Abstract
The deammonification process represents one of the most convenient pathways for nitrogen removal from wastewater. A great deal of scientific articles dwells on the treatment of sidestream fluxes, whereas applications to mainstream waters represent a novel field. Among the general challenges of deammonification, one of the most important is the effective selection of ammonia oxidizers (AOB) over nitrite oxidizers (NOB), but also the typical slow start-up periods. In addition to such issues, mainstream deammonification has to face water temperatures and alkalinity reserves lower than those of sidestream fluxes and higher content of organic matter. An attempt was made to tackle such challenges by employing a lab-scale plant; low dissolved oxygen (DO) values (average 0.78 mg/L) and granular AOB-enriched biomass were used in order to address exclusion of nitrite oxidizers. The granules also allowed better biomass retention. The hydraulic retention time (HRT) was established initially at 24 h and later decreased to 12 h, as to possibly enhance the performance of the reactor. After 52 days of operation, Anammox biomass was also inoculated to the reactor. The results showed a maximum nitrogen removal efficiency of 54%. Moreover, little quantities of nitrates were observed throughout the experiment (<5 mg N/L twice, under the limit of quantification the rest of the sampling days), meaning that NOB out-selection techniques worked properly. Retention of biomass was also positively addressed and yielded a final SRT value of 15.6 days. Therefore, the proposed solution for mainstream deammonification was demonstrated to be promising and more research would be necessary to optimize it.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
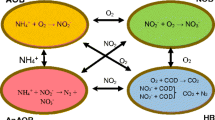
Deammonification techniques represent some of the most interesting solutions in the field of wastewater nitrogen removal: compared to traditional nitrification and denitrification, they allow for about 60% reduction in aeration-related costs, while no organic carbon donor is needed. Also, sludge handling and transportation costs are cut down by 90%, and the emission of N2O is reduced (Abbas et al. 2014). This technology is made up of two steps, occurring in the same system: partial nitrification takes place first, and consists of conversion of ammonia to nitrites, and then the Anammox biomass employs the nitrites as an electron acceptor for reduction of ammonium ions to gaseous N2, which easily escapes the reaction environment by contact with the atmosphere.
Most of the available research on deammonification technologies deals with high N-strength waters, as in the case of sidestream fluxes of municipal wastewater treatment plants (WWTPs) (Lackner et al. 2014); on the contrary, the implementation of such process to the mainstream lines of municipal WWTPs, notably characterized by lower temperatures, alkalinity reserves, and N content, is still in its infancy (Frijns et al. 2013). This is mainly related to a series of challenges that arise in these cases: first of all, the typically high ratios between organic matter (or equivalently, chemical oxygen demand; COD) and nitrogen content, referred to as COD/N ratios, lead to inhibition of the Anammox biomass (Jin et al. 2012) and subsequent out competition by denitrifiers (Lackner et al. 2008). Secondly, Anammox bacteria are hard to cultivate and retain due to their slow maximum growth rate and large doubling time; this is especially relevant since the N content of mainstream fluxes is low, and the lack of substrate can further impair the bacterial growth process. As a guideline, Table 1 reports the results of some experiments with mainstream wastewater in terms of sludge retention time (SRT). Table 2, instead, offers a brief overview of some available results in scientific literature, with regard to start-up and first Anammox activity detection times for a number of different plant configurations operated close to the optimum temperature. Another problem can be represented by the flow temperature, which can turn out to be too low, thus entailing considerable heating costs. As for alkalinity, this can represent an obstacle for the partial nitrification reaction, as an efficient buffer system is required to contrast abrupt pH changes. Other typical challenges of deammonification include difficulties in effectively retaining ammonia-oxidizing bacteria (AOB) over nitrite-oxidizing bacteria (NOB); this is particularly important as it allows for the process to stop at the step of nitrites, avoiding further oxidation to nitrates, which would result in unavailable substrate for the Anammox bacteria. The typical low N content of mainstream waters represents a further obstacle to the effective exclusion of NOB, since the substrate for AOB is limited. Finally, biodegradable organic matter can also represent a problem for partial nitrification, as investigated by Mosquera-Corral et al. (2005), who found that COD/N ratios bigger than 0.3 result in detrimental effects for AOB, due to a competition between autotrophic and heterotrophic bacterial groups; moreover, as mentioned above, Anammox bacteria are inhibited by excessive concentration of organic matter.
The experiment presented in this article attempted to address some of these hurdles; more specifically, keeping low dissolved oxygen (DO) levels was regarded as a means to favor AOB over NOB. The employment of already AOB-enriched granular biomass, on the other side, tentatively provided an answer to the normally long start-up times for deammonification, also trying to achieve satisfactory biomass retention values. As a term of comparison, Table 3 includes some relevant examples of nitrogen removal rates and efficiency values for several nitrogen removal plant configurations treating mainstream wastewater.
