not only provides high biomass concentration, but also possesses high porosity inside the reactor to reach the maximum value in performance. Sensitivity analysis indicates that when the granules concentration is lower than 37.8 g VSS L−1, the best way to improve reactor performance is to increase the sludge concentration; otherwise, it is more advisable to enhance substrate loading to achieve higher substrate conversion rate in the high-rate Anammox UASB reactor (Tang et al. 2010a).
Dissolved oxygen
As aforementioned, the Anammox granules possess a stratified ecological structure for resisting external detrimental effects. Usually, the operation of Anammox reactors is not strictly anaerobic. Thus, oxygen was accompanied in influent without oxygen elimination methods, or diffused into reactor from sampling ports. Thus, the Anammox granules would face the oxygen penetration during the long-term operation. The intrusion of oxygen resulted in the stratification of microbial species. The anoxic pockets in the interior of the granules are suitable to harbor Anammox bacteria that are strictly anaerobic bacteria and totally inhibited by 0.5% air saturation (Strous et al. 1997). While in the outer layer (or surface) of Anammox granule, AOB (ammonium-oxidizing bacteria) would consume excess oxygen. The stratification structure of AOB on surface and Anammox bacteria in inner section of Anammox granules contributes to stable and high-rate operation of Anammox reactors especially under the non-strictly anaerobic conditions.
Flotation of Anammox granules and control strategies
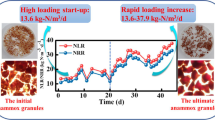
After granulation of Anammox biomass was realized, flotation of Anammox granules could occur. The increase in nitrogen removal rate was usually accompanied with increased hydraulic loading rate and nitrogen gas production, resulting in extensive mixing between gas bubbles and granules. Thus, the nitrogen gas bubbles produced by Anammox bacteria were trapped inside the granules (Tang et al. 2009a; Chen et al. 2010, 2014). As a result, the density of the granules decreased resulting in the sludge flotation and even sludge washout. In this situation, reactor performance eventually deteriorated during the continuous elevation of nitrogen loading rate. Moreover, the floated granules assembled continuously in the settler and blocked up the effluent pipe, resulting in malfunction of the reactor operation (Song et al. 2017). Therefore, the flotation of Anammox granules is a significant problem that affects the high rate and stable operation of Anammox reactors (Tang et al. 2009a; Chen et al. 2010, 2014; Song et al. 2017).
The flotation of Anammox granules has attracted a great deal of attention in recent years (Tang et al. 2009a, 2011b; Chen et al. 2010, 2014; Song et al. 2017). It seems that flotation of granules is inevitable during the operation of high-rate Anammox reactors, yet the mechanism of flotation still remains unclear. Most researchers insisted that the physical property of Anammox is the key reason. For example, Chen et al. (2010) reported that flotation occurred due to the blocking of gas tunnels and thus ineffective exhaust of nitrogen gas to the outside. Tang et al. (2009a) applied force analysis to evaluate the bubble column inside UASB reactor, and confirmed that gas accumulation led to the whole granules flotation. Our recent study compared the physical, chemical, and microbial properties of settled and floated granules (Song et al. 2017). It was found that EPS secretion, especially the protein content, of Anammox granules increased with the increase in nitrogen removal rate, thereby imposing the adhesion and bridging ability to form big granules from small aggregates. Gas bubbles were trapped between the joint of aggregates consequently (Fig. 6), and the gas tunnels from inner part to the surface of granules were easily blocked by the high EPS secretion (Chen et al. 2010). Furthermore, the direct adhesion of hydrophobic bubbles on EPS-rich granular surface was also favored (as shown in Fig. 6b). Due to the decrease in granules density, flotation of Anammox granules inevitably occurred in upflow reactors and was further enhanced with increasing gas production rates. Consequently, deterioration of reactor performance prevailed.
In order to recover the reactor performance after granules’ flotation, several methods were taken to relieve the flotation. Chen et al. (2010) and Lu et al. (2012) proposed the breaking–returning strategy in which the floated granules were first taken out of the reactor, and then broken into small pieces to release the gas bubbles entrapped in granules (Fig. 7). The sludge density was thus increased and would settle down to the reaction zone after returning to upflow reactors. But it should be pointed out that the ecological structure of the granules was also destroyed after complete breaking. A long recovery time was required to reform the granular ecological structure and restore the performance of Anammox reactors (Chen et al. 2010). Tang et al. (2009a) started shear force enhancement by introducing high hydraulic loadings to break the large gas column into small gas bubbles. In this way, the granules’ flotation was also relieved. Manual stirring with a stick to drive out the gas bubbles from the floated Anammox granules was also tested (Song et al. 2017), which can drive away the bubbles attached on the surface of the granules but cannot drive away the bubbles inside the granules. Thus, sludge flotation re-occurred after re-adhesion of gas bubbles during the continuous operation under high nitrogen removal rate. It is clear that the strategies based on physical properties of Anammox granules are not final methods to overcome sludge flotation. With the existence of high EPS and functional groups on sludge surface, attaching chemical precipitates with relatively high density (e.g., CaCO3) onto sludge surface in an appropriate content would increase the sludge density and enhance the granules settling ability significantly (Trigo et al. 2006; Xiong et al. 2013; Ali et al. 2016b; Song et al. 2017). In addition to these solutions, promising alternatives to control Anammox granular flotation are strongly recommended in future studies.
Mechanisms of granulation (a) and flotation (b) of Anammox biomass. Anammox bacteria first aggregate to form small Anammox cell cluster. Then, small cluster grow to large cluster and subsequent a small granule. After blocking the gas tunnels to the granular surface, nitrogen gas pocket(s) would be formed thus reducing the density of the granules. Flotation and washout of Anammox granules consequently occurs Lu et al. (2012)
Full-scale application of Anammox granules in wastewater treatment
Anammox process has been widely used for nitrogen removal from ammonium-rich wastewater (Kartal et al. 2010; Tang et al. 2008, 2011b; Wang et al. 2010b; Lackner et al. 2014). The development of granule-based Anammox reactors with NRR higher than 70 kg m−3 d−1 further increased the application significance of Anammox process for treatment of wastewaters including sludge liquor, landfill leachate, monosodium glutamate (MSG) wastewater and pharmaceutical effluents (van der Star et al. 2007; Wang et al. 2010b; Tang et al. 2011a; Lackner et al. 2014; Zhang et al. 2015). However, due to the slow growth rate of the Anammox bacteria, the application of full-scale Anammox-based process required an extremely long period, mainly due to the slow startup course (van der Star et al. 2007; Lackner et al. 2014). For the first full-scale Anammox reactor (70 m3) located in Rotterdam, Netherland (NL), the startup lasted 3.5 years (van der Star et al. 2007). It took about 4 years for enrichment of Anammox biomass in the first full-scale Anammox reactor (60 m3) implemented in China for treatment of MSG wastewater (built by Zhejiang University). In order to accelerate the startup, an upscaling strategy of “lab-pilot-full-scale” Anammox biomass enrichment has been proposed by Wett (2006), van der Star et al. (2007) and Tang et al. (2008), as shown in Fig. 8. Previous studies demonstrate that it was a prerequisite to enrich a large amount of Anammox biomass in lab scale as well as pilot-scale reactors. Then, the harvested Anammox biomass were used as seeding sludge to continuously feed full-scale reactors for enhanced Anammox reaction (Wett 2006; van der Star et al. 2007; Tang et al. 2008).
