Abstract
A simple potentiostat was constructed as a strategy to enhance solvent production in a mediatorless and oxygen-exposed fermentation inoculated with the aerotolerant strain Clostridium sp. C10. Elevated n-butanol and acetone titers were recorded in all fermentations with either glucose or xylose in the presence of electrodes poised at + 500 mV (+ 814 mV vs SHE) relative to cells plus substrate only controls. Respective butanol titers and volumetric butanol productivities in studies performed with 30 g/L glucose or 30 g/L xylose were 1.67 and 2.27 times and 1.90 and 6.13 times greater in the presence of electrodes compared to controls. Glucose and xylose utilization in the presence of electrodes was 61 and 125% greater than no-electrode controls, respectively. Increasing substrate concentrations to 60 g/L decreased the butanol yields relative to the studies performed at 30 g/L. These data suggest that it may be more efficient to alter reactor reduction potential than increase substrate concentration for solvent output during industrial fermentations, which favors higher yield with few additional inputs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bioelectrochemical systems (BES) are frequently applied to biological processes as a strategy to elucidate or control dynamics in microbial electrophysiology, but the cost prohibitive nature of most BES acts as a barrier to more extensive proliferation of this tool, particularly in industrial applications. Research groups have previously investigated the development and implementation of low-cost, open source electrochemical systems with great success in applications such as cyclic voltammetry and microbial respirometry [6, 29]. However, the feasibility of applying simple BES as a strategy to enhance native microbial metabolism is still not well understood, and additional investigation is warranted. Previous data suggest that anodic electron stripping enhances cellular bioenergetics in both prokaryotes and eukaryotes, likely by generating thermodynamically favorable metabolic intermediates and by acting as a solid-state surrogate for terminal electron accepting processes or as an electron sink alternative to molecular hydrogen [19, 20, 32, 34]. Electron-mediated enhancement of metabolite production has been studied extensively [12, 25, 26, 28, 32]. However, mediatorless or direct electrode stimulation is still developing as a strategy for stimulating wild-type metabolism [3, 11, 17, 38].
Solventogenic Clostridium are valuable biocatalysts for the production of commodity chemicals, such as acetone and butanol (ABE fermentations), but shortcomings associated with poor substrate utilization and solventogenic productivity in wild-type cells necessitate the development of “drop-in” treatments to overcome these limitations [9, 10, 31, 37]. Control of extracellular redox cycling has been previously described as a potential strategy to augment wild-type ABE fermentative metabolism where either glucose or xylose was used as the sole fermentable substrate [2, 8, 36]. Xylose has become a focus of fermentative manipulations, because it is regarded as a lower value lignocellulosic product, but it still retains a significant amount of energy content for downstream reactions. Eventual industrial-scale modifications that can increase xylose utilization are a goal of biofuel research and development with several wild-type phyla [5, 25, 39].
Butanol is a four-carbon (C4) solvent used in many commercial applications for the plastic, resin, pharmaceutical, automotive, and paint industries [8, 14]. ABE-derived butanol has been investigated as a viable alternative to directly replace gasoline and/or replace current gasoline oxygenates, such as ethanol, since it can be utilized in internal combustion engines without engine modification [33]. Additionally, researchers have been investigating its use as a precursor for military-grade JP-5 jet fuel, further proving the utility of the solvent across multiple platforms [13]. As described previously, butanol fermentations have several physiological limitations with respect to final solvent titers that include productivity, feedstock utilization, and maintenance of process performance without strict control of reactor conditions, such as anoxia and sterility [25, 37, 39].
The focus of this report is to demonstrate the capacity for a simple electrode system to increase solventogenesis (primarily butanol) and xylose utilization in fermentations using the aerotolerant, solventogenic Clostridium sp. strain C10 without the use of electron mediators, which are typically used to enhance electron transfer interactions between cells and electrodes [27, 30]. The purpose is to begin lowering the overall process cost surrounding industrial n-butanol production, primarily with xylose as the sole carbon substrate assuming laboratory processes can be scaled up in future reactors.