One of the most interesting solutions that have been recently put forward in the field of municipal wastewater treatment is the possibility of converting most of the biodegradable organic matter arriving to the plant into energy (i.e., biogas), because that biodegradable COD is not anymore necessary to denitrify. This is possible by virtue of the autotrophic nature of nitrifying and Anammox organisms; also, it exploits the fact that organic matter has to be removed in order to obtain lower COD/N ratios and avoid competition between heterotroph denitrifiers and Anammox bacteria. Since the reactor sections can operate independently, no more complex trade-offs between N and COD contents are required. Jetten et al. (1997) put forward a solution involving primary coagulation of colloidal parts and COD, only requiring the use of some flocculant agents, in spite of aeration; besides, this step can provide efficient phosphorus removal. Estimations show that up to 0.5 kg of CH4 can be produced per unit kilo of COD sludge; apart from this major advantage, minimal energy is required, and amounts to 0.2 kg instead of 0.6 kg of O2 per kilo of COD (Jetten et al. 1997). After this step, two streams are identified as follows: the sludge treatment effluent, consisting of a warm concentrated flux, and the wastewater itself, featuring colder temperatures and diluted ionic concentrations. The authors identified the deammonification technique as a suitable solution for further treatments of the effluents, only requiring 1.7 kg of O2 per kilogram of NH4 +-N removed, and no external carbon source; on the other hand, traditional nitrification and denitrification requires 4.6 kg of O2 and 4.5 kg of COD per kilogram of NH4 +-N (Jetten et al. 1997). In their research, Xu et al. (2015) consider 0.5 as an upper limit for COD/N ratios; once again, the aim is to avoid competition between Anammox and denitrifying bacteria. In this case, the technical possibilities for organic matter removal are represented by chemically enhanced primary treatment (CEPT) or high-rate activated sludge (HRAS). Later on, the removed and concentrated COD is sent to an anaerobic digester (AD) for production of biogas. Finally, Gao et al. (2014) investigated the possibility of carrying out direct AD treatment at lower temperatures. As for the AD step, in spite of a simpler configuration of the system, and regardless of the fact that lower temperature values corresponded to higher fractions of COD being converted to soluble, unavailable methane rather than gaseous methane, no significant variations in the percentage of total produced methane were observed. The conditions in the partial nitrification reactor were not optimal for growth rate control, since DO values were in between 2 and 7 mg/L, and the influent NH4 +-N was lower than 60 mg/L, thus giving FA concentrations lower than 1 mg/L at a pH level of 7.5. Temperature management was not a viable solution either, since the commonly accepted threshold for NOB exclusion is set at 25 °C (van Hulle et al. 2010). Therefore, the applied strategy consisted in defining different aeration patterns, depending on the inlet soluble COD, temperature, and content in NH4 +-N. Generally speaking, higher NH4 +-N values or lower temperatures corresponded to longer aeration times, accordingly to the Arrhenius’s equation. Major attention had to be paid to the COD removal efficiency of the reactor, since elevated COD/N ratios can turn out to be inhibitory for the Anammox activity in the last step. As a consequence, longer aeration times might be required to contrast this competition, entailing higher costs. Despite the maintenance of the Anammox reactor at a temperature of about 12–15 °C, they reported a relatively high N removal rate, with daily values of 0.83 kg/m3. The same authors refer that although related studies have reported successful results for higher loading rates and at modestly low temperatures, the majority of them focused on high strength, and mainly synthetic wastewater. Instead, their results prove that Anammox bacteria can grow under low temperatures and low nitrogen loads, as in the case of municipal mainstream fluxes. Some relevant operational variables in the context of deammonification plants are reported hereafter, along with a brief explanation and suggested value ranges.
1.1 Temperature
The importance of such parameter lies in its ability to influence the reaction environment and bacterial growth rates. As for AOB, their optimal range is included in between 25 and 35 °C (Guo et al. 2010); while operation at higher temperatures is seldom carried out, mainly because of increased operational costs, values lower than 5 °C normally correspond to complete inhibition of bacteria. On the other hand, the optimum range for Anammox activity is reported to be in between 30 and 40 °C; temperatures higher than 45 °C trigger irreversible reduction of the bacterial activity (Dosta et al. 2008). The same authors suggest that a decrease in temperature can lead to progressively lower nitrogen removal rates until a value of 15 °C is met, at which nitrites start to accumulate conducting to an ultimate loss of process stability. Considering that in their natural environment Anammox bacteria operate within a quite wide temperature range, with measured activity at −2 °C (Gao et al. 2014), low-temperature operation is probably possible through proper acclimation. The main drawbacks to this possibility are the reduced removal rates, along with the increased concentrations of free NH3. Maintenance of high temperatures, specifically above 25 °C, can be an effective technique for NOB out selection, as in such conditions their growth rate is lower than that of AOB. For a temperature of 35 °C, the maximum growth rate of NOB is only half of that of AOB, resulting in values of 0.5 and 1 d−1, respectively (Khin and Annachatre 2004). However, this technique is not possible at mainstream conditions with wastewaters usually significantly colder than 35 °C. Recently, Isanta et al. (2015) have reported that the combined control of DO and ammonium concentration in the reaction medium can be used as an alternative to keep stable partial nitritation when the temperature of the wastewater is relatively low.