Upscaling enrichment strategy for startup of Anammox process treating monosodium glutamate wastewater. A series of lab–scale Anammox reactors (1 L) were utilized to enrich seed matured Anammox granules with high activity; then the seed matured granules were used to inoculate pilot-scale reactors (2.5 m3) to amplify the production of Anammox biomass which were finally used as the inocula to startup full-scale reactors (60 m3). This lab-pilot–full-scale Anammox system, located in Yiwu City, was initially built in 2005
Once the Anammox reaction initiates inside reactors, the startup performance would be significantly accelerated (Wett 2006; van der Star et al. 2007; Tang et al. 2013a; Xiong et al. 2013). Therefore, after successful implementation of the first full-scale reactor, the Anammox biomass has been largely enriched, resulting in the widespread installations of full-scale Anammox-based processes. By the year 2014, there were more than 100 full-scale Anammox–based processes around the world, among which more than 50% are sequencing batch reactors (SBR), 88% being operated as single-stage systems, and 75% for side-stream treatment of municipal wastewater (Lackner et al. 2014). Although the granular systems only consisted 20% of all full-scale applications, the nitrogen removal performance was substantially higher (Lackner et al. 2014). For example, the nitrogen removal rate of the first full-scale Anammox reactor (designed by Paques) with granular sludge reached 9.5 kg m−3 d−1, while the average nitrogen loading for full-scale SBR (without granular sludge) is about 1.7 kg m−3 d−1 (Lackner et al. 2014). Since 2006, Paques has designed granular reactors as one-stage implementations, with the majority of their systems applied for industrial wastewater treatments. This shift from two- to one-stage installations was mainly driven by the lower investment costs (Lackner et al. 2014).
Most of the full-scale Anammox processes were applied to treat reject water with low COD (chemical oxygen demand)/N ratio, especially in Europe (van der Star et al. 2007; Lackner et al. 2014). For example, the first full-scale reactor (70 m3) in Rotterdam, NL (van der Star et al. 2007) and the largest full-scale reactor (22,000 m3) in Blue Plains, USA (Lackner et al. 2014) were all used to treat reject water from wastewater treatment plants. The Anammox process was then expanded to treat landfill leachate, slaughterhouse effluents, potato processing wastewater, distillery effluent, and even metal processing wastewater (Lackner et al. 2014).
Speth et al. (2016) investigated the Anammox biomass (Fig. 9) in a full-scale partial nitritation and Anammox (PNA) process (600 m3) in Olburgen, NL. They discovered 23 near-complete draft genomes that represent the majority of the microbial community, among which 19 have no close relatives being previously cultivated or sequenced and 6 belong to bacterial phyla without any cultivated members. A metabolism diagram (Fig. 9c) of the granules in the full-scale reactor has been proposed. As shown in Fig. 9c, organic carbon (C–org) was oxidized to carbon dioxide (CO2) on the granule surface by autotrophs. Also, on the granule surface, \({\text{NH}}_{4}^{ + }\) was oxidized to nitrite (\({\text{NO}}_{2}^{ - }\)) that was either reduced to nitric oxide (NO) or further oxidized to nitrate (\({\text{NO}}_{3}^{ - }\)). The \({\text{NO}}_{3}^{ - }\) formed in the granules was reduced again in the anaerobic core, either with C–org or molecular hydrogen (H2) as electron donor. H2 can be formed through fermentation of organic carbon by CHB2 and CFX1 (two hydrogenase–encoding organisms). This cyclic feeding resulted in additional organic carbon removal from the system. NO formed from \({\text{NO}}_{2}^{ - }\) could combine with \({\text{NH}}_{4}^{ + }\), producing dinitrogen gas by Anammox.
Biomass from the Olburgen partial nitritation and Anammox reactor. a Untreated sample consisting of both flocculent and granular biomass; b Washed sample containing predominantly granular biomass; and c Schematic diagram of nitrogen conversions in the Olburgen PNA reactor. Organic carbon (C–org) was oxidized to carbon dioxide (CO2) on the granule surface by autotrophs. Also, on the granule surface, \({\text{NH}}_{4}^{ + }\) was oxidized to nitrite (\({\text{NO}}_{2}^{ - }\)) that was either reduced to nitric oxide (NO) or further oxidized to nitrate (\({\text{NO}}_{3}^{ - }\)). The \({\text{NO}}_{3}^{ - }\) formed in the granules was reduced again in the anaerobic core, either with C–org or molecular hydrogen (H2) as electron donor. H2 can be formed through fermentation of organic carbon by CHB2 and CFX1 (two hydrogenase-encoding organisms). This cyclic feeding resulted in additional organic carbon removal from the system. NO formed from \({\text{NO}}_{2}^{ - }\) could combine with \({\text{NH}}_{4}^{ + }\) producing dinitrogen gas by Anammox Speth et al. (2016)
Gonzalez-Gil et al. (2015) analyzed the microbial community composition of the granules taken from the lower port of the first full-scale Anammox reactor, NL. Pyrosequencing results showed that, besides Anammox bacteria (Brocadiacea, 32%), substantial numbers of heterotrophic bacteria Ignavibacteriacea (18%) and Anaerolinea (7%) along with heterotrophic denitrifiers (15%) existed in the granules. These bacteria may form a network in which heterotrophic denitrifiers could cooperate to achieve a well-functioning denitrification system as they can utilize the nitrate intrinsically produced by Anammox reaction. It is possible that the Anammox process in a full-scale reactor triggers various reactions overall leading to efficient denitrification and a sink of carbon as biomass in Anammox granules.
