Abstract
The genes maeA and maeB, encoding NADH- and NADPH-dependent malic enzymes, have been deleted in a recombinant Escherichia coli strain with inactivated mixed-acid fermentation pathways and a modified system of glucose transport and phosphorylation upon the heterological expression of the pyruvate carboxylase gene. During anaerobic glucose utilization, the parental strain synthesized malic, fumaric, and succinic acids as the main fermentation end products, while pyruvic acid was accumulated as the main by-product resulting from the functioning of the pyruvate–oxaloacetate–malate–pyruvate futile cycle. Upon individual deletions of the maeA and maeB genes, the mutant strains converted glucose into four-carbon dicarboxylic acids with increased efficiency still secreting notable amounts of pyruvic acid. The combined inactivation of both malic enzymes in the constructed strain significantly elevated the portion of malic, fumaric, and succinic acids among the fermentation end products with a concomitant decrease in the secretion of pyruvic acid and other by-products due to the abolishment of the action of the futile cycle competing with the target biosynthetic processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Four-carbon dicarboxylic acids, in particular, malic, fumaric, and succinic, are industrially important chemicals that can serve as universal building blocks in the organic synthesis of a wide range of high value-added products [1]. At present, these acids are synthesized petrochemically. However, being conservative intermediates of the metabolism of huge variety of organisms, the corresponding compounds can be produced by microbial synthesis from renewable plant raw materials that can represent an ecologically reasonable alternative to traditional resource- and energy-consuming processes.
Aspergillus flavus, Aspergillus niger [2], Rhizopus oryzae [3], Actinobacillus succinogenes [4], Anaerobiospirillum succiniciproducens [5], and Mannheimia succiniciproducens are natural producers of malic, fumaric, and succinic acids [6]. However, nowadays the characteristics of known natural producers [7] cannot ensure economic feasibility for bio-based production of the corresponding dicarboxylates at industrial scale. At the same time, due to the convenience and availability of precise genetic editing tools, significant progress has been achieved in the development of recombinant producers of the respective compounds using Escherichia coli as a biosynthetic chassis [8].
The facultative anaerobic bacterium E. coli can form malic, fumaric, and succinic acids during carbon substrate utilization under both anaerobic and aerobic conditions. In the absence of oxygen, the reactions of the reductive branch of the tricarboxylic acid (TCA) cycle serve in E. coli as the main pathway of the biosynthesis of these acids whereas upon aeration the respective compounds are synthesized through the entire oxidative TCA cycle. The anaerobic synthesis of four-carbon dicarboxylates is more advantageous, since it can ensure a high yield of the target products due to CO2 fixation of at the stage of oxaloacetate (OAA) formation, the key precursor metabolite of the reductive branch of the TCA cycle.
Nevertheless, up to date the anaerobic conversion of glucose, the traditional substrate of microbial biotechnology, to the target dicarboxylic acid with yield values close to the theoretical maximum has been achieved using engineered E. coli strains only in the case of succinic acid, whose efficient biosynthesis implies participation of not only the reductive branch of the TCA cycle, but also the reactions of the glyoxylate shunt (GS) [9‒11]. Thus, the development of approaches for the construction of highly effecient microbial producers of not only succinic but also malic and fumaric acids remains an actual task.
Standard approaches aimed to ensure efficient anaerobic production of the four-carbon dicarboxylic acids in E. coli include increasing intracellular OAA availability for target biosynthetic reactions of the reductive branch of the TCA cycle. The corresponding effect is usually achieved resulting from the overexpression of genes encoding anaplerotic enzymes [12, 13]. The key bacterial anaplerotic enzymes responsible for the formation of the OAA from glycolytic precursors include phosphoenolpyruvate (PEP)-carboxylating PEP carboxylase (EC 4.1.1.31) and PEP carboxykinase (EC 4.1.1.49) as well as pyruvate carboxylase (EC 6.4.1.1), which uses pyruvic acid as a substrate [14].
E. coli cells possess both PEP carboxylating enzymes, whereas pyruvate carboxylase activity is absent in this bacterium. Consequently, the presence of heterologous pyruvate carboxylase activity increases the flexibility and efficiency of anaplerotic processes in recombinant E. coli strains [14, 15]. However, it was shown that the heterologous expression of the pyruvate carboxylase gene in E. coli strains engineered for the anaerobic conversion of glucose into four-carbon dicarboxylic acids provoked the appearance of a pyruvate-OAA-malate-pyruvate futile cycle, caused by the decarboxylating activity of cellular malic enzymes (EC 1.1.1.39/40) [16].
