Abstract
Bioethanol (fuel alcohol) has been produced by industrial alcoholic fermentation processes in Brazil since the beginning of the twentieth century. Currently, 432 mills and distilleries crush about 625 million tons of sugarcane per crop, producing about 27 billion liters of ethanol and 38.7 million tons of sugar. The production of bioethanol from sugarcane represents a major large-scale technology capable of producing biofuel efficiently and economically, providing viable substitutes to gasoline. The combination of immobilization of CO2 by sugarcane crops by photosynthesis into biomass together with alcoholic fermentation of this biomass has allowed production of a clean and high-quality liquid fuel that contains 93% of the original energy found in sugar. Over the last 30 years, several innovations have been introduced to Brazilian alcohol distilleries resulting in the improvement of plant efficiency and economic competitiveness. Currently, the main scientific challenges are to develop new technologies for bioethanol production from first and second generation feedstocks that exhibit positive energy balances and appropriately meet environmental sustainability criteria. This review focuses on these aspects and provides special emphasis on the selection of new yeast strains, genetic breeding, and recombinant DNA technology, as applied to bioethanol production processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brazil is the most competitive producer of bioethanol in the world with a well-developed domestic market increasingly stimulated by growing sales of flex fuel cars (Ibeto et al. 2011). The production of bioethanol has been supported by the development of new sugarcane varieties, favorable weather, fertile soils, and agricultural technologies (Leite et al. 2009). However, until 1975, bioethanol production was marginal, totaling less than 600 million liters per year. Two years after the world oil crisis of 1973, the Brazilian government launched the “ProAlcool” program with the aim of reducing high fuel prices and the dependency on oil imports (Zanin et al. 2000; Amorim and Lopes 2005; Goldemberg 2008; Macedo et al. 2008). Initially, the priority was to produce anhydrous bioethanol to be mixed with gasoline (Amorim and Leão 2005). After the 1979 world oil crisis, the main challenge was to initiate the manufacture of ethanol-powered vehicles. The success of bioethanol production stimulated the production of cars that ran on hydrated ethanol, which culminated in an increase of such vehicles in Brazil to reach 98%. In the 1980s, oil prices decreased, and the Brazilian government removed some subsidies given to the distilleries encouraging several of them to also produce sugar.
To overcome the low prices in the market and its costs of production, the sugar and alcohol industries improved their fermentation processes. New technologies were developed and transferred to distilleries allowing them to survive different crises over the last 20 years (Amorim 2006). The incorporation of these innovative technologies played a key role in increasing industrial efficiency as well as reducing the environmental impact of these distilleries. In 2008, bioethanol consumption as a fuel surpassed gasoline in Brazil (Chaddad 2010). Currently, more than 95% of all cars sold in Brazil are “flex-fuel” (cars that can use any blend of gasoline and ethanol; BNDES, CGEE 2008). Gasoline sold in Brazil now contains 25% anhydrous bioethanol. The percentage of bioethanol in gasoline is controlled by the Brazilian government according to market demand, strategic stores of bioethanol, and price. The expansion of ethanol consumption due to the growing fleet of light vehicles, mainly flex fuel cars (Porto et al. 2009; Chaddad 2010), and increased exports has opened new opportunities for industrial growth with several trading groups investing in Brazilian distilleries. However, the success of these industries depends on how they overcome the new scientific challenges faced. This review will focus on first and second generation bioethanol production and sustainability.
A description of the current fermentation process
The Brazilian National Company of Food Supply estimated that 432 Brazilian mills and distilleries crushed a total of 625 million tons of sugarcane and produced 27 billion liters of bioethanol as well as 38.7 million tons of sugar in 2010 (Porto et al. 2011; http://www.conab.gov.br/conabweb/download/safra/boletim_cana_ingles.pdf).
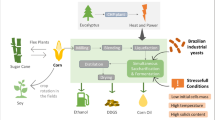
The actual process of fermentation was developed in the 1930s by Firmino Boinot in the French distilleries of the Melle region, and the technology was patented in 1937 (see Fig. 1). In the last 30 years, this process was improved to allow Brazilian distilleries to achieve fermentation yields of 92–93% compared with 75–80% recorded at the beginning of the “ProAlcool” program. This yield refers to ethanol production from sugar; however, the yeast cells also produce glycerol, cellular biomass, succinate, and malate during the alcoholic fermentation. For this reason, industrial processes of alcoholic fermentation may achieve 92–93% of the theoretical yield, while the other 7–8% is directed towards cellular metabolism. The Brazilian process of bioethanol production is characterized by industrial fermentation in very large tanks (0.5 to 3 million liters) and high yeast cell densities (10–15% w/v). Around 85% of these distilleries are fed-batch processes, and just 15% are continuous fermentation processes (Godoy et al. 2008). These fermentations are carried out with musts of sugarcane juice, water-diluted molasses, or a mix of both within a short period of 6–12 h. At the end of fermentation, the alcohol concentration reaches 7–11% (v/v), and residual sugar levels left in the wine remain below 0.1%. Then, the fermented must is centrifuged for separation of yeast and wine. The wine goes to the distillation step while concentrated (viscous suspension) yeast cells receive a treatment with sulfuric acid (pH 2.0–2.5) that aims to reduce bacterial contamination (Fig. 1). After 2–3 h, the yeast cells return to fermentation tanks to start a new cycle (Fig. 1; Wheals et al. 1999; Amorim et al. 2010). For this reason, very large amounts of yeast cell biomass are recycled by the distilleries every day. This process takes 200 to 300 days, depending on various factors, including: the region, weather conditions, sugarcane variety, and market demands. Yeast cells are recycled 400 to 600 times during sugarcane harvest season, which occurs from April to November or September to March in Brazil South–Center or Northeast regions, respectively.
