Abstract
Clostridium beijerinckii optinoii is a Clostridium species that produces butanol, isopropanol and small amounts of ethanol. This study compared the performances of batch and continuous immobilized cell fermentations, investigating how media flow rates and nutritional modification affected solvent yields and productivity. In 96-h batch cultures, with 80 % of the 30 g L−1 glucose consumed in synthetic media, solvent concentration was 9.45 g L−1 with 66.0 % as butanol. In a continuous fermentation using immobilized C. beijerinckii optinoii cells, also with 80 % of 30 g L−1 glucose utilization, solvent productivity increased to 1.03 g L−1 h−1. Solvent concentration reached 12.14 g L−1 with 63.0 % as butanol. Adjusting the dilution rate from 0.085 to 0.050 h−1 to allow extended residence time in column was required when glucose concentration in fresh media was increased from 30 to 50 g L−1. When acetate was used to improve the buffer capacity in media, the solvent concentration reached 12.70 on 50 g L−1 glucose. This continuous fermentation using immobilized cells showed technical feasibility for solvent production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the steady increase in energy consumption and concerns over energy self-sufficiency, there is pressure to develop sustainable renewable fuels [1]. Bioethanol is currently the dominant liquid biofuel which has been extensively developed to support the current transportation infrastructure [2, 3]. However, there are limitations to bioethanol as a transportation fuel [4–6].
Butanol, a traditional industrial solvent, can be produced by fermentation of sugars using Clostridium sp.. Butanol has a liquid heating value (LHV) which is 86 % that of gasoline, 31 % greater than the LHV of ethanol, making it a more desirable liquid fuel in terms of energy density [7]. Unlike ethanol, butanol can be blended with gasoline at any proportion without alteration to the internal combustion engine, and it is compatible with the current pipeline infrastructure, making it an attractive liquid fuel [5]. Anaerobic production of acetone/butanol/ethanol (ABE fermentation) by Clostridium sp. was the second largest biotechnological process in scale, only smaller than ethanol production by yeast until replaced by chemical synthesis [8]. The ABE fermentation process has two phases: (1) acidogenesis during exponential growth, which mainly leads to acetic and butyric acid accumulation, and (2) solventogenesis after a transition from exponential to stationary growth when previously formed acids are used for solvent production [8–10]. Normally pH drops to below 5 during acidogenesis, and bounces back to be between 5 and 5.5 during solventogenesis [11]. The transmembrane pH gradient of fermenting Clostridium cells drops as butanol fermentation progresses, dissipating the proton motive force and leading to an acidogenesis and solventogenesis balanced equilibrium established by the microorganisms [11]. ABE fermentations are normally conducted using C. acetobutylicum, although several C. beijerinckii sp., having an additional primary/secondary alcohol dehydrogenase, can convert acetone to isopropanol [8, 9]. Isopropanol is traditionally an important organic solvent used in laboratory and printing, and it can also be used as a fuel additive to reduce carbon monoxide emission [12].
Low productivity and product inhibition are problems that have limited expansion of butanol production through fermentation. Continuous fermentation and using immobilized cells have been applied to C. beijerinckii sp. to improve the solvent fermentation performance. Survase et al. [12] reported a solvent concentration of 7.51 g L−1 made up of 39.4 % isopropanol and 60.6 % butanol in a two-stage continuous culture with C. beijerinckii DSM 6423. Qureshi et al. [13] found that total solvent concentration reached 7.9 g L−1 at a dilution rate of 2.0 h−1 for C. beijerinckii BA101 immobilized on brick pieces. Acetate, the intermediate in butanol fermentation, has been reported to enhance solvent production and prevent degeneration of C. beijerinckii during ABE fermentation [14, 15]. The microorganism used in this study was C. beijerinckii optinoii, a Clostridium species producing high butanol and isopropanol but limited amounts of ethanol. The objective of this study was to compare batch with immobilized C. beijerinckii optinoii in continuous fermentation for butanol and isopropanol production.