It is obvious that the functioning of the revealed futile cycle, wastefully consuming pyruvic and oxaloacetic acid, is competitive with the biosynthesis of the target dicarboxylates through the reductive branch of the TCA cycle, and may affect the efficiency of their anaerobic production from glucose.
The goal of the work was to study the effect of the inactivation of malic enzymes on the anaerobic production of four-carbon dicarboxylic acids by recombinant Escherichia coli strains expressing pyruvate carboxylase.
MATERIALS AND METHODS
Bacterial Strains, Plasmids, and Media
Table 1 presents the strains, plasmids, and primers used in the work.
The E. coli strain K-12 MG1655 (VKPM B-6195) and the previously constructed E. coli strain MSG1.0 ΔfrdAB ΔpflB [17] (designated as FP), possessing a modified system of glucose transport and phosphorylation, deleted pyruvate formate lyase (EC 2.3.1.54), and inactivated pathways of mixed acid fermentation, were used as parent strains for the consrtuction of all strains obtained in the work. Bacteria were cultured in rich media LB, SOB, and SOC, as well as in minimal M9 medium [18] with the addition of 100 μg/mL ampicillin (Synthesis, Russia) or 30 μg/mL chloramphenicol (Sigma, United States) if necessary.
Reagents
Taq-DNA polymerase (Thermo Scientific, Lithuania) was used in the work. Oligonucleotide primers (Table 1) were synthesized by Evrogen (Russia). Nutrient media components, salts, and other reagents were from Panreac (Spain) and Sigma (United States). PCR products were purified by agarose gel electrophoresis and isolated with a QIAquick Gel Extraction Kit (Qiagen, United States).
Construction of Strains and Plasmids
All chromosomal modifications were performed with a modified [19] method developed by Datsenko and Wanner [20]. Linear DNA fragments for inactivation of the maeA and maeB genes containing the chloramphenicol resistance marker (cat gene) were obtained by PCR with two primer pairs (P1, P2 and P3, P4) and pMW118-(λattL-Cm-λattR) plasmid [19] as a template (see Table 1). The resulting DNA fragments were individually integrated into the chromosome of E. coli strain MG1655, carrying the pKD46 helper plasmid [20]. The correspondence between the expected and experimentally obtained chromosome structures of the selected strains with individually inactivated maeA and maeB genes was confirmed by PCR with pairs of locus-specific primers: P5, P6 and P7, P8.
Strains FP ΔmaeA, FP ΔmaeB, and FP ΔmaeAΔmaeB were obtained by the introduction of the individual modifications into the chromosome of the FP strain via P1-dependent transduction [18]. Removal of the marker, flanked by att-sites of lambda phage, from the chromosome of target strains was carried out using pMWts-Int/Xis plasmid as described previously [21]. Transformation of the strains with the pPYC plasmid [22] was carried out according to the standard procedure.
Cultivation of Strains for the Biosynthesis of Dicarboxylic Acids
The strains FP [pPYC], FP ΔmaeA [pPYC], FP ΔmaeB [pPYC], and FP ΔmaeA ΔmaeB [pPYC] were grown overnight in M9 medium containing 2 g/L glucose at 37°C. Five milliliters of the overnight culture was diluted ten times with 45 mL of M9 medium containing 10 g/L of glucose and 10 g/L of yeast extract. The resulting cultures were grown in 750-mL flasks at 37°C on a rotary shaker at 250 rpm for 8 h and then were centrifuged for 15 min at 2000 g and 4°C. The pellets were resuspended in 15 mL of M9 medium containing 10 g/L of glucose and 10 g/L of NaHCO3. Subsequently, the cultures were incubated in 15 mL tubes closed with screw caps on a rotary shaker (250 rpm) for 24 h at 37°C. All media additionally contained 100 μg/mL ampicillin.
The cell suspensions were then centrifugated at 10 000 g for 10 min, and the concentrations of secreted metabolites and residual glucose were determined in the resulting supernatants. All experiments were repeated at least three times, and the results of the repeated experiments varied in a range not exceeding 10%.