1.2 DO Values
The operational choice of setting low DO levels may seem counterproductive at first, since Anammox bacteria are strictly anaerobic. Reversible inhibition effects have been observed at values as low as 0.5 % of the air saturation level (Strous et al. 1997); other authors, like Egli et al. (2001), report similar threshold values, specifically 1%. As for irreversible inhibition, this was observed at levels of at least 18% (Jin et al. 2012). In fact, setting low oxygen levels works as a technique for NOB out selection because of the lower oxygen affinity of NOB compared to AOB; this is also the one of the key techniques for the whole experiment. Besides, it has the advantage of cutting down aeration-related expenses, which are among the main concerns whenever dealing with nitrogen removal techniques. Values lower than 0.5 mg/L have been shown beneficial in repressing NOB and have successfully been applied to waters both at low and high ammonium content (Fernández et al. 2012; Blackburne et al. 2008; Ruiz et al. 2003). Higher values, around 1.5 mg/L, still showed good results in the treatment of mainstream wastewater, as reported by Cao et al. (2013). Employing granular Anammox biomass for the experiment is intended as a positive enhancement on the process, as the structure itself of the granules isolates bacteria from oxygen, preventing further inhibition.
1.3 pH
A proper control of pH value is necessary for the process: first of all, it should ensure that a proper reaction environment is provided; secondly, appropriately intermediate ranges avoid the production of toxic compounds generated by nitrifiers, such as HNO2, which would be maximum at pH values lower than 6.5 (Kampschreur et al. 2009), and NH3, which typically appears for values above 8 (Anthonisen et al. 1976). Since the deammonification process unifies two different steps in the same reaction environment, a quite narrow variability range has to be set in order for the process to be successful. While Nitrosomonas have an optimum between 7.2 and 8, the desired range for Anammox bacteria has been identified in between 6.7 and 8 (Strous et al. 1999). High pH values, specifically around 7.5 and 8, are efficient in promoting out selection of NOB; in fact, at such values, the concentration of NH3 increases while that of HNO2 decreases, ultimately supporting the activity of AOB over NOB. However, as it was mentioned, such relatively high pH might contribute to nitrous oxides and ammonia gas emissions.
2 Materials and Methods
2.1 Reactor and Biomass
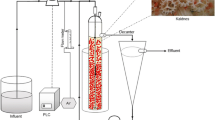
The experiment lasted for about 4 months. The employed reactor consisted of a SBR system; the main reaction environment was a semi-open Pyrex glass hollow container, shaped like a cylinder and with a rounded, semi-spherical bottom. The total volume was 1.7 L, whereas the amount of liquid retained in the system at every cycle was 0.9 L; this means that 0.8 L were expelled from the reactor at every step, giving a volume exchange ratio close to 50% per cycle. The feeding and the outflow system were devised using rubber pipes of about 5 mm in diameter and peristaltic pumps, allowing the flow to run as smooth as possible. The biomass for the partial nitrification step was highly AOB-enriched granular sludge obtained from a previous Ph.D. thesis work, conducted by López (2012), on a lab-scale SBR plant. As for the Anammox biomass, this was obtained from a full-scale plant located in the Netherlands. The first inoculation of nitrifying granules took place on day 24, and accounted for a volume of 200 mL; new biomass was added on day 52, in the form of 100 mL of nitrifying granules and 200 mL of Anammox sludge.
Aeration of the system was provided by means of an air pump, connected to a submerged stone diffuser, ensuring isotropic conditions for DO levels and efficient mixing of the reaction liquid. A second inlet tube provided gaseous N2 to the system and helped maintain a steady and appropriate volume percentage in DO. The reactor was started with a planned hydraulic retention time (HRT) of 24 h and a cycle time of 12 h, resulting in two cycles per day. The HRT was then decreased to 12 h on day 78, as to possibly enhance the reactor activity and study its performance under more stressful conditions. Aeration and feeding were continuous during the reaction time, which lasted for the most part of the cycle except the last 30 min, corresponding to settling and effluent withdrawal (15 min each). Temperature control was addressed by means of water at about 15 °C flowing through a rubber pipe spiraling around the outer side of the reactor.