Materials and methods
Clostridium strain and culture media
Clostridium beijerinckii sp. optinoii was originally isolated from a strain tentatively identified as Clostridium sp. Prazmowski 1880 AL (Code No. NCCBNr 84049), obtained from Centralbureau voor Schimmelcultures, Utrecht, Netherlands. It was cross listed as Clostridium saccharoperbutylacetonicum N1-504 (ATCC 27022). However, the carbohydrate utilization pattern and product profile of this isolate did not match Clostridium saccharoperbutylacetonicum N1-504, rather it was closer to Clostridium beijerinckii [16]. Therefore, it was renamed as Clostridium beijerinckii optinoii. This novel Clostridium strain has been deposited under ATCC Accession No. PTA-11285 (see patent US 2013/0149757 A1 for details).
An aliquot of the frozen C. beijerinckii optinoii spores was activated by heating at 80 °C for 10 min and then cooled to room temperature on ice. Five hundred microliters of the heat shocked spores was transferred to 9 mL sterilized Clostridium inoculation medium in a rubber stopper capped 10 mL serum bottle. The inoculation medium was composed of 5 g L−1 glucose, 5 g L−1 proteose peptone #3, 5 g L−1 yeast extract, 5 g L−1 sodium thioglycolate, 5 g L−1 KH2PO4, and 0.002 g L−1 methylene blue with pH adjusted to 6.5. The spore suspension was allowed to grow inside an anaerobic chamber at 36 °C with oxygen removed using a GasPak™ anaerobic sachet. After 7 days, 1 mL of an actively growing C. beijerinckii optinoii suspension was inoculated to 120 mL serum bottle containing 100 mL sterilized P2 medium which is composed of 30 g L−1 glucose, 1 g L−1 yeast extract, 1 g L−1 tryptone, 0.002 g L−1 methylene blue, and 1 mL filter-sterilized nutrient stock solution that is added prior to inoculation [17]. A 100-fold concentrate of nutrient stock solution contains 50 g L−1 KH2PO4, 50 g L−1 K2HPO4, 220 g L−1 ammonium acetate, 0.1 g L−1 para-amino benzoic acid, 0.1 g L−1 thiamin, 0.001 g L−1 biotin, 20 g L−1 MgSO4·7H2O, 1 g L−1 MnSO4·H2O, 1 g L−1 FeSO4·7H2O, and 1 g L−1 NaCl [18]. The culture was kept in 36 °C water bath for 48–72 h before being used as inoculum for subsequent batch and continuous cultures. Besides the standard P2 medium containing 30 g L−1 glucose, modified P2 medium with 50 g L−1 glucose was also prepared for both batch and continuous fermentations. Moreover, in another continuous fermentation experiment, the modified P2 medium containing 50 g L−1 glucose was further supplemented with sodium acetate as described later in the continuous fermentation section.
Batch fermentation
Batch fermentations were conducted using a 3 L New Brunswick BIOFLO® fermentor/bioreactor under a nitrogen flow at 8 mL min−1, with 150 rpm agitation at 36 °C. Two containers of the actively growing C. beijerinckii optinoii in 120 mL serum bottles were used as inoculum and added to freshly sterilized P2 medium containing 30 g L−1 glucose to make the total working volume of 1.5 L. A second batch fermentation was run with the same setup, but P2 medium contained 50 g L−1 glucose. During fermentations, 2 mL samples were collected at 0, 8, 16, 24, 36, 48, 72, and 96 h for substrate (glucose) and product (butanol, isopropanol, ethanol, acetic acid, butyric acid) analyses. A 6 mL sample was collected at the same time points for pH and absorbance (660 nm) measurement. Absorbance was measured using a Beckman and Coulter DU® 800 spectrophotometer. Each batch fermentation was run twice and the results were averaged.