Analytical Methods
The concentrations of organic acids in culture liquids freed from the biomass by centrifugation were determined by HPLC with the Waters HPLC system (Waters, United States). An ion-exclusion column Rezex ROA-Organic Acid H + (8%), 300 × 7.8 mm, 8 μm (Phenomenex United States) was used with detection at a wavelength of 210 nm. An aqueous solution of sulfuric acid (2.5 mM) was used as the mobile phase at a flow rate of 0.5 mL/min. For the glucose measurements, the system was equipped with a Waters 2414 refractive index detector and a Spherisorb-NH2 column, 4.6 × 250 mm, 5 μm (Waters). An acetonitrile-water mixture (volume ratio 75 : 25) served as the mobile phase at a flow rate of 1 mL/min.
RESULTS AND DISCUSSION
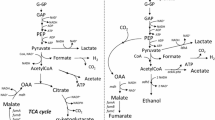
The FP strain was used as the chasis to study the effect of the inactivation of malic enzymes on the anaerobic production of four-carbon dicarboxylic acids by recombinant Escherichia coli strains expressing pyruvate carboxylase (see Table 1). This strain was previously engineered for the anaerobic production of malic, fumaric, and succinic acids from glucose through the reductive branch of the TCA cycle. The main pathways of mixed-acid fermentation in the strain were inactivated by the deletion of the ackA, pta, poxB, ldhA, and adhE genes, which encode key E. coli enzymes responsible for the formation of acetic acid, lactic acid, and ethanol.
The intracellular availability of PEP, the substrate of cellular OAA-synthesizing anaplerotic enzymes, was increased due to PEP-independent glucose transport and phosphorylation. The formation of the full range of four-carbon intermediates of the TCA cycle, malic, fumaric, and succinic acids, was ensured resulting from the deletion of frdAB genes, which encode the catalytic components of the fumarate reductase complex (EC 1.3.5.4). Anaerobic biosynthesis of the respective metabolites in FP strain and its derivatives suggested, therefore, the channelling of OAA through a series of residual fermentation reactions, including, in particular, the conversions catalyzed by malate dehydrogenase (EC 1.1.1.37), fumarases ( EC 4.2.1.2), and aerobically synthesized succinate dehydrogenase (EC 1.3.5.1) [16, 17]. At the same time, the contribution of GS reactions to the biosynthesis of malic and succinic acids in the strain was decreased due to inactivation of the pflB gene, which encodes pyruvate formate lyase (EC 2.3.1.54), the main enzyme providing the E. coli cell with acetyl-CoA under anaerobiosis.
During anaerobic utilization of glucose, the FP [pPYC] strain expressing pyruvate carboxylase of B. subtilis synthesized malic, fumaric, and succinic acids with the molar yields of about 0.11, 0.02, and 0.76 mol/mol, respectively (Fig. 1).
At the same time, the portion of four-carbon metabolites among fermentation products formed by the strain was only 57.4% (Table 2), and pyruvic acid was secreted as the main by-product (see the Fig. 1).
Considerable secretion of pyruvic acid by the strain was resulted from the functioning of the previously revealed pyruvate-OAA-malate-pyruvate futile cycle mediated by the action of cellular malic enzymes [16]. E. coli possesses two malic enzymes, NADH-dependent MaeA (EC 1.1.1.39) and NADPH-dependent MaeB (EC 1.1.1.40) [23]. The NADH-dependent MaeA could presumably served as the main malic enzyme responsible for the functioning of the futile cycle. Indeed, the basal activity of the NADH-dependent malic enzyme in E. coli is relatively high [24]. Moreover, besides the reports on the positive or neutral effect of maeA gene overexpression on the anaerobic production of succinic acid by E. coli strains [25], an increased biosynthesis of intermediates of the reductive branch of the TCA cycle caused by the inactivation of the corresponding gene has also been demonstrated [26, 27]. Thus, the gene encoding the NADH-dependent malic enzyme was initially inactivated in the FP [pPYC] strain.