The synthetic feeding solution was periodically prepared in the laboratory, with the aim of efficiently simulating the chemical composition of pretreated municipal mainstream wastewater. Since this work was focused on the effects of moderate temperatures and low nitrogen loading rates, no biodegradable organic matter was added to the feeding. More specifically, guideline concentration values were obtained from available technical literature (Table 4, Mosquera-Corral et al. 2005). The concentration of ammonium was lowered to adapt to the expected in mainstream conditions. Besides, slight changes in the concentrations of other components served as a means to actively manipulate the process. More specifically, NaHCO3 was sometimes added to the solution to contrast excessive changes in alkalinity or to regulate too low pH values.
Effective control of the reaction environment relied on the monitoring of some key parameters; this also ensured optimal conditions for the employed bacteria. Statistical analysis of the same data consisted of mean, maximum, and minimum values identification; these retrievals were useful for a comparison with literature data referring to similar reactor configurations.
2.2 Operational Conditions Analyses
Temperature was monitored because of its influence on the reaction advancement and on bacterial growth rates; levels were recorded daily, and the aim was to keep a value roughly in between 20 and 25 °C. The online measuring tool employed for temperature measurement also served as a means to get information on the pH levels; more specifically, a Crison pH 26 was chosen. DO levels were measured continuously and registered daily, always at the same time of cycle, both in the form of oxygen concentration and saturation percentage; the rationale here was to maintain a level as steady as possible, including between 0.3 and 0.7 mg/L. A WTW Oxi 340i measuring tool was employed. The outflow volume of the reactor was recorded every day in the first weeks of the experiment, as a means to monitor and control the HRT of the system. Moreover, the effluent samples allowed for the calculation of the sludge retention time (SRT), which reflects the average residence time of the biomass particles in the system.
2.3 Chemical Analyses
Chemical analyses were performed both on the inflow solution, which was regularly prepared in the laboratory, and the outflow, treated water; the monitoring activity regarded not only the solution pH levels, alkalinity, and electrical conductivity but also the content of relevant dissolved ions. It has to be noticed that the alkalinity measurements were conducted on the outflow only. First of all, pH levels were measured daily, employing the same online tool that was in use for temperature. Electrical conductivity was measured in the effluent once per week using a Crison CM 35 analyzer; ionic activity expresses the ability of a solution to transmit current, and it is directly influenced by the amount and valence of ions, as well as by the solution temperature (Szatkowska and Plaza 2005). As a consequence, the progress of the reaction system can be studied by means of electrical conductivity variations: higher values correspond to an increased number of ions, whereas lower ones are a symptom of a decreased amount of charged particles. Total alkalinity (TA) was measured twice per week, employing a Crison pH-Burette 24. The concentration of nitrogen ionic species (NH4 +, NO2 −, and NO3 −) apart from other ions, both in the influent and effluent solutions, was analyzed about twice per week using a Metrohm 861 Compact Ionic Chromatography analyzer, coupled with an 863 Compact autosampler.
2.4 Suspended Solid Analyses
The procedure for these analyses was derived from the Standard Methods for the Examination of Water and Wastewater (APHA 2012). Two samples from the outflow were collected and analyzed weekly; the sample volume was initially set at 50 mL, but later increased as to minimize the effect of random errors. The first part of the operation was carried out using a P Selecta oven, operating at 105 °C; the second step employed a Hobersal HD-230 oven, at an operating temperature of 550 °C. Weight analyses were conducted using a Sartorius TE214S precision scale and employed to obtain the amount of total suspended solids (TSS) and ultimately volatile suspended solids (VSS) in the outflow. Such data was employed to gain information on the biomass retention capacity of the system; also, a sample collected directly from the reactor allowed for the calculation of the sludge retention time (SRT), reflecting the average residence time of the biomass particles in the system.
3 Results
The studies reported in this article were carried out with the aim of investigating partial nitrification and Anammox techniques applied to mainstream municipal water; in order to present the results as clearly as possible, it was chosen to group them into three separate sections, depending on the examined parameters.
3.1 Operational Conditions
Fluctuations of temperature and pH levels were recorded throughout the whole experiment (Fig. 1); during the partial nitrification step, they were coupled with a consistent and almost immediate decrease in the alkalinity levels. Although this was a symptom of correct progression of the reaction, attention was paid not to leave pH values go below their optimal level; more specifically, a threshold value of 7 was set. Apart from daily monitoring the reactor, modification of the feeding solution and direct correction of pH by addition of basic concentrates to the reactor proved to be useful and practical ways of keeping the system stability. Still, the advancement of the nitritation reaction seemed to aggressively consume all sources of carbonate species, which had to be added almost daily. The situation changed when the first clear symptoms of Anammox activity were recorded; this happened days after the inoculation of that biomass, and brought to the levels back up, according to the expected consumption of H+ ions by the Anammox bacteria. As it turned out, some variations of the pH levels did not follow the degrading pattern related to progressive nitrification. The peak and trough values for pH were 8.68 and 6.02, respectively, with an average level of 7.19. As for temperature, the parameter varied in between 18 and 31.6 °C, averaging 23.4 °C.