Continuous fermentation using immobilized cell columns
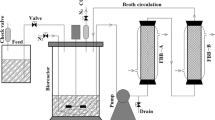
For continuous fermentation, a 400 mL jacketed immobilized cell column (50 × 300 mm jacketed, Ace Glass, Inc., Vineland, NJ, Cat. No. 5821-29) was sterilized by autoclaving. The porous ceramic Raschig rings (6 mm × 6 mm, Brewhaus Inc., Keller, TX) used as supports were washed twice with distilled water, autoclaved, and decanted into the sterilized column. The void volume of the column was 240 mL after filling. A sterile, one hundred gram per liter glucose solution was used to fill the column and it was set to stand for 72 h. The glucose solution was then drained from the column and two serum bottles of C. beijerinckii optinoii suspension (total 200 mL), at stationary phase containing little to no carbon source were poured into the column and allowed to incubate for 24 h. Then, sterilized 30 g L−1 glucose P2 solution with supplemental nutrients was fed continuously, by upflow, at 0.34 mL min−1, controlled by a Masterflex® pump (Model 77200-60). The spent media from the column was collected in a 4 L aspirator bottle with a bubbler air lock on top to maintain anaerobic conditions. The column was maintained at 36 °C using a temperature controlled circulating water bath. A 250 mL aspirator bottle was placed between the media reservoir and the pump to prevent back contamination from the column to the original reservoir. The setup schematic is shown in Fig. 1.
During fermentations, the effluent media were sampled between the column and the collection aspirator bottle through a Y-split tubing. Besides the standard 30 or 50 g L−1 glucose P2 media pumped at a dilution rate of 0.085 h−1 (flow rate of 0.34 mL min−1), 50 g L−1 glucose P2 media and 50 g L−1 glucose P2 media with 4.9 g L−1 sodium acetate trihydrate (CH3COONa·3H2O) supplementation were tested at a dilution rate of 0.050 h−1 (flow rate of 0.20 mL min−1) [14]. Conditions of all four continuous fermentations performed are listed in detail in Table 1. The starting pH of all media was adjusted to 6.5. All samples collected were filtered through 0.45 µm PTFE membrane before GC (gas chromatography) and HPLC (high performance liquid chromatography) analyses. All effluent samples collected were analyzed under microscope to monitor axenicity of the column.
Solvent and acid analyses
Solvents (butanol, isopropanol, and ethanol) and acids (acetic and butyric) were determined using an Agilent 7890A gas chromatography system equipped with a flame ionization detector. The column used was a Zebron™ ZB-WAXplus capillary one, 60 m × 0.25 mm ID with 0.25 µm film thickness. A split ratio of 40:1 was set up at injection with a constant flow of helium at 1.4 mL min−1. The oven temperature program (34.125 min per cycle) was set as: holding at 35 °C for 1 min, increasing at 10 °C min−1 up to 150 °C and holding for 10 min, increasing again at 10 °C min−1 up to 180 °C and holding for 5 min, and finally decreasing at 40 °C min−1 to 35 °C. A series of standards containing a mixture of butanol, isopropanol, ethanol, acetic acid, and butyric acid at known concentrations were prepared and run with fermentation samples. If the ethanol concentration was less than 5 % that of either isopropanol or butanol, it was not reported in results.
Glucose analysis
Glucose was analyzed using Agilent 1200 series HPLC equipped with a refractive index detector and a Bio-Rad Aminex® HPX-87 K column. Deionized water was the mobile phase running at 0.6 mL min−1. Column temperature was set at 85 °C for a 20 min run and each injection volume was 20 µL. A series of glucose solutions at known concentrations was prepared and used as standards in fermentation sample analysis.
Calculations of bioprocess parameters
For both batch and continuous fermentations, substrate utilization in percent was calculated by dividing the consumed glucose (initial minus remaining concentration) by the initial glucose concentration in input media. Solvent yield (g g−1) was calculated by dividing the increase in solvent concentration (only butanol and isopropanol) by the corresponding consumed glucose concentration. Isopropanol to butanol mass ratio was calculated by dividing the detectable concentration of these two solvents. For continuous culture, the dilution rate (h−1) was calculated by dividing the media flow rate by the void volume of the reactor. Solvent productivity (g L−1 h−1) was calculated by multiplying the solvent concentration (individual or total solvent) by the dilution rate. Solvent productivity in batch culture was calculated by dividing the solvent concentration by the entire duration of batch fermentation process of 96 h.