Materials and methods
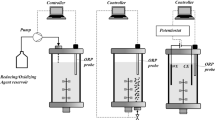
Potentiostat construction
A three-electrode, analog potentiostat prototype was constructed to control Clostridium sp. C10 batch fermentations. A simple potentiostat circuit was created on a prototyping shield which used an LM324 quadruple operational amplifier to supply power to each electrode (S1 Fig). Voltage was regulated using a 10 kΩ dial potentiometer (S2 Fig). The part list for the circuit construction can be found in Table S2. Steady voltage was supplied to the circuit board using the 5 V output voltage pin from an Arduino Uno powered by a D-link multi-port USB [1].
Graphite rods for the working and counter electrodes were retrieved from a 6 V battery cell, and these were cut into smaller pieces with a Dremel tool. The pieces were soaked overnight in 1 N HCl to remove residual metals, and these were subsequently rinsed with NanoPure water. Electrodes were flamed with a butane hand torch after drying to combust residual carbon. Holes (5/64″) were drilled into the top of each electrode, and 22 AWG exposed copper wire was inserted into each hole. Solder was applied to the cavity, and the graphite was flamed until the solder melted. Epoxy was applied to the remaining exposed wire within the electrode after cooling. Electrically insulating heat shrink tubing was used to further encapsulate the wire and increase the strength of the joint after the epoxy hardened. The exposed surface areas for the counter and working electrodes were 780.7 and 472.8 mm2, respectively (S3 Fig). Opposing ends of the wires were stripped and soldered to jumper wires to facilitate more seamless integration with the prototyping board.
The reference electrode was constructed by scoring and cracking the tip from a 1 mL Pasteur pipette using a ceramic gas chromatography (GC) column cutter, followed by adding a 4 Å molecular sieve to the tip of the electrode. The 4 Å molecular sieve was bonded to the glass pipette tip using silicone waterproofing sealant. Glass beads (0.1 mm) were added over the top of the molecular sieve after the silicone sealant dried. The liquid-tight electrode was filled with a 50 mM copper sulfate solution in NanoPure water. Three inches of insulation was stripped from the copper wire, and the exposed portion was inserted into a rubber plug using an 18 Gauge needle as a guide. The plug containing the wire was inserted into the Pasteur pipette containing the copper solution. A portion of insulation on the opposing side of the wire was stripped and soldered to a jumper wire to provide more seamless integration into the circuit board. Electrically insulating heat shrink tubing was used to cover this joint. New wire and copper sulfate solution were prepared prior to the start of each experiment, since wire oxidation and copper deposition within the electrode were visible at the end of each fermentation.
The total energy used by the electrode system over the course of 5-day fermentations was simplified by calculating the energy required to supply the LM324 operational amplifier alone, using characteristics described for + 5.0 V input, at which the typical input bias current was 45 nA (Eqs. 1, 2) [18]. The number of Coulombs (C) were determined by dividing input current (A; amperes) over the time-course of the fermentation. Units were converted to Joules (J) and subtracted from the total energy content of butanol generated (29.2 MJ/L) at the end of each fermentation:
Culture maintenance and fermentation conditions
Clostridium sp. C10 was isolated from a crystalline cellulose-fed woodland marsh sediment enrichment culture. Phylogenetic analysis indicated that strain C10 shared 99% 16S rRNA sequence similarity with other solventogenic Clostridium species (Supporting Information Table 3). Aerotolerant growth was confirmed in three separate physiological tests during strain characterization, and this was further confirmed in oxygen-exposed shake flasks (Supporting Information Figure 8).
Spores from aerotolerant Clostridium sp. C10 were stored at − 20 °C in NanoPure water. A 0.1 mL volume of the thawed spore suspension was used to inoculate an oxygen-exposed, 100 mL screw top Pyrex bottle containing TYG media at room temperature for each batch study. TYG media was prepared by adding 30 g/L tryptone, 20 g/L glucose, and 10 g/L yeast extract to NanoPure water. Liquid volume was brought up to 100 mL, and this was autoclaved on a liquid cycle at 121 °C for 30 min. Cells growing in TYG were incubated at 37 °C in the dark for 26 h prior to the start of each batch study.