Simplified illustration showing bioethanol production and the main scientific challenges. a Introduction of new feedstocks. b Production of second generation bioethanol from bagasse. c Selection of new yeast strains more adapted to stressing conditions of industrial fermentations. d Bacterial and yeast contamination in the fermentation process with cell recycling. e Reduction of vinasse volume
Tolerance to recycling and stressful conditions
Recycling results in the yeast cells being constantly submitted to stressful conditions such as temperature, ethanol, low pH, inhibitory compounds from molasses, metabolites produced by bacteria, excess of salts, mineral deficiency, and others (Basso et al. 2008). The comprehension of how these parameters affect the yeast cells and the fermentation process represents the first challenge. Nowadays, only a handful of laboratories are able to reproduce properly these conditions at bench scale. It is very difficult to understand how the yeast cells respond to the stressful conditions of industrial fermentation without precisely reproducing these conditions. Thus, the results of investigations about other fermentation processes of bioethanol production (from corn, wheat, sugar beet molasses as well as fermentations to produce wine, beer, sake, whisky, and other fermented beverages) cannot be directly transferred to Brazilian distilleries because the conditions are completely different (Amorim and Lopes 2004).
Yeast cell recycling represents an advantage to the industry because the reuse of living cell biomass saves sugar and increases fermentation yield. Instead of the yeast converting the sugar into the cellular biomass, more sugar is converted into ethanol than other processes without yeast recycling (Amorim and Lopes 2005). For this reason, other processes worldwide that do not recycle yeast cells cannot compete with Brazilian distilleries because of their low fermentation yields. Brazilian distilleries take advantage not only of sugarcane as a feedstock but also industrial fermentation that prioritizes yield and very short fermentation times (Basso et al. 2008).
It is generally accepted that alcoholic fermentation is a well-known and mastered procedure. However, the knowledge about the yeast responses to stress under the conditions adopted by Brazilian distilleries is still largely unknown. Industrial fermentations with cell recycling and continuous exposure of the yeast cells to stressing agents represent another challenge (Amorim et al. 2009). Many of these stressing factors simply do not occur in fermentative processes other than Brazilian distilleries.
Bacterial and yeast contamination
Industrial fermentations are subjected to contamination by bacteria and wild yeast (Saccharomyces and non-Saccharomyces species). Both contaminants compete with selected yeast strains to survive in the fermentors (Cabrini and Gallo 1999). Successive recycling of tons of yeast cells every day and the difficulties to sterilize large volumes of juice and water allow contaminating microorganisms to enter into the process, competing with the selected yeast strains. These contaminating microorganisms have developed different strategies of survival and competition in alcoholic fermentation processes.
Gram-positive bacterial species such as Lactobacillus and Bacillus are among the main contaminants of alcoholic fermentation processes (Lucena et al. 2010), while contaminations by Gram-negative species are less frequent, but difficult to control (Gallo 1990; Lopes et al. 2004; Lucena et al. 2010). Bacterial populations are controlled with acid treatment, antibiotics, hop products, and chemical biocides that do not affect yeast cells (Amorim et al. 2009). However, some bacteria are more resistant to these compounds than others, making them difficult to control during the recycling process. In some cases, contamination reaches 108 bacterial rods per milliliter causing losses of 10,000 to 30,000 l of bioethanol per day for a distillery that produces one million liters a day. In addition, high bacterial contaminations cause yeast to flocculate, besides inhibiting the fermentation. Yeast flocculation is one of the worst problems presented to processes that recycle yeast by centrifugation. Flocculant yeast strains may cause several prejudices to industrial fermentations with cell recycle by centrifugation. In the Brazilian process of alcoholic fermentation, large quantities of yeast biomass need to be centrifuged at the end of each fermentation cycle. This cellular biomass (yeast cream) is diluted with water and sent to acid treatment at pH 1.8–2.5 for 2–3 h before returning to fermentation tanks and starting a new fermentative cycle (Fig. 1c). The flocculation caused by yeast strains may reduce the efficiency of centrifugation, and consequently, a less-concentrated yeast cream goes to acid treatment. A less-concentrated yeast cream means that more wine goes to acid treatment. This wine has a buffering capacity, and distilleries spend more on sulfuric acid to reduce the pH. Consequently, when submitted to a more stressing acid treatment, yeast cells may reduce their activity or die. Moreover, flocculant cells can also decant at the bottom of fermentors leaving residual sugars in the wine without fermenting and thus increasing the fermentation time.