Results
Batch fermentation
The cell growth (measured by optical density at 660 nm), pH, substrate consumption and solvent production patterns for batch fermentations on 30 g L−1 glucose in P2 media are shown in Fig. 2. The lag phase of C. beijerinckii optinoii lasted about 8 h followed by a 24 h exponential growth phase, reaching an A660 of 2.0. The exponential growth started when the blue color of media disappeared, meaning an anaerobic environment was established. After an initial drop in pH from 5.8 to 5.1, pH fluctuated for 30 h and then stabled at 5.1.
Glucose as the carbohydrate source was consumed until 80.0 % of it was utilized with a residual glucose of 6.0 g L−1. Solvent production started with exponential cell growth, and by late stationary phase, solvent yield reached 0.39 g g−1 with a final solvent concentration being 9.45 g L−1 in the spent media. Butanol concentration was 6.24 g L−1, 66.0 % of the total solvent. The overall solvent productivity was 0.10 g L−1 h−1 and butanol productivity was 0.07 g L−1 h−1. The isopropanol: butanol mass ratio stayed consistent at 0.51 from 48 to 96 h. Final acetic acid concentration was 1.34 g L−1 and butyric acid was 0.46 g L−1. There was no significant acid accumulation observed during the entire culture period.
The changes of cell growth (measured by optical density at 660 nm), pH, glucose, and solvents during a batch fermentation using 50 g L−1 glucose in P2 media are illustrated in Fig. 3. The exponential growth phase lasted 16 h until growth reached an A660 of 1.9. There was a rapid drop in pH from 6.4 to 5.5 during the first 8 h prior to the start of exponential growth, then pH remained between 5.5 and 5.7 for 64 h before finally dropping to 5.2.
With a carbohydrate consumption of 45.3 %, 27.35 g L−1 glucose remained in the spent media at the end of the fermentation. Solvent production coincided with the start of exponential phase yielding a final solvent concentration of 8.05 g L−1 with a 0.36 g g−1 solvent yield. Butanol concentration was 5.02 g L−1 representing 62.4 % of the total solvents. The overall solvent productivity was 0.08 g L−1 h−1 and butanol productivity was 0.05 g L−1 h−1. The isopropanol: butanol mass ratio decreased from 0.89 at 16 h to 0.60 at 96 h. Final acetic acid concentration was 2.03 g L−1 and butyric acid was 1.19 g L−1.
Continuous fermentation
A summary of all bioprocess parameters calculated from continuous fermentations is presented in Table 1. There was no contamination in the production column as verified by microscopy.
When fed with P2 media (30 g L−1 glucose) at a dilution rate of 0.085 h−1, a C. beijerinckii optinoii immobilized cell column consumed 80.0 % of the input glucose, producing a solvent concentration of 12.14 g L−1 of which 63.0 % was butanol. Solvent yield and solvent productivity were 0.51 g g−1 and 1.03 g L−1 h−1, respectively. Butyric and acetic acids were not detected. When the glucose concentration was increased to 50 g L−1 at the same dilution rate, both acids accumulated with acetic acid being 3.00 g L−1 and butyric acid being 6.01 g L−1, and the effluent pH dropped to 4.58. Solvents were not produced. Glucose consumption was 30 % of the input. When the dilution rate was reduced to 0.050 h−1, increasing the residence time of feed, solvent production improved [12]. At 0.050 h−1 dilution rate, with 50 g L−1 glucose feed, substrate utilization was 52.2 % and solvent concentration reached 9.26 g L−1 of which 68.9 % was butanol. Solvent yield was 0.35 g g−1. Solvent productivity was 0.46 g L−1 h−1 and acid concentration was below 5.0 g L−1 of which 1.28 g L−1 was acetic and 3.37 g L−1 was butyric.