Minimal control with respect to media preparation, sterility, and atmospheric maintenance (i.e., strictly anoxic conditions), was sustained throughout the study to simulate conditions which would ultimately foster decreased operational costs, should this process eventually be industrially scaled. Prior characterization of Clostridium sp. C10 indicated robust biomass growth and solvent production from xylose or glucose in oxygen-exposed atmospheres in the presence and absence of agitation, making it ideal for use under these conditions [24]. Experimental media for this study were prepared immediately prior to inoculation with vegetative cultures of strain C10. Strain C10 fermentation broth was prepared by mixing 2 g/L ammonium acetate, 1 g/L yeast extract, 20 mM KH2PO4, and either glucose or xylose in NanoPure water. Sterile media was aliquoted into 250 mL Pyrex reactors without degassing or pH adjustment. This was inoculated with a 6% (v/v) inoculum of an actively growing culture of strain C10 to start the experiment. Final fermentation volumes were 200 mL. All control reactors were run in duplicate. Electrodes were added to single reactors which contained the same media constituents as the controls. The exposed graphite portions of both working and counter electrodes were flamed with a hand torch for 5 s each, and after cooling, the electrodes were grouped together with a plastic zip tie and placed in the fermentation vessel. Proper care was ensured that no contact was observed between any of the electroconductive portions of the electrodes into prevent short-circuiting. Reactors containing the electrode bundle were covered with a layer of Parafilm, while control reactors were run with loosened caps to provide open atmosphere exposure. The potential was set at 500 mV (+ 814 mV vs SHE) for all electrode studies using a 10 kΩ dial potentiometer. Initial voltage differences between the working and counter electrodes in the bulk fermentation liquid were measured using a digital multimeter. Fermentations were incubated in the dark at 37 °C with minimal agitation and mixing. Samples (5 mL) were withdrawn periodically over a 120 h period.
Sample collection and analytical methods
Liquid samples for metabolite analysis were filtered (0.2 μm) into autosampler vials containing 250 μL glass inserts (Lab Supply Distributors). Vials were sealed with screw top caps containing PTFE-lined septa and stored at 4 °C until chromatographic analysis. Remaining sample volumes were aliquoted into polystyrene cuvettes (VWR) for optical density analysis and clean, 15 mL Falcon tubes for pH analysis.
Acetone and butanol were analyzed on a Shimadzu GC-2014 equipped with a flame ionization detector and a DB-FFAP column (30 m × 0.250 mm; 0.25 μm film thickness). Helium was used as the carrier gas, and the linear velocity was set to 80.3 cm/s. Sample volumes of 1 μL were withdrawn from the vials using an AOC20i+S autosampler. Following injection, the syringe was washed three times with NanoPure water. The oven temperature program included a 40 °C dwell (2 min) and a temperature ramp of 50 °C/min until the oven reached 220 °C. The oven temperature was held at 220 °C for 1 min until returning back to the initial dwell temperature. The injector and detector temperatures were set to 200 °C and 300 °C, respectively.
Glucose and xylose were separated with a Dionex HPLC equipped with a Bio-Rad HP-Aminex column and were analyzed using a RefractoMax 521 refractive index detector (Thermo Scientific). The mobile phase (degassed 5 mM H2SO4 in NanoPure water) was set at a constant flow of 0.6 mL/min, and the column oven was set to 60 °C. Sample volumes of 0.25 μL were used for the analysis.
Optical density was measured using a Genesys 10S UV–Vis spectrophotometer (Thermo). The pH was using a Thermo Scientific Orion Star A111 pH meter equipped with an Orion 9107BNMD probe. This was calibrated prior to each measurement using BDH general pH buffer solutions, ranging from pH 4–10.