Conclusion
Anammox process is considered to be the most sustainable nitrogen removal technology for ammonium-rich wastewater treatments due to the high nitrogen removal performance, low operational cost, and reduced sludge production. However, due to the extremely slow growth rate of the chemolithoautotrophic Anammox bacteria, the enrichment of Anammox biomass is quite difficult, and the process is easily inhibited by environmental conditions, which significantly restricts the application of this environmental-friendly process. Granulation of Anammox biomass provides an effective method for stable and high-rate operation of Anammox upflow reactors. Anammox bacteria could secrete large amount of extracellular polymeric substances and many hydrophobic functional groups, which significantly contributes to Anammox biomass granulation. The Anammox granules feature a stable ecological structure with AOB on the surface and Anammox bacteria in the inner section of granules, which also improve the stability of Anammox reactors. Unfortunately, flotation of Anammox granules occurs inevitably under high shear stress and high nitrogen loading conditions. Floated granules assemble continuously in the settler and block up the effluent pipe, resulting in malfunction of the reactor operation. However, the mechanism behind the flotation still remains unclear, and effective control methods are highly desirable during the subsequent investigations. Since the granular Anammox process exhibits much higher nitrogen removal performance, more full-scale granular Anammox upflow reactor for treatment of ammonium-rich wastewater should be installed in future.
References
Adav SS, Lee DJ, Tay JH (2008) Extracellular polymeric substances and structural stability of aerobic granule. Water Res 42:1644–1650. doi:10.1016/j.watres.2007.10.013
Ali M, Chai LY, Tang CJ, Zheng P, Min XB, Yang ZH, Xiong L, Song YX (2013) The increasing interest of anammox research in China: bacteria, process development, and application. BioMed Res Int, article ID: 134914
Ali M, Chai LY, Min XB, Tang CJ, Afrin S, Liao Q, Wang HY, Peng C, Song YX, Zheng P (2016a) Performance and characteristics of a nitritation air-lift reactor under long-term HRT shortening. Int Biodeterior Biodegr 111:45–53. doi:10.1016/j.watres.2007.10.013
Ali M, Chai LY, Wang HY, Tang CJ, Min XB, Yan X, Peng C, Song YX, Zheng P (2016b) Enhanced short-cut nitrification in an airlift reactor by CaCO3 attachment on biomass under high bicarbonate condition. Biodegradation 27:131–144. doi:10.1007/s10532-016-9761-x
Amine-Khodja A, Richard C, Lavédrine B, Guyot G, Trubetskaya O, Trubetskoj O (2006) Water-soluble fractions of composts for the photodegradation of organic pollutants in solar light. Environ Chem Lett 4:249. doi:10.1007/s10311-006-0074-x
Arrojo B, Mosquera-Corral A, Campos JL, Méndez R (2006) Effects of mechanical stress on Anammox granules in a sequencing batch reactor (SBR). J Biotechnol 123:453–463. doi:10.1016/j.jbiotec.2005.12.023
Brandsma J, Van de Vossenberg J, Risgaard-Petersen N, Schmid MC, Engström P, Eurenius K, Hulth S, Jaeschke A, Abbas B, Hopmans EC, Strous M, Schouten S, Jetten MSM, Sinninghe Damsté JS (2011) A multi-proxy study of anaerobic ammonium oxidation in marine sediments of the Gullmar Fjord, Sweden. Environ Microbiol Rep 3:360–366. doi:10.1111/j.1758-2229.2010.00233.x
Cai M, Zhu Y, Wei Z, Hu JQ, Pan SD, Xiao R, Dong CY, Jin MC (2016) Rapid decolorization of dye Orange G by microwave enhanced Fenton-like reaction with delafossite-type CuFeO2: a kinetic and mechanistic study. Sci Total Environ. doi:10.1016/j.scitotenv.2016.12.047
Carneiro J, Cardenas LM, Hatch DJ, Trindade H, Scholefield D, Clegg CD, Hobbs P (2010) Effect of the nitrification inhibitor dicyandiamide on microbial communities and N2O from an arable soil fertilized with ammonium sulphate. Environ Chem Lett 8:237–246. doi:10.1007/s10311-009-0212-3
Chai LY, Min XB, Tang N, Wang YY (2009) Mechanism and kinetics of Zn(II) removal from wastewater by immobilized beads of SRB sludge. Int J Environ Pollut 37:20–33
Chai L, Wang Q, Li Q, Yang Z, Wang Y (2010) Enhanced removal of Hg(II) from acidic aqueous solution using thiol-functionalized biomass. Water Sci Technol 62:2157–2166. doi:10.2166/wst.2010.385
Chai LY, Wang YY, Zhao N, Yang W, You XY (2013) Sulfate-doped Fe3O4/Al2O3 nanoparticles as a novel adsorbent for fluoride removal from drinking water. Water Res 47:4040–4049. doi:10.1016/j.watres.2013.02.057
Chai LY, Yan X, Li QZ, Yang BT, Wang QW (2014) A comparative study of abiological granular sludge (ABGS) formation in different processes for zinc removal from wastewater. Environ Sci Pollut Res 21:12436–12444. doi:10.1007/s11356-014-3184-1
Chai LY, Ali M, Min XB, Song YX, Tang CJ, Wang HY, Yu C (2015) Partial nitrification in an air-lift reactor with long-term feeding of increasing ammonium concentrations. Bioresour Technol 185:134–142. doi:10.1016/j.biortech.2015.02.091
Chen MY, Lee DJ, Tay JH, Show KY (2007) Staining of extracellular polymeric substances and cells in bioaggregates. Appl Microbiol Biotechnol 75:467–474. doi:10.1007/s00253-006-0816-5
Chen JW, Ji QX, Zheng P, Chen TT, Wang CH, Mahmood Q (2010) Floatation and control of granular sludge in a high-rate anammox reactor. Water Res 44:3321–3328. doi:10.1016/j.watres.2010.03.016
Chen H, Ma C, Yang GF, Wang HZ, Yu ZM, Jin RC (2014) Floatation of flocculent and granular sludge in a high-loaded anammox reactor. Bioresour Technol 169:409–415. doi:10.1016/j.biortech.2014.06.063
Chen D, Szostak P, Wei Z, Xiao R (2016a) Reduction of orthophosphates loss in agricultural soil by nano calcium sulfate. Sci Total Environ 539:381–387. doi:10.1016/j.scitotenv.2015.09.028