The record of DO levels showed an initial instability; nonetheless, an average value was defined. The trend observed in Fig. 2 demonstrates that with the development of the partial nitrification process, DO tended to a stable level; minimum and maximum values were 0.09 and 1.44 mg/L, with an average of 0.78 mg/L. As for electrical conductivity in the effluent, a steady increase was observed in the first nitrification phase. Later on, a slight and moderate decrease was noticed, which started roughly at the moment when the Anammox biomass was inserted into the system. The peak and trough conductivity values in the effluent were 569 and 1720 μS, with an average of 1273 μS (Fig. 3). Alkalinity levels were quite variable, with almost constant low values and sudden peaks; the range spanned from 11.5 to 523.2 mg/L, with an average of 126 mg/L (Fig. 4).
3.2 Nitrogen Ions
Figure 5 reports the temporal variability of the nitrogen compounds related to NH4 + ions for the inlet and the outlet; also, the concentration pertaining to NO2 − ions in the outflow is plotted. The concentration of ammonia ions in the inflow averaged 57.5 mg N/L, with peak and trough values of 73.6 and 28.7 mg N/L. As for the outflow, the average value was 31.8 mg N/L, with a minimum of 7.4 mg and a maximum of 49.8 mg N/L. Nitrites yielded peak and trough values of 47.3 and 5.9, with an average of 29.9 mg N/L; since the TN in the outflow was observed to be slightly greater than in the inflow for the peak point, it felt necessary to compute the random error brought in at the chromatography step. This was assessed at ±6.9% by comparison of the inflow and outflow TN values; furthermore, the overall conversion percentage of NH4 +-N to NO2 −-N for the same point was calculated at 38%, with the same margin of error. The choice of expressing the mentioned levels in terms of nitrogen content allowed for the definition of Fig. 6, reporting TN values for the inflow and the outflow; besides, this made possible the assessment of the overall nitrogen removal efficiency. More specifically, the average TN levels for the inflow and outflow, respectively, were 67.4 and 61.5 mg N/L. The maximum nitrogen removal efficiency was assessed at 55.2%; this value was reached at the very last sampling point of the experiment. As for nitrates, their levels in the outflow were quantifiable only two times along the whole experimental period, but still quite low (<5 mg N/L); hence, they were not reported in the above diagrams. Lastly, information on the nitrogen loading rate was also obtained by averaging the related chromatography results, giving about 0.06 g N/(L d) for a HRT value of 24 h and about 0.12 g N/(L d) for the second phase, which had a HRT of 12 h.
3.3 Biomass Retention
Chemical analyses regarding the VSS content in the effluent are available in Fig. 7. As it turned out, VSS levels were not quantifiable in the first step of the experiment, but rose during the second part of it, reaching 0.014% (w/v) of the total weight content. The SRT for the last sampling point was evaluated after measuring the VSS content in the reactor environment, which yielded a value of 0.046%, obtained by averaging the results given by two different samples; subsequently, the final SRT was assessed at 15.6 days.
4 Discussion
Monitoring of the presented operational values for the reactor proved that the reaction system efficiently progressed in the desired direction. pH levels were consistent with both the partial nitrification and Anammox reaction equations; in fact, the former consumes protons, while the latter features them as products. The temperature in the reaction environment rose over time, demonstrating that the thermostatic system devised for the experiment was not able to cope with the intense changes in atmospheric temperature conditions; however, the values were kept moderate and close to the range expected in the mainstream flow of a WWTP located in the Mediterranean coast of Spain during the spring or summer. Anyway, except for the last period of operation, the temperature of operation was lower than the optimum for partial nitrification (30–40 °C; van Dongen et al. 2001) and Anammox (35–40 °C; Dosta et al. 2008). Maintenance of a steady DO value was achieved within values close to those reported in other works (Table 1), yet in the first part of the experiment, a wide variability was clearly noticeable, and probably due to the operational difficulty of regulating the inlet faucet for N2. The variation of electrical conductivity levels in the effluent accommodated the advance in the reaction system. An increase was observed in the first part of the experiment. At least the first part of that increase could be related to the increase of combined concentration of ionic nitrogenous species in the effluent (Figs. 5 and 6). Later on, after day 78 of operation, the conductivity values decreased. According to the literature (Szatkowska and Plaza 2005), it was expected that when the Anammox reaction was established and the ionic nitrogen species converted into nitrogen gas, conductivity in the effluent was decreasing, so we could attribute the behavior to the start-up of the Anammox reaction. Alkalinity levels generally showed to be low, as it was expected from mainstream municipal wastewater. Regular addition of carbonate was necessary in order for the partial nitrification not to stop; this is also the reason for the sudden changes and shifts in the alkalinity diagram. As expected, the low alkalinity levels could be one of the limitations of this application.