Kinetic analysis
Time-course butanol data were fitted using a modified Gompertz equation. Rate constants were generated using non-linear regression. This model’s utility has been described in the previous reports [15, 21, 22, 25, 40]. Volumetric butanol productivities of the batch fermentations were calculated in SigmaPlot statistical software, and units are listed in g/L h:
Specific butanol production was determined using the Luedeking–Piret equation for mixed growth associated metabolite production, where α is the growth associated coefficient for product formation (g/g), X denotes biomass concentration (g/L), t denotes time (h), and β is non-growth associated production (Eq. 4) [23]. Units are described in h−1:
Results and discussion
Influence on xylose utilization and solventogenesis
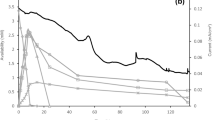
Both xylose utilization and butanol production increased in fermentations subjected to a consistent, steady-state potential of + 500 mV (+ 814 mV vs SHE), relative to controls which were run in the presence of a physical electrode but without the reduction potential being manipulated (Fig. 1). As such the increased activity was attributed to the reduction potential differences between the experimental and control incubations, and not merely the presence of a reactive solid surface being present in the cell suspension. However, the solid-phase electron transfer was critical to the overall reaction dynamics.
Solvent production in xylose-containing batch fermentations in the presence and absence of the electrode system. Fermentations were fed with either 30 g/L (a) or 60 g/L (b) xylose. Results for the controls are presented as the average of two parallel replicates, while electrode-containing incubations are presented as a single experimental reactors
The mechanism for both xylose utilization and butanol production has been reported previously [25]. Briefly, using ferric iron to strip electrons from C. beijerinckii redirected electron flow from the hydrogenase pathway to the low-molecular-mass organic acids (acetic acid and butyric acid), and ultimately the ethanol and butanol pathways. In addition, the cells conserved slightly more ATP in the presence of ferric iron mediated electron stripping, which may have increased ATP-dependent xylose transport into the cell.
Butanol titers in the presence of the electrode system increased 2.3-times (5.0 g/L) relative to the cells plus xylose controls (2.2 g/L) after 5 days of growth in fermentations containing 30 g/L xylose (Fig. 1a). Acetone concentrations also increased by 59.7% (0.6-times higher) in electrode-subjected fermentations (1.1 g/L). Both solvents also increased when 60 g/L xylose was amended as the sole fermentable substrate. Again, the butanol titers (5.8 g/L) were 1.9-times greater in the electrode-subjected incubations when compared to the cells plus xylose controls (3.1 g/L), while acetone concentrations were 1.5-times greater than that in the absence of electrodes (Fig. 1b).
Butanol productivity (volumetric and specific) and xylose consumption were enhanced in electrode-subjected fermentations (Fig. 1). Specific and volumetric butanol production was increased by 337.5 and 512.5%, respectively, in the presence of the electrodes for fermentations containing 30 g/L xylose. Both parameters also increased in fermentations containing 60 g/L xylose, but the percentage increase was lower than the 30 g/L xylose incubations. Specific and volumetric butanol productivities were 228.6 and 284.6% greater than that of the control, respectively. Butanol yield did not increase, because as indicated below the substrate (xylose) utilization increased in the presence of the electrode.
Xylose utilization increased in the presence of electrodes. Xylose consumption increased from 11.0 to 24.8 g/L in the 30 g/L electrode-subjected incubations, and from 24.1 to 34.1 g/L in the 60 g/L electrode-subjected incubations (Table 1). Amongst the controls, merely increasing the concentration of xylose from 30 to 60 g/L did enhance substrate consumption (in the controls); 11.0 g/L xylose was consumed in 30 g/L xylose-fed fermentations and 24.1 g/L xylose consumed in controls containing 60 g/L xylose (Table 1). Prior reports indicate that C. acetobutylicum alters its xylose consumption pathway under high xylose concentrations by enabling the phosphoketolase pathway to supplement pentose metabolism [16]. However, induction of this pathway under high xylose stress has not confirmed with Clostridium sp. strain C10. Butanol yields decreased in all fermentations containing 60 g/L xylose relative to those with 30 g/L xylose because of the increase in substrate utilization for these treatments.
Strain C10 is an aerotolerant culture, and control incubations were run to confirm that the electrode, and not oxygen, was enhancing both xylose utilization and butanol production. Given that the electron stripping mechanism of the electrodes is similar to that of ferric iron—a ferric iron-amended series was run to determine if oxygen and ferric iron would have similar fermentation dynamics.