Chen QQ, Sun FQ, Guo Q, Shen YY, Zhu WQ, Jin RC (2016b) Process stability in an anammox UASB reactor with individual and combined thiocyanate and hydraulic shocks. Sep Purif Technol 173:165–173. doi:10.1016/j.seppur.2016.09.005
Cirpus IEY, De Been M, Op den Camp HJM, Strous M, Le Paslier D, Kuenen GJ, Jetten SM (2005) A new soluble 10 kDa monoheme cytochrome c-552 from the anammox bacterium Candidatus “Kuenenia stuttgartiensis”. FEMS Microbiol Lett 252:273–278. doi:10.1016/j.femsle.2005.09.007
Cirpus IE, Geerts W, Hermans JH, Opden Camp HJ, Strous M, Kuenen JG, Jetten MS (2006) Challenging protein purification from anammox bacteria. Int J Biol Macromol 39:88–94. doi:10.1016/j.ijbiomac.2006.02.018
Dai HJ, Huang ZJ, Deng QH, Li Y, Xiao T, Ning XP, Lu Y, Yuan H (2015) The effects of lead exposure on serum uric acid and Hyperuricemia in Chinese adults: a cross-sectional study. Int J Environ Res Public Health 12:9672–9682. doi:10.3390/ijerph120809672
Dalsgaard T, Thamdrup B (2002) Factors controlling anaerobic ammonium oxidation with nitrite in marine sediments. Appl Environ Microbiol 68:3802–3808. doi:10.1128/AEM.68.8.3802-3808.2002
Dang HY, Chen RP, Wang L, Guo LZ, Chen PP, Tang ZW, Tian F, Li SZ, Klotz Martin G (2010) Environmental factors shape sediment Anammox bacterial communities in hypernutrified Jiaozhou Bay, China. Appl Environ Microbiol 76:7036–7047. doi:10.1128/AEM.01264-10
Dang HY, Zhou HX, Zhang ZN, Yu ZS, Hua E, Liu XS, Jiao NZ (2013) Molecular detection of Candidatus Scalindua pacifica and environmental responses of Sediment Anammox bacterial community in the Bohai Sea, China. PLoS One 8:e61330. doi:10.1371/journal.pone.0061330
Deng J, Gu YF, Zhang J, Xue K, Qin YJ, Yuan MT, Yi HQ, He ZL, Wu LY, Schuur EAG, Tiedje JM, Zhou JZ (2015) Shifts of tundra bacterial and Archaeal communities along a permafrost thaw gradient in Alaska. Mol Ecol 24:222–234. doi:10.1111/mec.13015
Di Iaconi C, Ramadori R, Lopez A, Passino R (2006) Influence of hydrodynamic shear force on properties of granular biomass in a sequencing batch biofilter reactor. J Biochem Eng 30:152–157. doi:10.1016/j.bej.2006.03.002
Franco A, Roca E, Lema JM (2006) Granulation in high-load denitrifying upflow sludge bed pulsed reactors. Water Res 40:871–880. doi:10.1016/j.watres.2005.11.044
Fuerst JA (2005) Intracellular compartmentation in Planctomycetes. Annu Rev Microbiol 59:299–328. doi:10.1146/annurev.micro.59.030804.121258
Gonzalez-Gil G, Sougrat R, Behzad AR, Lens PNL, Saikaly PE (2015) Microbial community composition and ultrastructure of granules from a full-scale Anammox reactor. Microb Ecol 70:118–131. doi:10.1007/s00248-014-0546-7
Hong YG, Li M, Cao HL, Gu JD (2011) Residence of habitat-specific Anammox bacteria in the deep-sea subsurface sediments of the South China Sea: analyses of marker gene abundance with physical chemical parameters. Microb Ecol 62:36–47. doi:10.1007/s00248-011-9849-0
Hori K, Matsumoto S (2010) Bacterial adhesion: from mechanism to control. Biochem Eng J 48(3):424–434. doi:10.1016/j.bej.2009.11.014
Hou XL, Liu ST, Zhang ZT (2015) Role of extracellular polymeric substance in determining the high aggregation ability of anammox sludge. Water Res 75:51–62. doi:10.1016/j.watres.2015.02.031
Hu BL, Zheng P, Tang CJ, Chen JW (2010) Identification and quantification of anammox bacteria in eight nitrogen removal reactors. Water Res 44:5014–5020. doi:10.1016/j.watres.2010.07.021
Hulshoff Pol LW, de Castro Lopes SI, Lettinga G, Lens PN (2004) Anaerobic sludge granulation. Water Res 38:1376–1389. doi:10.1016/j.watres.2003.12.002
Ismail SB, de La Parra CJ, Temmink H, van Lier JB (2010) Extracellular polymeric substances (EPS) in upflow anaerobic sludge blanket (UASB) reactors operated under high salinity conditions. Water Res 44:1909–1917. doi:10.1016/j.watres.2009.11.039
Jetten MSM, van Niftrik L, Strous M, Kartal B, Keltjens JT, Op den Camp HJ (2009) Biochemistry and molecular biology of anammox bacteria. Crit Rev Biochem Mol Biol 44:65–84. doi:10.1080/10409230902722783
Jiang K, Zhou KG, Yang YC, Du H (2013) A pilot-scale study of cryolite precipitation from high fluoride-containing wastewater in a reaction-separation integrated reactor. J Environ Sci 25:1331–1337. doi:10.1016/S1001-0742(12)60204-6
Jiang K, Zhou KG, Yang YC, Du H (2014) Growth kinetics of calcium fluoride at high super saturation in a fluidized bed reactor. Environ Technol 35:82–88. doi:10.1080/09593330.2013.811542
Jin RC, Zhang QQ, Liu JH, Yang BE, Wu K, Zheng P (2013) Performance and stability of the partial nitrification process for nitrogen removal from monosodium glutamate wastewater. Sep Purif Technol 103:195–202. doi:10.1016/j.seppur.2012.10.042
Kanakaraju D, Glass BD, Oelgemöller M (2014) Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ Chem Lett 12:27–47. doi:10.1007/s10311-013-0428-0
Kartal B, Rattray J, van Niftrik LA (2007) Candidatus “Anammoxoglobus propionicus” a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Syst Appl Microbiol 30:39–49. doi:10.1016/j.syapm.2006.03.004
Kartal B, van Niftrik L, Rattray J, de Vossenberg J, Schmid MC, Damste JS, Jetten MSM, Strous M (2008) Candidatus “Brocadia fulgida”: an autofluorescent anaerobic ammonium oxidizing bacterium. FEMS Microbiol Ecol 63:46–55. doi:10.1111/j.1574-6941.2007.00408.x
Kartal B, Kuenen JG, van Loosdrecht MCM (2010) Sewage treatment with Anammox. Science 328:702–703. doi:10.1126/science.1185941
Klotz MG, Schmid MC, Strous M, Op den Camp HJM, Jetten MSM, Hooper AB (2008) Evolution of an octahaem cytochrome c protein family that is key to aerobic and anaerobic ammonia oxidation by bacteria. Environ Microbiol 10:3150–3163. doi:10.1111/j.1462-2920.2008.01733.x