The theoretical nitrogen load that was devised for the system was about 0.14 g/(L d); this value was approximately reached when the HRT time was changed to 12 h. Average values obtained by chromatography test gave a load about 0.12 g/(L d) for that HRT. This means that the influent TN was slightly lower than planned according to the feeding composition in Table 4, as a consequence of imprecision in weighting the feeding compounds but also of oxidation of NH4 + from NH4Cl by contact with the atmosphere in the feeding storage tanks. Anyway, the applied loading rate was comparable (i.e., the same order of magnitude) with the removal rates reported in Table 3. It was also lower but comparable to the nitrogen removal rate reported (0.3–0.4 g N/(L d)) by Isanta et al. (2015) for an airlift granular reactor performing partial nitrification at mainstream conditions. Chromatography results proved that good accumulation of NO2 − was achieved. Nitrite concentrations rose over the partial nitrification step, according to the stoichiometric equation; on the other hand, the Anammox reaction lead to a decrease in this value, due to the steady consumption for the reduction reaction. NO3 − concentrations values were almost always too low to be noticed, suggesting that the choice of setting low DO values can be appropriate for NOB out selection. Besides, the use of a highly AOB-enriched biomass seems to be a suitable strategy. Still, other parameters could have a role in determining unsuitable conditions for nitrite oxidizers and would require further study. For example, the rising operational temperatures observed throughout the second half of the experiment, above 25 °C, may have had a role in favoring the activity of ammonia oxidizers. Diagrams derived from chromatography results showed that the NH4 +:NOx ratio was close to the desirable target of 1:1 before the inoculation of the Anammox biomass. In fact, such a ratio is intended to avoid nitrite excess and subsequent inhibition of the Anammox activity. Still, it is frequent for full-scale plants to follow a ratio dictated by the Anammox stoichiometry, to avoid having unreacted ammonium in the effluent. Activity of the Anammox bacteria was detected about 1 month after the inoculation; this value is almost double compared to literature results (at near optimum conditions) reported in Table 2. This could be explained by the lower temperatures employed in the present work, compared to the works in the table. Another possible contribution can come from the early decrease of the HRT, which probably also happened too abruptly; also the nitrogen loading rate (NLR) was too low and did not allow for proper growth and development of the bacteria. In any case, taking into account, these limitations due to the operation under low nitrogen load and moderate temperature and a detection time which is the double of the corresponding to near optimum conditions seems a promising value. Besides, the experiment was conducted for a period of time not long enough to reach steady-state conditions for Anammox nitrogen removal; however, it seems that extending the experiment further in time could have probably led to constant TN removal efficiency level, as suggested by the overall degrading trend in Fig. 6.
Tests on the content of VSS lead to the definition of Fig. 7; the evident shift in the values is attributed to the insertion of Anammox biomass and confirms the expected difficulty in retaining this kind of bacteria. Still, a SRT of 15.6 days was calculated for the last sampling point. Such value was considered to be a symptom of efficient biomass retention, when compared to other values retrieved in scientific literature (Table 1).
Apart from allowing for the evaluation of the process feasibility, the experiment gave rise to a series of remarks that could be considered for further research. For instance, more research should dwell on the possibility of speeding up the start-up phase for industrial Anammox-related technologies; oxygen affinities of AOB and NOB should be studied in detail, along with oxygen diffusion mechanisms, as they could play a bigger part in NOB out-selection solutions. Finally, studies about possible emissions of N2O from Anammox-based systems should be undertaken in order to reduce the impact of greenhouse gasses. More specifically, aeration and gas circulation techniques should be focused on.
5 Conclusions
The experiment featured in this article suggests that deammonification techniques applied to municipal mainstream wastewater can be feasible, pinpointing the possibility of applying low DO values combined with highly NOB-enriched granular biomass as a means to avoid nitrite oxidation and achieve good bacterial retention. It is clear that an efficient process has to be met by fine tuning of some key operational parameters: temperature, pH, and DO levels. Using a lab-scale reactor, a final TN removal efficiency of 54% was reached on day 126, along with very low yield of nitrates and high accumulation of nitrites peaking at 38 ± 6.9% of total NH4 + conversion. The use of granular biomass also proved to be successful in enhancing the bacterial retention capacity of the system, yielding a final SRT value of 15.4 days. Still, the first signs of Anammox activity were detected about 1 month after the inoculation of the biomass, requiring double the time if compared to other similar experiments.
Based on the obtained results, it seems that some the typical issues of biomass retention and NOB out selection for mainstream deammonification can be approached by employing granular biomass and operating at low DO values. Still, some other demanding aspects of the process, like long start-up times and possible influence of toxic agents, were not dealt with in this experiment and call for further research, possibly addressing all the presented issues and studying their correlation.
The conducted literature review also highlighted the feasibility of conducting mainstream deammonification in the perspective of saving organic carbon to convert it into usable energy, also at low temperatures.