During the anaerobic glucose utilization, the resulting strain FP ΔmaeA [pPYC] synthesized malic acid with a yield of ~0.2 mol/mol, increased mainly at the expense of pyruvate and lactate secretion (see Fig. 1). The yields of fumaric and succinic acids were also slightly increased, and the total fraction of the four-carbon dicarboxylates formed by the strain reached 65.7% of all fermentation products (see Table 2). Thus, the inactivation of the NADH-dependent malic enzyme reliably decreased the activity of the pyruvate-OAA-malate-pyruvate futile cycle in the FP ΔmaeA [pPYC] strain, leading to relatively enhanced synthesis of the target four-carbon dicarboxylic acids. However, the distribution of the metabolites synthesized by the strain indicated that the glycolytic carbon flux was not completely channeled toward the reductive branch of the TCA cycle. At the same time, continued notable production of lactic acid indicated the inability of the strain to efficiently reoxidize reducing equivalents exclusively via the NADH-dependent reactions of malic, fumaric, and succinic acid formation. The secretion of lactic acid by strains lacking fermentative lactate dehydrogenase LdhA (EC 1.1.1.28) indicated activation of the respiratory lactate dehydrogenases Dld (EC 1.1.5.12) and/or LldD to compensate for the insufficiency of fermentation processes for the maintenance of the intracellular redox balance. Nevertheless, the contribution of respiratory enzymes to the formation of reduced products of anaerobic glucose utilization by the FP ΔmaeA [pPYC] strain decreased compared to the parent FP [pPYC] strain, and the portion of the four-carbon metabolites among these compounds reached 84.8% (Table 2).
Accordingly, it could not be excluded that NADPH-dependent malic enzyme contributed to the functioning of pyruvate-OAA-malate-pyruvate futile cycle to match the requirements for the maintenance of intracellular NADH/NADPH balance during anaerobiosis. To verify this assumption, the gene encoding this enzyme was also inactivated in the core producing strain.
The profile of metabolites synthesized by the resulting strain FP ΔmaeB [pPYC] during anaerobic glucose utilization was similar to that of the FP ΔmaeA [pPYC] strain (Fig. 1).
The molar yield of malic, fumaric, and succinic acids was slightly lower than that of the FP ΔmaeA [pPYC] strain, exceeding, nevertheless, the value demonstrated by the parent FP [pPYC] strain and indicating a negative impact of NADPH-dependent malic enzyme activity on the anaerobic formation of four-carbon intermediates of the TCA cycle.
Thus, both genes encoding malic enzymes were inactivated in the core strain. The production of pyruvic and lactic acids synthesized by the FP ΔmaeA ΔmaeB [pPYC] strain during anaerobic glucose utilization sharply decreased, not only when compared to the core strain FP [pPYC], but also with control strains FP ΔmaeA [pPYC] and FP ΔmaeB [pPYC] (see Fig. 1). The secretion of malic acid by the strain concomitantly increased, and the molar yield of this dicarboxylate from glucose reached ~0.36 mol/mol. The yield of fumaric acid raised up to two times (Fig. 1). As a result, though the level of succinic acid synthesis was almost unchanged, the portion of the target four-carbon dicarboxylates among the fermentation products formed by the FP ΔmaeA ΔmaeB [pPYC] strain increased to 77.6%, while their portion among the reduced metabolites reached 91.5% (Table 2). This result indicates that the abolishment of the functioning of the of pyruvate-OAA-malate-pyruvate futile cycle allowed the FP ΔmaeA ΔmaeB [pPYC] strain to efficiently reoxidize the glycolically formed NADH in the reactions of the reductive branch of the TCA cycle upon the enhanced formation of the required precursor (OAA) by the combined action of both PEP- and pyruvate-carboxylating anaplerotic enzymes. Since the respective fermentation process is directly coupled with the biosynthesis of malic, fumaric, and succinic acids, the opening of the futile cycle eventually led to increased anaerobic synthesis of the target dicarboxylates by the constructed strain.
Residual secretion of pyruvic, lactic, and acetic acids by the strain was apparently caused the suboptimal distribution of carbon fluxes in the metabolic node PEP-pyruvic acid-acetyl-CoA. Efficient coordination between the reactions constituting the corresponding metabolic node, favorable for OAA formation, can be further achieved by the overexpression of the ppc and/or pckA genes, which code for phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase, and by diminishing of the pyruvate to acetyl-CoA conversion by pyruvate dehydrogenase.
CONCLUSIONS
The results of this study indicate that the inactivation of malic enzymes promotes the synthesis of four-carbon dicarboxylic acids through the reductive branch of the TCA cycle in engineered E. coli strains upon overexpression of OAA-forming pyruvate carboxylase, and it could be considered an obligatory requirement for enabling an efficient redox balanced anaerobic production of target compounds from glucose.