Kuenen JG, Jetten MSM (2001) Extraordinary anaerobic ammonium-oxidizing bacteria. ASM News 67:456–463
Kuenen JG, Robertson LA (1994) Combined nitrification–denitrification processes. FEMS Microbiol Rev 15:109–117. doi:10.1111/j.1574-6976.1994.tb00129.x
Kuypers MM, Sliekers AO, Lavik G, Schmid M, Jørgensen BB, Kuenen JG, Sinninghe Damsté JS, Strous M, Jetten MS (2003) Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature 422:608–611. doi:10.1038/nature01472
Lackner S, Gilbert EM, Vlaeminck SE, Joss A, Horn H, van Loosdrecht MCM (2014) Full-scale partial nitritation/anammox experiences: an application survey. Water Res 55:292–303. doi:10.1016/j.watres.2014.02.032
Li M, Gu JD (2016) The diversity and distribution of anammox bacteria in the marine aquaculture zones. Appl Microbiol Biotechnol 100:8943–8953. doi:10.1007/s00253-016-7690-6
Li H, Chen S, Mu BZ, Gu JD (2010) Molecular detection of anaerobic ammonium-oxidizing (Anammox) bacteria in high-temperature petroleum reservoirs. Microb Ecol 60:771–783. doi:10.1007/s00248-010-9733-3
Li XF, Barnes D, Chen J (2011) Performance of struvite precipitation during pretreatment of raw landfill leachate and its biological validation. Environ Chem Lett 9:71–75. doi:10.1007/s10311-009-0248-4
Li MM, Zhu JY, Gan M, Wang QF, Shi QJ, Chai LY (2014) Characteristics of chromium coprecipitation mediated by acidithiobacillus ferrooxidans DC. Water Air Soil Pollut 225:2071. doi:10.1007/s11270-014-2071-1
Li PJ, Xia JL, Yang S, Nie ZY, Su DL, Gao QR, Zhang C, Ma YL (2015) Optimizing production of pectinase from orange peel by penicilliumoxalicum PJ02 using response surface methodology. Waste Biomass Valoriz 6:13–22. doi:10.1007/s12649-014-9317-4
Lindsay MR, Webb RI, Strous M, Jetten MS, Butler MK, Forde RJ, Fuerst JA (2001) Cell compartmentalisation in planctomycetes: novel types of structural organisation for the bacterial cell. Arch Microbiol 175:413–429. doi:10.1007/s002030100280
Liu Y, Tay JH (2002) The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Res 36:1653–1665. doi:10.1016/S0043-1354(01)00379-7
Liu YQ, Liu Y, Tay JH (2004) The effects of extracellular polymeric substances on the formation and stability of biogranules. Appl Microbiol Biotechnol 65:143–148. doi:10.1007/s00253-004-1657-8
Liu ST, Yang FL, Gong Z, Meng FG, Chen HH, Xue Y, Furukawa K (2008) Application of anaerobic ammonium-oxidizing consortium to achieve completely autotrophic ammonium and sulfate removal. Bioresour Technol 99:6817–6825. doi:10.1016/j.biortech.2008.01.054
Liu XW, Sheng GP, Yu HQ (2009) Physicochemical characteristics of microbial granules. Biotechnol Adv 27:1061–1070. doi:10.1016/j.biotechadv.2009.05.020
Lu HF, Zheng P, Ji QX, Zhang HT, Ji JY, Wang L, Ding S, Chen TT, Zhang JQ, Tang CJ, Chen JW (2012) The structure, density and settlability of anammox granular sludge in high-rate reactors. Bioresour Technol 123:312–317. doi:10.1016/j.biortech.2012.07.003
Lu HF, Ji QX, Ding S, Zheng P (2013) The morphological and settling properties of ANAMMOX granular sludge in high-rate reactors. Bioresour Technol 143:592–597. doi:10.1016/j.biortech.2013.06.046
Ma YG, Hira D, Li ZG, Chen C, Furukawa K (2011) Nitrogen removal performance of a hybrid anammox reactor. Bioresour Technol 102:6650–6656. doi:10.1016/j.biortech.2011.03.081
Ma B, Wang SY, Cao SB, Miao Y, Jia F, Du R, Peng YZ (2016) Biological nitrogen removal from sewage via anammox. Bioresour Technol 200:981–990. doi:10.1016/j.biortech.2015.10.074
Maciej D (2000) Activities in nonpoint pollution control in rural areas of Poland. Ecol Eng 14:429––434. doi:10.1016/S0925-8574(99)00066-X
Martínez F, Lema J, Mendez R, Cuervo-Lopez F, Gomez J (2004) Role of exopolymeric protein on the settleability of nitrifying sludges. Bioresour Technol 94:43–48
Mizuto K, Okabe S (2014) Ecophysiology of anammox bacterium ‘Candidatus Scalindua japonica’. Master Thesis
Mu Y, Yu HQ (2006) Biological hydrogen production in a UASB reactor with granules. I: physicochemical characteristics of hydrogen-producing granules. Biotechnol Bioeng 94:980–987
Ni BJ, Chen YP, Liu SY, Fang F, Xie WM, Yu HQ (2009) Modeling a granule-based anaerobic ammonium oxidizing (ANAMMOX) process. Biotechnol Bioeng 103:490–499. doi:10.1002/bit.22279
Ni BJ, Hu BL, Fang F, Xie WM, Kartal B, Liu XW, Sheng GP, Jetten M, Zheng P, Yu HQ (2010) Microbial and physicochemical characteristics of compact anaerobic ammonium-oxidizing granules in an upflow anaerobic sludge blanket reactor. Appl Environ Microbiol 76:2652––2656. doi:10.1128/AEM.02271-09
Ni SQ, Sun N, Yang HL, Zhang J, Ngo HH (2015) Distribution of extracellular polymeric substances in anammox granules and their important roles during anammox granulation. Biochem Eng J 101:126–133. doi:10.1016/j.bej.2015.05.014
Peng C, Chai LY, Tang CJ, Min XB, Ali M, Song YX, Qi WM (2017a) Feasibility and enhancement of copper and ammonia removal from wastewater using struvite formation: a comparative research. J Chem Technol Biotechnol 92:325–333. doi:10.1002/jctb.5009
Peng C, Chai LY, Tang CJ, Min XB, Duan CS, Yu C (2017b) Study on the mechanism of copper–ammonia complex decomposition in struvite formation process and enhanced ammonia and copper removal. J Environ Sci. doi:10.1016/j.jes.2016.06.020
Qiao S, Yamamoto T, Misaka M (2010) High-rate nitrogen removal from livestock manure digester liquor by combined partial nitritation-anammox process. Biodegradation 21:11–20. doi:10.1007/s10532-009-9277-8