Addition of 4.9 g L−1 sodium acetate trihydrate as a buffering compound held the effluent pH at 5.32. Glucose utilization increased to 64.2 % and the glucose consumption was 32.1 g L−1. The solvent concentration was 12.70 g L−1 of which butanol was 8.17 g L−1 (64.3 % of total solvents). Solvent productivity was 0.64 g L−1 h−1, higher than previous with 50 g L−1 glucose media feed at the same dilution rate of 0.050 h−1 without acetate addition. Even with acetate supplementation, the final acetic acid of 2.22 g L−1 was lower than the acetic acid concentration detected when 50 g L−1 glucose was fed at a dilution rate of 0.085 h−1 as shown in Table 1. Meanwhile, butyric acid was 3.37 g L−1 with acetate addition.
Continuous fermentation runs reached steady state in 10–14 days (data not shown) except the run fed with 50 g L−1 glucose with a dilution rate of 0.050 h−1. Glucose, acid, solvent and pH profiles of this specific 50 g L−1 glucose run are illustrated in Fig. S1. All glucose, solvent and acid concentrations as well as pH presented in Table 1 are mean values obtained from 5-day readings at steady state conditions.
Overall, C. beijerinckii optinoii consumed 24 g L−1 (80 % of the 30 g L−1 glucose) in either batch or continuous fermentations. However, solvent concentration reached 12.14 g L−1 in continuous immobilized culture, higher than 9.45 g L−1 obtained in 96-h batch fermentation. When glucose concentration 50 g L−1 was used in continuous fermentation experiments at a dilution rate of 0.050 h−1, 52.2 % of glucose was utilized with a solvent concentration of 9.26 g L−1 compared to 45.3 % glucose utilization in batch cultures with 8.05 g L−1 solvents. With acetate added into the media, the pH remained above 5 in the continuous fermentation and the solvent productivity increased from 0.46 to 0.64 g L−1 h−1, compared to its unsupplemented counterpart. The highest solvent concentration of 12.70 g L−1 was reached with acetate addition.
Discussion
Clostridium beijerinckii optinoii is a novel Clostridium strain and a high butanol and isopropanol producer that produces small amounts of ethanol. This characteristic is beneficial for downstream solvent separations. Continuous fermentation with immobilized cells produced higher solvent concentrations with enhanced productivity compared to batch fermentations. Acetate supplementation to the feed improved the buffering capacity of the media which led to a higher solvent and butanol productivity.
For both batch and continuous fermentations, greater glucose utilization led to a higher solvent yield, indicating a more effective and robust glucose-to-solvent conversion and greater solvent productivity. In batch culture, solvent production initiated with exponential growth of C. beijerinckii optinoii, and productivity decreased as growth approached late stationary phase. The pH remained above 5 across the entire culture period following an initial drop at the start of the exponential growth, possibly due to dominant acid formation seen in the early stages of solvent synthesis in Clostridia. In continuous fermentation, pH decreased across the column from 6.5 in feed to 4.58–5.32 in the effluent. Solvent production was maintained when effluent pH was above 5. Acid accumulation without effective pH control inhibited solvent production. When dilution rate was not adjusted accordingly after a substantial increase in glucose in the feeding media, residence time of glucose in the column was too short, the solvent production was shut down and only acids were produced, resulting in acidogenesis without solventogenesis. For solventogenic Clostridia, such acid crash can significantly decrease the solvent yields [8, 10]. The cause of acid crash in ABE batch fermentation without any pH control is the high concentration of undissociated acids which accumulate in the culture broth [18]. No acid crash was detected in both batch fermentations in this study as the solvent production continued and the total undissociated acid concentration was well below the suggested threshold concentration of 57–60 mmol L−1 [19]. There was incomplete consumption of glucose in both batch and continuous fermentation using P2 media, and similar results were reported by Lee et al. [14] and Bankar et al. [20]. Besides being the main substrate, glucose was also an energy source as it provided the required reducing power for a series of enzymes involved in solventogenesis. Therefore, the residual glucose in spent media was necessary to meet the energy requirement of Clostridium cells and to ensure continuous solvent production through acid utilization [21].