References
Abbas, G., Zheng, P., Wang, L., Li, W., Shahzad, K., Zhang, H., Hashmi, M. Z., Zhang, J., & Zhang, M. (2014). Ammonia nitrogen removal by single-stage process: a review. Journal of the Chemical Society of Pakistan, 36(4), 775–782.
American Public Health Association (APHA). (2012). Standard methods for the examination of water and wastewater (22nd ed.). Washington D. C: American Public Health Association, American Water Works Association, Water Environment Federation publication. APHA.
Anthonisen, A. C., Loehr, R. C., Prakasam, T. B. S., & Srinath, E. G. (1976). Inhibition of nitrification by ammonia and nitrous acid. Journal of the Water Pollution Control Federation, 48(5), 835–852.
Bi, Z., Qiao, S., Zhou, J., Tang, X., & Zhang, J. (2014). Fast start-up of Anammox process with appropriate ferrous concentration. Bioresource Technology, 170, 506–512.
Blackburne, R., Yuan, Z., & Keller, J. (2008). Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation, 19, 303–312.
Cao, Y., Kwok, B. H., Yong, W. H., Chua, S. C., Wah, Y. L., & Ghani, Y. (2013). Mainstream partial nitritation—Anammox nitrogen removal in the largest full-scale activated sludge process in Singapore: process analysis. Canada: In WEF/IWA Nutrient Removal and Recovery. Presented at the WEF/IWA Nutrient Removal and Recovery.
Chen, H., Liu, S., Yang, F., Xue, Y., & Wang, T. (2009). The development of simultaneous partial nitrification, Anammox and denitrification (SNAD) process in a single reactor for nitrogen removal. Bioresource Technology, 100(4), 1548–1554.
De Clippeleir, H., Vlaeminck, S. E., De Wilde, F., Daeninck, K., Mosquera, M., Boeckx, P., Verstraete, W., & Boon, N. (2013). One stage partial nitritation/Anammox at 15 °C on pretreated sewage: feasibility demonstration at lab-scale. Applied Microbiology and Biotechnology, 97(23), 10199–10210.
Dosta, J., Fernández, I., Vázquez-Padin, J. R., Mosquera-Corral, A., Campos, J. L., Mata-Álvarez, J., & Méndez, R. (2008). Short- and long-term effects of temperature on the Anammox process. Journal of Hazardous Materials, 154(1–3), 688–693.
Egli, K., Fanger, U., Alvarez, P. J. J., Siegrist, H., van der Meer, J. R., & Zehnder, A. J. B. (2001). Enrichment and characterization of an Anammox bacterium from a rotating biological contractor treating ammonium-rich leachate. Archives of Microbiology, 175(3), 198–207.
Fernández, I., Dosta, J., Fajardo, C., Campos, J. L., Mosquera-Corral, A., & Méndez, R. (2012). Short- and long-term effects of ammonium and nitrite on the Anammox process. Journal of Environmental Management, 95, 170–174.
Frijns, J., Hofman, J., & Nederlof, M. (2013). The potential of (waste) water as energy carrier. Energy Conversion and Management., 65, 357–363.
Gao, D. W., Lu, J. C., & Liang, H. (2014). Simultaneous energy recovery and autotrophic nitrogen removal from sewage at moderately low temperatures. Applied Microbiology and Biotechnology, 98(6), 2637–2645.
Gong, Z., Yang, F., Liu, S., Bao, H., Hu, S., & Furukawa, K. (2007). Feasibility of a membrane-aerated biofilm reactor to achieve single-stage autotrophic nitrogen removal based on Anammox. Chemosphere, 69(5), 776–784.
Guo, J., Peng, Y., Huang, H., Wang, S., Ge, S., Zhang, J., & Wang, Z. (2010). Short- and long-term effects of temperature on partial nitrification in a sequencing batch reactor treating domestic wastewater. Journal of Hazardous Materials, 179(1), 471–479.
Hu, Z., Lotti, T., de Kreuk, M., Kleerebezem, R., van Loosdrecht, M., Kruit, J., Jetten, M. S. M., & Kartal, B. (2013). Nitrogen removal by a nitritation-Anammox bioreactor at low temperature. Applied Environmental Microbiology, 79(8), 2807–2812.
Isanta, E., Reino, C., Carrera, J., & Pérez, J. (2015). Stable partial nitritation for low-strength wastewater at low temperature in an aerobic granular reactor. Water Research, 80, 149–158.
Jetten, M. S. M., Horn, S. J., & van Loosdrecht, M. C. M. (1997). Towards a more sustainable municipal wastewater treatment system. Water Science and Technology, 35(9), 171–180.
Jin, R. C., Yang, G. F., Yu, J. J., & Zheng, P. (2012). The inhibition of the Anammox process: a review. Chemical Engineering Journal, 197, 67–79.