Quan ZX, Rhee SK, Zuo JE, Bae JW, Park JR, Lee ST, Park JH (2008) Diversity of ammonium-oxidizing bacteria in a granular sludge anaerobic ammonium-oxidizing (anammox) reactor. Environ Microbiol 10:3130–3139. doi:10.1111/j.1462-2920.2008.01642.x
Schalk J, De VS, Kuenen JG, Jetten MS (2000) Involvement of a novel hydroxylamine oxidoreductase in anaerobic ammonium oxidation. Biochemistry 39:5405–5412. doi:10.1021/bi992721k
Schluter J, Nadell Carey D, Bassler BL, Foster KR (2015) Adhesion as a weapon in microbial competition. ISME J 9:139–149. doi:10.1038/ismej.2014.174
Schmid M, Twachtmann U, Klein M, Strous M, Juretschko S, Jetten M, Metzger JW, Schleifer KH, Wagner M (2000) Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst Appl Microbiol 23:93–106. doi:10.1016/S0723-2020(00)80050-8
Schmid M, Walsh K, Webb R, Rijpstra WI, van de Pas-Schoonen K, Verbruggen MJ, Hill T, Moffett B, Fuerst J, Schouten S, Damsté JS, Harris J, Shaw P, Jetten M, Strous M (2003) Candidatus “Scalindua brodae”, sp. Nov., Candidatus “Scalindua wagneri”, sp. Nov., two new species of anaerobic ammonium oxidizing bacteria. Syst Appl Microbiol 26:529–538. doi:10.1078/072320203770865837
Schmid MC, Hooper AB, Klotz MG, Woebken D, Lam P, Kuypers MMM, Pommerening RA, op den Camp HJM, Jetten MSM (2008) Environmental detection of octahaem cytochrome c hydroxylamine/hydrazine oxidoreductase genes of aerobic and anaerobic ammonium-oxidizing bacteria. Environ Microbiol 10:3140–3149. doi:10.1111/j.1462-2920.2008.01732.x
Shen LD, Hu AH, Jin RC, Cheng DQ, Zheng P, Xu XY, Hu BL (2012) Enrichment of anammox bacteria from three sludge sources for the startup of monosodium glutamate industrial wastewater treatment system. J Hazard Mater 199–200:193–199. doi:10.1016/j.jhazmat.2011.10.081
Shimamura M, Nishiyama T, Shigetomo H (2007) Isolation of a multiheme protein with features of a hydrazine-oxidizing enzyme from an anaerobic ammonium- oxidizing enrichment culture. Appl Environ Microbiol 73:1065–1072. doi:10.1128/AEM.01978-06
Shimamura M, Nishiyama T, Shinya K, Kawahara Y, Furukawa K, Fujii T (2008) Another multiheme protein, hydroxylamine oxidoreductase abundantly produced in an anammox bacterium besides the hydrazine-oxidizing enzyme. J Biosci Bioeng 105:243–248. doi:10.1263/jbb.105.432
Sinninghe Damste JS, Strous M, Rijpstra WIC, Hopmans EC, Geenevasen JAJ, Van Duin ACT, Van Niftrik LA, Jetten MSM (2002) Linearly concatenated cyclobutane lipids form adense bacterial membrane. Nature 419:708–712. doi:10.1038/nature01128
Song YX, Liao Q, Chai LY, Yu C, Duan CS, Peng C, Tang CJ (2017) Physicochemical and microbial properties of settled and floated anammox granules in upflow reactor. Biochem Eng J. Accepted
Speth DR, Guerrero-Cruz S, Dutilh BE, Jetten MSM (2016) Genome-based microbial ecology of anammox granules in a full-scale wastewater treatment system. Nature Comm 7:11172. doi:10.1038/ncomms11172
Strous M, Van Gerven E, Kuenen JG, Jetten M (1997) Effects of aerobic and microaerobic conditions on anaerobic ammonium-oxidizing (Anammox) sludge. App Environ Microbiol 63:2446–2448.
Strous M, Heijnen JJ, Kuenen JG, Jetten MSM (1998) The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl Microbiol Biotechnol 50:589–596. doi:10.1007/s002530051340
Strous M, Pelletier E, Mangenot S et al (2006) Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440:790–794. doi:10.1038/nature04647
Sultan M (2016) Polyurethane for removal of organic dyes from textile wastewater. Environ Chem Lett. doi:10.1007/s10311-016-0597-8
Tang CJ, Zheng P, Zhang L (2008) Seeding sludge and start-up strategy for Anammox bioreactor. China Water Wastewater 24:15–20 (in Chinese)
Tang CJ, Zheng P, Mohmmod Q, Chen JW (2009a) Start-up and inhibition analysis of the Anammox process seeded with anaerobic granular sludge. J Ind Microbiol Biotechnol 36:1093–1100. doi:10.1007/s10295-009-0593-0
Tang CJ, Zheng P, Qaisar M (2009b) The shear force amendments on the slugging behavior of upflow Anammox granular sludge bed reactor. Sep Purif Technol 69:262–268. doi:10.1016/j.seppur.2009.07.029
Tang CJ, Zheng P, Hu BL, Chen JW, Wang CH (2010a) Influence of substrates on nitrogen removal performance and microbiology of anaerobic ammonium oxidation by operating two UASB reactors fed with different substrate levels. J Hazard Mater 181:19–26. doi:10.1016/j.jhazmat.2010.04.015
Tang CJ, Zheng P, Wang CH, Mohmmod Q (2010b) Suppression of anaerobic ammonium-oxidizers under high organic content in high-rate Anammox UASB reactor. Bioresour Technol 101:1762–1768. doi:10.1016/j.biortech.2009.10.032
Tang CJ, Zheng P, Zhang L, Chen JW, Mohmmod Q, Chen XG, Hu BL, Wang CH, Yu Y (2010c) Enrichment features of anammox consortia from methanogenic granules loaded with high organic and methanol content. Chemosphere 79:613–619. doi:10.1016/j.chemosphere.2010.02.045
Tang CJ, Zheng P, Chen TT, Qaisar M, Zhang JQ, Ding S, Chen XG, Chen JW, Wu DT (2011a) Enhanced nitrogen removal from pharmaceutical wastewater using SBA-ANAMMOX process. Water Res 45:201–210. doi:10.1016/j.watres.2010.08.036
Tang CJ, Zheng P, Wang CH, Mohmmod Q, Zhang JQ, Chen XG, Zhang L, Chen JW (2011b) Performance of high-loaded ANAMMOX UASB reactors containing granular sludge. Water Res 45:135–144. doi:10.1016/j.watres.2010.08.018
Tang CJ, He R, Zheng P, Chai LY, Min XB (2013a) Mathematical modeling of high-rate Anammox UASB reactor based on granular packing patterns. J Hazard Mater 250–251:1–8. doi:10.1016/j.jhazmat.2013.01.058
Tang CJ, Zheng P, Chai LY, Min XB (2013b) Characterization and quantification of anammox start-up in UASB reactors seeded with conventional activated sludge. Int Biodeterior Biodegr 82:141–148. doi:10.1016/j.ibiod.2013.02.014