The relatively high solvent yield reached in continuous fermentation on 30 g L−1 glucose can possibly be because of acetate as the co-substrate for solvent production. Acetate leads to the generation of acetyl-CoA, an intermediate compound in butanol fermentation pathway. If ammonium acetate in P2 media is fully converted to butanol, it can contribute to 1.08 g/L butanol. No acetate was detected in the final fermentation broth under this condition, indicating a complete conversion. In addition, getting a similarly high solvent/glucose yield has been extensively reported in other studies. For example, Survase et al. [12] set up a two-stage continuous fermentation of C. beijerinckii DSM 6423 to produce butanol and isopropanol and obtained a solvent yield of 0.53 g per g glucose in the second bioreactor. Meanwhile, no acetic or butyric acids were detected under their conditions, the same as when 0.51 g/g solvent/glucose yield was reached in this study. For ABE fermentations, yield at 0.44 g butanol per g glucose were found when fermenting immobilized C. beijerinckii NCIMB 8052 cells [14].
The continuous fermentation system operated for more than 120 days without contamination problems or Clostridium degeneration, which indicates potential technical and economic feasibility for larger scale applications. Similar process sustainability has been reported for other continuous butanol fermentation studies [14, 20, 22].
Butanol productivity increased with acetate supplementation to a value similar to that reported for butyrate supplementation, at a similar dilution rate for continuous ABE fermentations [14]. Acetate supplementation improved the buffering capacity of glucose media as the phosphate buffer system did not control the pH well over the range of 4.0–5.5. When the pH in the column was maintained between 5.3 and 5.4, the environment was suitable for effective solventogenesis. In addition, when acetate was added to the media, the sol operon, containing genes encoding a series of enzymes involved in solvent production pathway for Clostridium beijerinckii, was highly expressed [15]. Therefore, acetate can enhance solvent production and prevent the Clostridium species from degeneration through the enduring expression of the sol operon [15]. Acetate could also be a co-substrate for butanol production.
Mutschlechner et al. [22] reported on a design for continuous two-stage ABE fermentation where acidogenesis took place in the first reactor (first stage) followed by solventogenesis in a second reactor (second stage). However, in this study, both acidogenesis and solventogenesis took place in a single column when glucose concentration and feeding rate were balanced. Further studies on its design and parameter control will be very useful for process optimization in both continuous lab and pilot plant scale butanol fermentation.
Continuous fermentation with immobilized cells proved to be a technically and economically feasible approach to solvent production.
References
U.S. Energy Information Administration (2014) Annual energy outlook 2014. http://www.eia.gov/forecasts/aeo/pdf/0383(2014).pdf. Accessed 2 Mar 2015
Perlack RD, Wright LL, Turhollow AF, Graham RL, Stokes BJ, Erbach DC (2005) Biomass as feedstock for a bioenergy and bioproducts industry: the technical feasibility of a billion-ton annual supply. Oak Ridge National Laboratory, U.S. Department of Energy. https://www1.eere.energy.gov/bioenergy/pdfs/final_billionton_vision_report2.pdf. Accessed 23 Dec 2015
Yang Y, Sharma-Shivappa R, Burns JC, Cheng JJ (2009) Dilute acid pretreatment of oven-dried switchgrass germplasms for bioethanol production. Energy Fuel 23:3759–3766
Somerville C (2007) Biofuels. Curr Biol 17(4):R115–R119
Swana J, Yang Y, Behnam M, Thompson R (2011) An analysis of net energy production and feedstock availability for biobutanol and bioethanol. Bioresour Technol 102:2112–2117