Kampschreur, M. J., Temmink, H., Kleerebezem, R., Jetten, M. S. M., & van Loosdrecht, M. C. M. (2009). Nitrous oxide emission during wastewater treatment. Water Research, 43(17), 4093–4103.
Khin, T., & Annachatre, A. P. (2004). Novel microbial nitrogen removal processes. Biotechnology Advances, 22(7), 519–532.
Lackner, S., Terada, A., & Smets, B. F. (2008). Heterotrophic activity compromises autotrophic nitrogen removal in membrane-aerated biofilms: results of a modeling study. Water Research, 42(4), 1102–1112.
Lackner, S., Gilbert, E. M., Vlaeminck, S. E., Joss, A., Horn, H., & van Loosdrecht, M. C. M. (2014). Full-scale partial nitritation/anammox experiences—an application survey. Water Research, 55, 292–303.
López, S. (2012). Biological granulation technology for wastewater treatment. PhD thesis. Barcelona: Department of Chemical Engineering, Universitat de Barcelona.
Malovanyy, A., Yang, J., Trela, J., & Plaza, E. (2015). Combination of upflow anaerobic sludge blanket (UASB) and partial nitritation/Anammox moving-bed biofilm reactor for municipal wastewater treatment. Bioresource Technology, 180, 144–153.
Mosquera-Corral, A., González, F., Campos, J. L., & Méndez, R. (2005). Partial nitrification in a SHARON reactor in the presence of salts and organic carbon compounds. Process Biochemistry, 40(9), 3109–3118.
Regmi, P., Miller, M. W., Holgate, B., Bunce, R., Park, H., Chandran, K., Wett, B., Murthy, S., & Bott, C. B. (2014). Control of aeration, aerobic SRT and COD input for mainstream nitritation/denitritation. Water Research, 57, 162–171.
Ruiz, G., Jeison, D., & Chamy, R. (2003). Nitrification with high nitrite accumulation for the treatment of wastewater with high ammonia concentration. Water Resources, 37(6), 1371–1377.
Sliekers, A. O., Derwort, N., Campos Gomez, J. L., Strous, M., Kuenen, J. G., & Jetten, M. S. M. (2002). Completely autotrophic nitrogen removal over nitrite in one single reactor. Water research, 36(10), 2475–2482.
Strous, M., Kuenen, J. G., & Jetten, M. S. M. (1999). Key physiology of anaerobic ammonium oxidation. Applied and Environmental Microbiology, 65(7), 3248–3250.
Strous, M., Van Gerven, E., Kuenen, J. G., & Jetten, M. (1997). Effects of aerobic and microaerobic conditions on anaerobic ammonium-oxidizing (anammox) sludge. Applied and Environmental Microbiology, 63(6), 2446–2448.
Sultana, R. (2014). Partial nitritation/Anammox process in a moving bed biofilm reactor operated at low temperatures. PhD thesis. Stockholm: Department of Land and Water Engineering, KTH.
Szatkowska, B., & Plaza, E. (2005). Partial nitritation/Anammox and CANON nitrogen removal systems followed by conductivity measurements. Cracow: In Proceedings of Polish/Swedish seminars.
van Dongen, U., Jetten, M. S. M., & van Loosdrecht, M. C. M. (2001). The SHARON®-Anammox® process for treatment of ammonium rich wastewater. Water Science and Technology, 44(1), 153–160.
van Hulle, S. W. H., Vandeweyer, H. J. P., Meesschaert, B. D., Vanrolleghem, P. A., Dejans, P., & Dumoulin, A. (2010). Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams. Chemical Engineering Journal, 162(1), 1–20.
Wang, T., Zang, H., Yang, F., Liu, S., Fu, Z., & Chen, H. (2009). Start-up of the Anammox process from the conventional activated sludge in a membrane bioreactor. Bioresource Technology, 100(9), 2501–2506.
Wett, B., Omari, A., Podmirseg, S. M., Han, M., Akintayo, O., Gómez, B. M., Murthy, S., Bott, C., Hell, M., Takács, I., Nyhuis, G., & O’Shaughnessy, M. (2013). Going for mainstream deammonification from bench to full scale for maximized resource efficiency. Water Science and Technology, 68(2), 283–289.
Xu, G., Zhou, Y., Yang, Q., Lee, Z. M., Gu, J., Lay, W., Cao, Y., & Liu, Y. (2015). The challenges of mainstream deammonification process for municipal used wastewater treatment. Applied Microbiology and Biotechnology, 99(6), 2485–2490.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salmistraro, M., Fernández, I., Dosta, J. et al. Mainstream Deammonification: Preliminary Experience Employing Granular AOB-Enriched Biomass at Low DO Values. Water Air Soil Pollut 228, 178 (2017). https://doi.org/10.1007/s11270-017-3253-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3253-4