REFERENCES
Werpy, T., Petersen, G., Aden, A., Bozell, J., Holladay, J., White, J., Manheim, A., Eliot, D., Lasure, L., and Jones, S., Top Value Added Chemicals from Biomass, vol. 1: Results of Screening for Potential Candidates from Sugars and Synthesis Gas, Washington DC, USA: Pacific Northwest National Laboratory, National Renewable Energy Laboratory and Department of Energy, 2004.
West, T.P., Malic acid production from thin stillage by Aspergillus species, Biotechnol. Lett., 2011, vol. 33, no. 12, pp. 2463– 2467. doi 10.1007/s10529-011-0720-7
Roa, EngelC.A., Straathof, A.J., Zijlmans, T.W., et al., Fumaric acid production by fermentation, Appl. Microbiol. Biotechnol., 2008, vol. 78, no. 3, pp. 379–389. doi 10.1007/s00253-007-1341-x
Guettler, M.V., Rumler, D., and Jain, M.K., Actinobacillus succinogenes sp. nov., a novel succinic-acid-producing strain from the bovine rumen, Int. J. Syst. Bacteriol., 1999, vol. 49, no. 1, pp. 207–216. doi 10.1099/00207713-49-1-207
Nghiem, N.P., Davison, B.H., Suttle, B.E., and Richardson, G.R., Production of succinic acid by Anaerobiospirillum succiniciproducens, Appl. Biochem. Biotechnol., 1997, vol. 63-65, pp. 565–576. doi 10.1007/bf02920454
Lee, P.C., Lee, S.Y., Hong, S.H., and Chang, H.N., Isolation and characterization of a new succinic acid-producing bacterium, Mannheimia succiniciproducens MBEL55E, from bovine rumen, Appl. Microbiol. Biotechnol., 2002, vol. 58, no. 5, pp. 663–668. doi 10.1007/s00253-002-0935-6
Yin, X., Li, J., Shin, H.D., et al., Metabolic engineering in the biotechnological production of organic acids in the tricarboxylic acid cycle of microorganisms: advances and prospects, Biotechnol. Adv., 2015, vol. 33, no. 6, pp. 830–841. doi 10.1016/j.biotechadv.2015.04.006
Liu, J., Li, J., Shin, H.D., et al., Protein and metabolic engineering for the production of organic acids, Bioresour. Technol., 2017, vol. 239, pp. 412–421. doi 10.1016/j.biortech.2017.04.052
Vemuri, G.N., Eiteman, M.A., and Altman, E., Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli, Appl. Environ. Microbiol., 2002, vol. 68, no. 4, pp. 1715–1727. doi 10.1128/AEM.68.4.1715-1727.2002
Sanchez, A.M., Bennett, G.N., and San, K.Y., Novel pathway engineering design of the anaerobic central metabolic pathway in Escherichia coli to increase succinate yield and productivity, Metab. Eng., 2005, vol. 7, no. 3, pp. 229–239. doi 10.1016/j.ymben.2005.03.001
Skorokhodova, A.Y., Morzhakova, A.A., Gulevich, A.Y., and Debabov, V.G., Manipulating pyruvate to acetyl-CoA conversion in Escherichia coli for anaerobic succinate biosynthesis from glucose with the yield close to the stoichiometric maximum, J. Biotechnol., 2015, vol. 214, pp. 33–42. doi 10.1016/j.jbiotec.2015.09.003
Cao, Y., Cao, Y., and Lin, X., Metabolically engineered Escherichia coli for biotechnological production of four-carbon 1,4-dicarboxylic acids, J. Ind. Microbiol. Biotechnol., 2011, vol. 38, no. 6, pp. 649–656. doi 10.1007/s10295-010-0913-4
Thakker, C., Martinez, I., Li, W., et al., Metabolic engineering of carbon and redox flow in the production of small organic acids, J. Ind. Microbiol. Biotechnol., 2015, vol. 42, no. 3, pp. 403–422. doi 10.1007/s10295-014-1560-y
Sauer, U. and Eikmanns, B.J., The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria, FEMS Microbiol. Rev., 2005, vol. 29, no. 4, pp. 765–794. doi 10.1016/j.femsre.2004.11.002
Gokarn, R.R., Evans, J.D., Walker, J.R., et al., The physiological effects and metabolic alterations caused by the expression of Rhizobium etli pyruvate carboxylase in Escherichia coli, Appl. Microbiol. Biotechnol., 2001, vol. 56, nos. 1–2, pp. 188–195. doi 10.1007/s002530100661
Skorokhodova, A.Yu., Stasenko, A.A., Gulevich, A.Yu., and Debabov, V.G., Effect of anaplerotic pathways activation on CO2-dependent anaerobic glucose utilization by Escherichia coli strains deficient in the main pathways of mixed acid fermentation, Appl. Biochem. Microbiol., 2018, vol. 54, no. 2, pp. 141–148.