Tang CJ, Zheng P, Chai LY, Min XB (2013c) Thermodynamic and kinetic investigation of anaerobic bioprocesses on ANAMMOX under high organic conditions. Chem Eng J 230:149–157. doi:10.1016/j.cej.2013.06.047
Tang CJ, Zheng P, Ding S, Lu HF (2014) Enhanced nitrogen removal from ammonium-rich wastewater containing high organic contents by coupling with novel high-rate ANAMMOX granules addition. Chem Eng J 240:454–461. doi:10.1016/j.cej.2013.11.052
Tappin AD, Loughnane JP, McCarthy AJ, Fitzsimons MF (2016) Unexpected removal of the most neutral cationic pharmaceutical in river waters. Environ Chem Lett 14:455. doi:10.1007/s10311-016-0582-2
Trigo C, Campos JL, Garrido JM, Mendez R (2006) Start-up of the Anammox process in a membrane bioreactor. J Biotechnol 126:475–487. doi:10.1016/j.jbiotec.2006.05.008
Trubetskaya OE, Richard C, Trubetskoj OA (2016) High amounts of free aromatic amino acids in the protein-like fluorescence of water-dissolved organic matter. Environ Chem Lett 14:495–500. doi:10.1007/s10311-016-0556-4
Tsuneda S, Nagano T, Hoshino T (2003) Characterization of nitrifying granules produced in an aerobic upflow fluidized bed reactor. Water Res 37:4965–4973. doi:10.1016/j.watres.2003.08.017
Tsushima I, Ogasawara Y, Kindaichi T, Okabe S (2007) Development of high-rate anaerobic ammonium-oxidizing (Anammox) biofilm reactors. Water Res 41:1623–1634. doi:10.1016/j.watres.2007.01.050
Van De Vossenberg J, Woebken D, Maalcke WJ, Wessels HJ, Dutilh BE, Kartal B, Janssen-Megens EM, Roeselers G, Yan J, Speth D, Gloerich J, Geerts W, Van Der Biezen E, Pluk W, Francoijs KJ, Russ L, Lam P, Malfatti SA, Tringe SG, Haaijer SC, Op den Camp HJ, Stunnenberg HG, Amann R, Kuypers MM, Jetten MS (2013) The metagenome of the marine anammox bacterium ‘Candidatus Scalindua profunda’ illustrates the versatility of this globally important nitrogen cycle bacterium. Environ Microbiol 15:1275–1289. doi:10.1111/j.1462-2920.2012.02774.x
Van der Star WRL, Abma WR, Bolmmers D, Mulder JW, Tokutomi T, Strous M, Picioreanu C, van Loosdrecht MCM (2007) Startup of reactors for anoxic ammonium oxidation: experiences from the first full-scale anammox reactor in Rotterdam. Water Res 41:4149–4163. doi:10.1016/j.watres.2009.09.051
Van Loosdrecht MCM, Jetten M (1998) Microbiological conversions in nitrogen removal. Water Sci Technol 38:1–7. doi:10.1016/S0273-1223(98)00383-7
Van Niftrik L, Van Helden M, Kirchen S, van Donselaar EG, Harhangi HR, Webb RI, Fuerst JA, Op den Camp HJ, Jetten MS, Strous M (2010) Intracellular localization of membrane-bound ATPases in the compartmentalized anammox bacterium ‘Candidatus Kuenenia stuttgartiensis’. Molecul Microbiol 77:701–715. doi:10.1111/j.1365-2958.2010.07242.x
Vazquez-Padin J, Mosquera-Corral A, Campos JL, Mendez R, Revsbech NP (2010) Microbial community distribution and activity dynamics of granular biomass in a CANON reactor. Water Res 44:4359–4370. doi:10.1016/j.watres.2010.05.041
Vlaeminck S, Terada A, Smets BF, De Clippeleir H, Schaubroeck T, Bolca S, Demeestere L, Mast J, Boon N, Carballa M, Verstraete W (2010) Aggregate size and architecture determine microbial activity balance for one-stage partial nitritation and Anammox. Appl Environ Microbiol 76:900–909. doi:10.1128/AEM.02337-09
Wang C, Lee P, Kumar M, Huang YT, Sung S, Lin JG (2010a) Simultaneous partial nitrification, anaerobic ammonium oxidation and dentrification (SNAD) in a full-scale landfill leachate treatment plant. J Hazard Mater 175:622–628. doi:10.1016/j.jhazmat.2009.10.052
Wang ZX, Chai LY, Yang ZH, Wang YY, Wang HY (2010b) Identifying sources and assessing potential risk of heavy metals in soils from direct exposure to children in a mine-impacted city, Changsha, China. J Environ Qual 39:1616–1623. doi:10.2134/jeq2010.0007
Wang QW, Qin WQ, Chai LY, Li QZ (2014) Understanding the formation of colloidal mercury in acidic wastewater with high concentration of chloride ions by electrocapillary curves. Environ Sci Pollut Res 21:3866–3872. doi:10.1007/s11356-013-2379-1
Wang SL, Hong YG, Wu JP, Xu XR, Bin LY, Pan YP, Guan FJ, Wen JL (2015a) Comparative analysis of two 16S rRNA gene-based PCR primer sets provides insight into the diversity distribution patterns of anammox bacteria in different environments. Appl Microbiol Biotechnol 99:8163–8176. doi:10.1007/s00253-015-6814-8
Wang T, Zhang LY, Li CF, Yang WC, Song TT, Tang CJ, Meng Y, Dai S, Wang HY, Chai LY, Luo J (2015b) Synthesis of core-shell magnetic Fe3O4@poly(m-Phenylenediamine) particles for chromium reduction and adsorption. Environ Sci Technol 49(9):5654–5662. doi:10.1021/es5061275
Wang SH, Guo JB, Lian J, Ngo HH, Guo WS, Liu YM, Song YY (2016) Rapid start-up of the anammox process by denitrifying granular sludge and the mechanism of the anammox electron transport chain. Biochem Eng J 115:101–107. doi:10.1016/j.bej.2016.09.001
Wei Z, Spinney R, Ke R, Yang Z, Xiao R (2016) Effect of pH on the sonochemical degradation of organic pollutants. Environ Chem Lett 14(2):163–182. doi:10.1007/s10311-016-0557-3
Wett B (2006) Solved upscaling problems for implementing deammonification of rejection water. Water Sci Technol 53:121–128. doi:10.2166/wst.2006.413
Wiszniowski J, Robert D, Surmacz-Gorska J, Miksch K, Weber JV (2006) Landfill leachate treatment methods: a review. Environ Chem Lett 4:51–61. doi:10.1007/s10311-005-0016-z
Woebken D, Lam P, Kuypers MM, Naqvi SW, Kartal B, Strous M, Jetten MS, Fuchs BM, Amann R (2008) A microdiversity study of anammox bacteria reveals a novel Candidatus Scalindua phylotype in marine oxygen minimum zones. Environ Microbiol 10:3106–3119. doi:10.1111/j.1462-2920.2008.01640.x
Wu J, Zhou HM, Li HZ, Zhang PC, Jiang J (2009) Impacts of hydrodynamic shear force on nucleation of flocculent sludge in anaerobic reactor. Water Res 43:3029–3036