Five-day, oxygen-exposed batch fermentations containing optimized media conditions were performed in the presence of electron shuttles (AQDS) alone, ferrihydrite alone, or ferrihydrite plus AQDS using 60 g/L glucose as the sole fermentable substrate to investigate whether the supplementation of soluble and insoluble electron sinks in oxygen-exposed Clostridium sp. C10 fermentations will enhance solventogenesis similarly to that which was observed in a previous study [25]. Butanol production was stimulated in the presence of insoluble 20 mM ferrihydrite (FeGel), relative to the cells plus glucose control (Supporting Information Figure 8). Five-day butanol titers for these treatments were 6.18 and 4.59 g/L, respectively (Supporting Information Table 2).
Volumetric butanol productivity was the highest in the oxygen-exposed fermentation containing 20 mM ferrihydrite (0.201 g/L/h), and this value was 2.48 times higher than the cells plus glucose control (oxygen-exposed) and 1.31 times higher than the anoxic treatment containing 20 mM ferrihydrite (Supporting Information Table 2). Additionally, glucose consumption increased in the aerobic preparation of the treatment containing ferrihydrite without compromising the butanol yield (0.23 g/g). In the cells plus glucose control, respective increases in butanol productivity, yield, and substrate consumption were elevated in the presence of oxygen in comparison to the strictly anoxic control, indicating that the presence of oxygen may slightly enhance glucose metabolism and butanol evolution in strain C10. However, oxygen-exposed cultures never generated butanol or consumed xylose at levels comparable to ferric iron-amended incubations. Ferric iron amendment was most similar to the electrode-enhanced incubations presented here.
Glucose as the sole fermentable substrate
Butanol titers increased by 66.6% relative to the control (4.12 g/L) in 30 g/L glucose-fed fermentations that were subjected to the working electrode. Increasing glucose concentrations had inhibitory effects on solventogenesis, which was opposite to the effects with xylose (Fig. 2 and Table 2). Butanol and acetone titers decreased by 6.1 and 20.7%, respectively, at 60 g/L glucose (Fig. 2, no-electrode controls). Furthermore, butanol productivity and yield decreased at 60 g/L glucose concentrations in fermentations treated with the working electrode system (Table 2). Acetone titers increased minimally in the presence of the electrode system at both 30 and 60 g/L glucose concentrations (Fig. 2), and these data are consistent with a previous study which showed that the presence of the electrode system had the most pronounced effect on butanol producing pathways with xylose [35].
Solvent production in batch fermentations containing 30 g/L (a) and 60 g/L (b) glucose in the presence and absence of the potentiostat. Results for the controls are presented as the average of two parallel replicates, while electrode-containing incubations are presented as a single experimental reactors
Initial and final substrate concentrations were quantified to determine the extent of glucose utilization in the presence and absence of the electrode system (Table 2). Glucose consumption in the absence of electrodes was greater than that was observed in the controls containing xylose, and glucose consumption reached a limit of 28.9–30.4 g/L in the presence of electrodes, while consumption was similar (18 g/L vs 19.9 g/L) between the 30 and 60 g/L glucose controls. Higher glucose consumption in the controls (relative to xylose consumption in the controls) was expected, as all previous data suggest that it is a preferential substrate for Clostridia fermentations [4, 5, 7].
Energy output
Total energy output per fermentation was calculated from the perspective of butanol produced versus the energy required to deliver voltage through the electrode system. Xylose-fed fermentations subjected to the working electrode were able to extract 179.5–208.0 kJ/L from the provided reducing sugars, which equates to an 89.0–127.2% increase in butanol energy output relative to the energy output in non-electrode-subjected fermentations (Table 3). Similar results were observed for glucose-fed fermentations. Observed increases in the total energy output for glucose-fed, electrode-enhanced fermentations was 64.3–66.4% greater than that of the controls.