Skorokhodova, A.Yu., Gulevich, A.Yu., and Debabov, V.G., Effect of extra- and intracellular sources of CO2 on anaerobic utilization of glucose by Escherichia coli strains deficient in carboxylation-independent fermentation pathways, Appl. Biochem. Microbiol., 2017, vol. 53, no. 3, pp. 304–309. doi 10.7868/S0555109917030151
Sambrook, J., Fritsch, E., and Maniatis, T., Molecular Cloning: A Laboratory Manual, New York, USA: Cold Spring Harbor Laboratory Press, 1989, 2nd ed.
Katashkina, Zh.I., Skorokhodova, A.Yu., Zimenkov, D.V., et al., Tuning the expression level of a gene located on a bacterial chromosome, Mol. Biol. (Moscow), 2005, vol. 39, no. 5, pp. 719–726.
Datsenko, K.A. and Wanner, B.L., One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products, Proc. Natl. Acad. Sci. U. S. A., 2000, vol. 97, no. 12, pp. 6640–6645. doi 10.1073/pnas.120163297
Gulevich, A.Yu, Skorokhodova, A.Yu., Ermishev, V.Yu., et al., A New method for the construction of translationally coupled operons in a bacterial chromosome, Mol. Biol. (Moscow), 2009, vol. 43, no. 3, pp. 505–514.
Skorokhodova, A.Yu., Gulevich, A.Yu., Morzhakova, A.A., et al., Anaerobic Synthesis of succinic acid by recombinant Escherichia coli strains with activated NAD+-reducing pyruvate dehydrogenase complex, Appl. Biochem. Microbiol., 2011, vol. 47, no. 4, pp. 373–380.
Bologna, F.P., Andreo, C.S., and Drincovich, M.F., Escherichia coli malic enzymes: two isoforms with substantial differences in kinetic properties, metabolic regulation, and structure, J. Bacteriol., 2007, vol. 189, no. 16, pp. 5937–5946. doi 10.1128/JB.00428-07
Kwon, Y.D., Kwon, O.H., Lee, H.S., and Kim, P., The effect of NADP-dependent malic enzyme expression and anaerobic C4 metabolism in Escherichia coli compared with other anaplerotic enzymes, J. Appl. Microbiol., 2007, vol. 103, no. 6, pp. 2340–2345. doi 10.1111/j.1365-2672.2007.03485.x
Hoefel, T., Faust, G., Reinecke, L., et al., Comparative reaction engineering studies for succinic acid production from sucrose by metabolically engineered Escherichia coli in fed-batch-operated stirred tank bioreactors, Biotechnol. J., 2012, vol. 7, no. 10, pp. 1277–1287. doi 10.1002/biot.201200046
Jantama, K., Zhang, X., Moore, J.C., et al., Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C, Biotechnol. Bioeng., 2008, vol. 101, no. 5, pp. 881–893. doi 10.1002/bit.22005
Zhang, X., Wang, X., Shanmugam, K.T., and Ingram, L.O., L-malate production by metabolically engineered Escherichia coli, Appl. Environ. Microbiol., 2011, vol. 77, no. 2, pp. 427–434. doi 10.1128/AEM.01971-10
ACKNOWLEDGMENTS
The work was supported by a grant from the Russian Science Foundation (project 16-14-10389).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by P. Kuchina
Rights and permissions
About this article
Cite this article
Skorokhodova, A.Y., Gulevich, A.Y. & Debabov, V.G. Inactivation of Malic Enzymes Improves the Anaerobic Production of Four-Carbon Dicarboxylic Acids by Recombinant Escherichia coli Strains Expressing Pyruvate Carboxylase. Appl Biochem Microbiol 54, 849–854 (2018). https://doi.org/10.1134/S0003683818090065
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683818090065