Wu Z, Hua Z, Yuan XZ, Wang H, Wang LL, Chen XH, Zeng GM, Wu W (2014) Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res 67:330–344. doi:10.1016/j.watres.2014.09.026
Wu YD, Zhou KG, Dong SY, Yu W, Zhang HQ (2015) Recovery of gallic acid from gallic acid processing wastewater. Environ Technol 36:661–666. doi:10.1080/09593330.2014.957246
Xiang KS, Liu H, Yang BT, Zhang C, Yang S, Liu ZL, Liu C, Xie XF, Chai LY, Min XB (2016) Selenium catalyzed Fe(III)-EDTA reduction by Na2SO3: a reaction-controlled phase transfer catalysis. Environ Sci Pollut Res 8:8113–8119. doi:10.1007/s11356-016-6267-3
Xiao R, Wei Z, Chen D, Weavers LK (2014) Kinetics and mechanism of sonochemical degradation of pharmaceuticals in municipal wastewater. Environ Sci Technol 48:9675–9683. doi:10.1021/es5016197
Xiao R, Arnot JR, MacLeod M (2015a) Towards an improved understanding of processes controlling absorption efficiency and biomagnification of organic chemicals by fish. Chemosphere 138:89–95. doi:10.1016/j.chemosphere.2015.05.053
Xiao R, Ye T, Wei Z, Luo S, Yang Z, Spinney R (2015b) Quantitative structure-activity relationship (QSAR) for the oxidation of trace organic contaminants by sulfate radical. Environ Sci Technol 49:13394–13402. doi:10.1021/acs.est.5b03078
Xiao R, Zammit I, Wei Z, MacLeod M, Spinney R (2015c) Kinetics and mechanism of the oxidation of cyclosiloxanes by hydroxyl radical in the gas phase: an experimental and theoretical study. Environ Sci Technol 49:13322–13330. doi:10.1021/acs.est.5b03744
Xie WM, Ni BJ, Seviour T, Yu HQ (2013) Evaluating the impact of operational parameters on the formation of soluble microbial products (SMP) by activated sludge. Water Res 47:1073–1079. doi:10.1016/j.watres.2012.11.022
Xing BS, Guo Q, Yang GF, Zhang ZZ, Li P, Guo LX, Jin RC (2015) The properties of anaerobic ammonium oxidation (anammox) granules: roles of ambient temperature, salinity and calcium concentration. Sep Purif Technol 147:311–318. doi:10.1016/j.seppur.2015.04.035
Xiong L, Wang YY, Tang CJ, Chai LY, Xu KQ, Song YX, Ali M, Zheng P (2013) Start-up characteristics of a granule-based Anammox UASB reactor seeded with anaerobic granular sludge. BioMed Res Int. doi:10.1155/2013/396487
Xue S, Ma Y, Zhou X, Liu F (2011) Screening and biological characteristics of a manganese tolerant microorganism. Environ Eng Manag J 10:881–885
Yan X, Li QZ, Chai LY, Yang BT, Wang QW (2014) Formation of abiological granular sludge – A facile and bioinspired proposal for improving sludge settling performance during heavy metal wastewater treatment. Chemosphere 113:36–41. doi:10.1016/j.chemosphere.2014.04.038
Yan X, Chai L, Li Q, Ye L, Yang B, Wang Q (2017) Abiological Granular Sludge Formation Benefit for Heavy Metal Wastewater Treatment Using Sulfide Precipitation. CLEAN-Soil, Air, Water, doi:10.1002/clen.201500730
Yang QX, Jia ZJ, Liu RY, Chen JJ (2007) Molecular diversity and anammox activity of novel planctomycete-like bacteria in the wastewater treatment system of a full-scale alcohol manufacturing plant. Process Biochem 42:180–187. doi:10.1016/j.procbio.2006.07.032
Yang J, Chai LY, Wang YY, He XW, Wang JL (2010) Transportation and distribution of chromium in the anaerobic sludge treating the chromium-containing wastewater. Int J Environ Pollut 38:256–266. doi:10.1504/IJEP.2009.027226
Yang Y, Liu H, Xiang XH (2013) Outline of occupational chromium poisoning in China. Bull Environ Contam Toxicol 90:742–749. doi:10.1007/s00128-013-0998-3
Yin L, Li X, Liu Y, Zhang D, Zhang S, Luo X (2012) Biodegradation of cypermethrin by rhodopseudomonas palustris GJ-22 isolated from activated sludge. Fresen Environ Bull 21:397–405
Yu C, Song YX, Chai LY, Duan CS, Tang CJ, Ali M, Peng C (2016) Comparative evaluation of short-term stress of Cd(II), Hg(II), Pb(II), As(III) and Cr(VI) on anammox granules by batch test. J Biosci Bioeng 122:722–729. doi:10.1016/j.jbiosc.2016.06.008
Yuan SJ, Sun M, Sheng GP, Li Y, Li WW, Yao RS, Yu HQ (2010) Identification of key constituents and structure of the extracellular polymeric substances excreted by Bacillus megaterium TF10 for their flocculation capacity. Environ Sci Technol 145:1152–1157. doi:10.1021/es1030905
Zhang Y, Xie JP, Liu MM, Tian Z, He ZL, Joy D, Nostrand Van, Ren LR, Zhou JZ, Yang M (2013) Microbial community functional structure in response to antibiotics in pharmaceutical wastewater treatment systems. Water Res 47:6298–6308. doi:10.1016/j.watres.2013.08.003
Zhang L, Zhang SJ, Peng YZ, Han XY, Gan YP (2015) Nitrogen removal performance and microbial distribution in pilot- and full-scale integrated fixed-biofilm activated sludge reactors based on nitritation-anammox process. Bioresour Technol 196:448–453. doi:10.1016/j.biortech.2015.07.090
Zhang ZZ, Xu JJ, Hu HY, Shi ZJ, Ji ZQ, Deng R, Shi ML, Jin RC (2016) Insight into the short- and long-term effects of inorganic phosphate on anammox granule property. Bioresour Technol 208:161–169. doi:10.1016/j.biortech.2016.02.097
Zheng YM, Yu HQ (2007) Determination of the pore size distribution and porosity of aerobic granules using size-exclusion chromatography. Water Res 41:39–46
Acknowledgements
Financial support of this work by the National Natural Science Foundations of China (51674305, 51204213) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tang, CJ., Duan, CS., Yu, C. et al. Removal of nitrogen from wastewaters by anaerobic ammonium oxidation (ANAMMOX) using granules in upflow reactors. Environ Chem Lett 15, 311–328 (2017). https://doi.org/10.1007/s10311-017-0607-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-017-0607-5