Conclusions
A simple potentiostat was constructed to modify the native redox environment of minimally controlled ABE fermentations containing either xylose or glucose as the sole fermentable substrates. Solventogenic enhancement for fermentations in the presence and absence of the potentiostat was examined from the perspective of overall solvent titers, volumetric and specific butanol productivity, substrate utilization, butanol yield from substrate, and total energy content produced per fermentation in the form of butanol. Increases in each of the previously listed metrics, excluding butanol yield, were vastly higher in fermentations subjected to the increased reduction potential of the working electrode system. However, the increase in xylose consumption may offset the yield data, because xylose has been reported to underperform as a growth substrate during industrial fermentations. These data indicate that this system may be deployed as a possible drop-in strategy to lower processing costs associated with ABE fermentations and increase butanol production with xylose as the primary fermentable substrate. However, this would first need to be tested at a much larger scale than laboratory continuous batch or fed-batch reactors.
References
Arduino SA (2015) Arduino. Arduino LLC
Cai X, Bennett GN (2011) Improving the Clostridium acetobutylicum butanol fermentation by engineering the strain for co-production of riboflavin. J Ind Microbiol Biotechnol 38:1013–1025
Chaudhuri SK, Lovley DR (2003) Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol 21:1229–1232
Chen Y, Zhou T, Liu D, Li A, Xu S, Liu Q et al (2013) Production of butanol from glucose and xylose with immobilized cells of Clostridium acetobutylicum. Biotechnol Bioprocess Eng 18:234–241
Finneran KT, Popovic J (2018) Solvent production from xylose. Appl Microbiol Biotechnol 102:8707–8715
Friedman ES, Rosenbaum MA, Lee AW, Lipson DA, Land BR, Angenent LT (2012) A cost-effective and field-ready potentiostat that poises subsurface electrodes to monitor bacterial respiration. Biosens Bioelectron 32:309–313
Grimmler C, Held C, Liebl W, Ehrenreich A (2010) Transcriptional analysis of catabolite repression in Clostridium acetobutylicum growing on mixtures of d-glucose and d-xylose. J Biotechnol 150:315–323
Harvey BG, Meylemans HA (2011) The role of butanol in the development of sustainable fuel technologies. J Chem Technol Biotechnol 86:2–9
Jeffries TW (1983) Utilization of xylose by bacteria, yeasts, and fungi. In: Pentoses and Lignin (pp 1–32) Springer, Berlin
Jojima T, Omumasaba CA, Inui M, Yukawa H (2010) Sugar transporters in efficient utilization of mixed sugar substrates: current knowledge and outlook. Appl Microbiol Biotechnol 85:471–480
Kim HJ, Park HS, Hyun MS, Chang IS, Kim M, Kim BH (2002) A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb Technol 30:145–152
Kim TS, Kim BH (1988) Electron flow shift in Clostridium acetobutylicum fermentation by electrochemically introduced reducing equivalent. Biotechnol Lett 10:123–128
Lee SK, Chou H, Ham TS, Lee TS, Keasling JD (2008) Metabolic engineering of microorganisms for biofuels production: from bugs to synthetic biology to fuels. Curr Opin Biotechnol 19:556–563
Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS (2008) Fermentative butanol production by Clostridia. Biotechnol Bioeng 101:209–228
Lin CY, Lay CH (2004) Effects of carbonate and phosphate concentrations on hydrogen production using anaerobic sewage sludge microflora. Int J Hydrog Energy 29:275–281
Liu L, Zhang L, Tang W, Gu Y, Hua Q, Yang S et al (2012) Phosphoketolase pathway for xylose catabolism in Clostridium acetobutylicum revealed by 13C metabolic flux analysis. J Bacteriol 194:5413–5422
Liu Y, Wang M, Zhao F, Xu Z, Dong S (2005) The direct electron transfer of glucose oxidase and glucose biosensor based on carbon nanotubes/chitosan matrix. Biosens Bioelectron 21:984–988
LMx24-N, LM2902-N Low-Power, Quad-Operational Amplifiers (2000). http://www.ti.com/lit/ds/symlink/lm124-n.pdf. Accessed 14 May 2016