Harvey BG, Quintana RL (2010) Synthesis of renewable jet and diesel fuels from 2-ethyl-1-hexene. Energy Environ Sci 3:352–357
Wu M, Wang M, Liu J, Huo H (2008) Assessment of potential life-cycle energy and greenhouse gas emission effects from using corn-based butanol as a transportation fuel. Biotechnol Progr 24(6):1204–1214
Dürre P (1998) New insights and novel developments in clostridial acetone/butanol/isopropanol fermentation. Appl Microbiol Biotechnol 49(6):639–648
Yan R-T, Zhu C-X, Golemboski C, Chen J-S (1988) Expression of solvent-forming enzymes and onset of solvent production in batch cultures of Clostridium beijerinckii. Appl Environ Microb 54(3):642–648
Ezeji TC, Qureshi N, Blaschek HP (2007) Bioproduction of butanol from biomass: from genes to bioreactors. Curr Opin Biotech 18:220–227
Terracciano JS, Kashket ER (1986) intracellular conditions required for initiation of solvent production by Clostridium acetobutylicum. Appl Environ Microb 52(1):86–91
Survase SA, Jurgens G, van Heiningen A, Granström T (2011) Continuous production of isopropanol and butanol using Clostridium beijerinckii DSM 6423. Appl Microbiol Biotechnol 91:1305–1313
Qureshi N, Schripsema J, Lienhardt J, Blaschek HP (2000) Continuous solvent production by Clostridium beijerinckii BA101 immobilized by adsorption onto brick. World J Microb Biotechnol 16:377–382
Lee S-M, Cho MO, Park CH, Chung Y-C, Kim JH, Sang B-I, Um Y (2008) Continuous butanol production using suspended and immobilized Clostridium beijerinckii NCIMB 8052 with supplementary butyrate. Energy Fuel 22:3459–3464
Chen C-K, Blaschek HP (1999) Acetate enhances solvent production and prevents degeneration in Clostridium beijerinckii BA 101. Appl Microbiol Biotechnol 52:170–173
Dürre P (2005) Formation of solvents in Clostridia. Handbook on Clostridia. Taylor & Francis Group, LLC, Boca Raton
Hoogewind A (2013) Production of 2-propanol, butanol and ethanol using Clostridium beijerinckii optinoii. Ph.D. Dissertation. Louisiana State University Electronic Thesis and Dissertation Collection etd-01162014-093302
Qureshi N, Blaschek HP (1999) Butanol recovery from model solution/fermentation broth by pervaporation: evaluation of membrane performance. Biomass Bioenergy 17(2):175–184
Maddox IS, Steiner E, Hirsch S, Wessner S, Gutierrez NA, Gapes JR, Schuster KC (2000) The cause of “acid crash” and “acidogenic fermentations” during the batch acetone-butanol-ethanol (ABE-) fermentation process. J Mol Microbiol Biotechnol 2:95–100
Bankar SB, Survase SA, Ojamo H, Granström T (2013) The two stage immobilized column reactor with an integrated solvent recovery module for enhanced ABE production. Bioresour Technol 140:269–276
Tashiro Y, Takeda K, Kobayashi G, Sonomoto K, Ishizaki A, Yoshino S (2004) High butanol production by Clostridium saccharoperbutylacetonicum N1-4 in fed-batch culture with pH-stat continuous butyric acid and glucose feeding method. J Biosci Bioeng 98(4):263–268
Mutschlechner O, Swoboda H, Gapes JR (2000) Continuous two-stage ABE-fermentation using Clostridium beijerinckii NRRL B592 operating with a growth rate in the first stage vessel close to its maximal value. J Mol Microbiol Biotechnol 2(1):101–105
Acknowledgments
This research was supported by a USDA Agriculture and Food Research Initiative Competitive Grant Award No. 2011-69005-30515. The authors appreciated the technical support with GC analysis by Dr. Derek Dorman at Audubon Sugar Institute of LSU AgCenter.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
pH, solvent, glucose and acid concentrations during continuous fermentation with immobilized C. beijerinckii optinoii on 50 g L−1 glucose P2 media at a dilution rate of 0.05 h−1 (TIFF 1894 kb)
Rights and permissions
About this article
Cite this article
Yang, Y., Hoogewind, A., Moon, Y.H. et al. Production of butanol and isopropanol with an immobilized Clostridium . Bioprocess Biosyst Eng 39, 421–428 (2016). https://doi.org/10.1007/s00449-015-1525-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1525-1