Lovley DR (2006) Bug juice: harvesting electricity with microorganisms. Nat Rev Microbiol 4:97–508
Lovley DR (2006) Microbial fuel cells: novel microbial physiologies and engineering approaches. Curr Opin Biotechnol 17:327–332
Mu Y, Yu HQ, Wang G (2007) A kinetic approach to anaerobic hydrogen-producing process. Water Res 41:1152–1160
Mu Y, Zheng XJ, Yu HQ, Zhu RF (2006) Biological hydrogen production by anaerobic sludge at various temperatures. Int J Hydrog Energy 31:780–785
Pazouki M, Najafpour G, Hosseini MR (2008) Kinetic models of cell growth, substrate utilization and bio-decolorization of distillery wastewater by Aspergillus fumigatus UB260. Afr J Biotechnol 7:1369–1376
Popovic J, Finneran KT (2014) Optimization of aerotolerant and mixed culture fermentations for biofuel production. In: American Society for Microbiology 114th general meeting, Boston, MA. USA
Popovic J, Ye X, Haluska A, Finneran KT (2017) Ferric iron and extracellular electron shuttling increase xylose utilization and butanol production during fermentation with multiple solventogenic bacteria. Appl Microbiol Biotechnol 101:8053–8061
Price-Whelan A, Dietrich LE, Newman DK (2006) Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nat Chem Biol 2:71–78
Rabaey K, Rozendal RA (2010) Microbial electrosynthesis—revisiting the electrical route for microbial production. Nat Rev Microbiol 8:706–716
Rao G, Mutharasan R (1986) Alcohol production by Clostridium acetobutylicum induced by methyl viologen. Biotechnol Lett 8:93–896
Rowe AA, Bonham AJ, White RJ, Zimmer MP, Yadgar RJ, Hobza TM et al (2011) CheapStat: an open-source, “Do-It-Yourself” potentiostat for analytical and educational applications. PLoS One 6:1–7
Schröder U (2007) Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys Chem Chem Phys 9:2619–2629
Servinsky MD, Kiel JT, Dupuy NF, Sund CJ (2010) Transcriptional analysis of differential carbohydrate utilization by Clostridium acetobutylicum. Microbiology 156:3478–3491
Shin H, Zeikus J, Jain M (2002) Electrically enhanced ethanol fermentation by Clostridium thermocellum and Saccharomyces cerevisiae. Appl Microbiol Biotechnol 58:476–481
Szulczyk KR (2010) Which is a better transportation fuel–butanol or ethanol? Int J Energy Environ 3:501–512
Thrash JC, Coates JD (2008) Direct and indirect electrical stimulation of microbial metabolism. Environ Sci Technol 42:3921–3931
Wang S, Zhu Y, Zhang Y, Li Y (2012) Controlling the oxidoreduction potential of the culture of Clostridium acetobutylicum leads to an earlier initiation of solventogenesis, thus increasing solvent productivity. Appl Microbiol Biotechnol 93:1021–1030
Wietzke M, Bahl H (2012) The redox-sensing protein Rex, a transcriptional regulator of solventogenesis in Clostridium acetobutylicum. Appl Microbiol Biotechnol 96:749–761
Xiao H, Gu Y, Ning Y, Yang Y, Mitchell WJ, Jiang W, Yang S (2011) Confirmation and elimination of xylose metabolism bottlenecks in glucose phosphoenolpyruvate-dependent phosphotransferase system-deficient Clostridium acetobutylicum for simultaneous utilization of glucose, xylose, and arabinose. Appl Environ Microbiol 77:7886–7895
Zebda A, Gondran C, Le Goff A, Holzinger M, Cinquin P, Cosnier S (2011) Mediatorless high-power glucose biofuel cells based on compressed carbon nanotube-enzyme electrodes. Nat Commun 2:1–6
Zheng J, Tashiro Y, Wang Q, Sonomoto K (2015) Recent advances to improve fermentative butanol production: genetic engineering and fermentation technology. J Biosci Bioeng 119:1–9
Zwietering MH, Jongenburger I, Rombouts FM, Van’t Riet KJ (1990) Modeling of the bacterial growth curve. Appl Environ Microbiol 56:1875–1881
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Popovic, J., Finneran, K.T. Enhancing xylose and glucose utilization as well as solvent production using a simplified three-electrode potentiostat system during Clostridium fermentation. J Ind Microbiol Biotechnol 47, 889–895 (2020). https://doi.org/10.1007/s10295-020-02313-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-020-02313-4