Nowadays, Brazilian distilleries have procedures to identify in just 6 h the best antibiotic to control bacterial contamination (Amorim 2006). But the greatest challenge is how to prevent bacterial contamination in industrial fermentations with yeast cell recycling. Moreover, some distilleries used to remove part of the yeast cells, which are dried and subsequently sold for animal feed (Amorim et al. 1998). These distilleries have avoided the use of traditional antibiotics because these compounds may leave residues in the dry yeast. New, safer, and environmentally friendly compounds need to be developed for bacterial control by distilleries that sell dry yeast.
Contaminations by wild yeast are more difficult to control than bacteria, although non-Saccharomyces contamination might be controlled by modifications in the process (de Souza Liberal et al. 2005; Basílio et al. 2008). On the other hand, wild Saccharomyces species are the most difficult to control because their metabolism is very similar to that of industrial yeast strains but carry inappropriate features such as cell flocculation, excessive foam production, low fermentation yield, and incomplete sugar fermentation (Basso et al. 2008). Nowadays, there is no product or process that can be used by the distilleries to reduce contaminations by wild Saccharomyces in the fermentation process without affecting the selected yeast strains.
Selection of new yeast strains
The first challenge to improve the first generation process is the selection of yeast strains able to survive these industrial conditions and compete with wild yeasts (Basso et al. 1993; Amorim et al. 2004). Several research projects are being conducted at bench scale worldwide without considering the parameters of Brazilian fermentation processes. In addition, these strains are not able to compete with tolerant wild Saccharomyces and non-Saccharomyces species. For this reason, yeast strains such as PE2 and CAT1, selected in the 1990s, have called the attention of researchers worldwide. These strains were selected from industrial processes (fed-batch and continuous fermentation) by a well-directed program of yeast selection (Basso et al. 2008). These strains were monitored using a molecular karyotyping technique, selected from industrial fermentations, evaluated under laboratory conditions, and reintroduced in their respective distilleries during successive years to prove their fermentative abilities. Characteristics such as dominance over other strains and permanence in the process during the sugarcane harvest season were taken into consideration when selecting these strains. Their fermentative abilities were evaluated at the laboratory scale under well-controlled conditions, mimicking the industrial fermentation process.
However, Brazilian distilleries have only five industrial yeast strains commercially available to the fermentation processes. Baker's yeast and laboratory and yeast strains selected from other fermentation processes do not survive more than 1 month in industrial fermentation with yeast cell recycling. Indeed, these yeasts are quickly replaced by wild Saccharomyces (Basso et al. 2008). Therefore, it is likely that programs to develop new strains for Brazilian distilleries may fail because they are based on strains that are not able to survive the recycling process, acid treatment, fast fermentations, and competition with wild yeasts.
Knowing the genetic background of industrial yeast strains
Selected yeast strains, PE2 and CAT1, have been used with success by Brazilian distilleries in their fermentation processes. During several years, these strains have shown the highest rate of dominance and persistence in industrial fermentations. PE2 and CAT1 are diploid and heterothallic strains that sporulate very well. In a few hours, these strains are able to resume meiosis producing asci with three or four viable spores (Lopes 2000). Chromosomal rearrangements have been described for these strains collected from industrial fermentations as well as after sporulation (Lopes et al. 2002). Spores from these strains can mate with themselves or with other yeasts. However, there is little information about the chromosomal constitution and cell behavior under meiosis and conjugation.
Another scientific challenge is to understand why these strains are more tolerant to industrial fermentation than baker's yeast and laboratory strains. It has been demonstrated that CAT1 cells multiply faster in a fermentation medium depleted of vitamins B1 and B6 in comparison with laboratory strain S288c (Stambuk et al. 2009). In addition, two groups have independently investigated special features of CAT1 (Stambuk et al. 2009) and PE2 (Argueso et al. 2009) genomes. However, there is still no information about gene expression under conditions of industrial fermentation. Besides genome characterization, it is equally important to understand how gene expression is controlled by these yeast strains during fermentation conditions. Baker's yeast and strains developed under laboratory conditions are destined to fail when introduced into industrial fermentation with cell recycling. These strains carry different genes and features but are not able to survive, or adapt to, the stressing conditions of the industrial alcoholic fermentation process. In addition, these strains do not have the ability to compete with wild Saccharomyces and are quickly replaced by more tolerant and adapted strains (Amorim et al. 2004, Basso et al. 2008, Stambuk et al. 2009; Lopes 2010). Industrial yeast strains can serve as a source to receive foreign genes. Recently, a PE2 strain was constructed where both alleles for URA3 were deleted (Gonçalo Guimaraes Pereira, personal communication). This strain can be used for further genetic improvement through DNA recombinant technology.
On the other hand, because of their heterothallic nature, sporulation, chromosomal recombination and segregation may affect the inheritance, balance, and expression of genes not only to produce bioethanol but to survive the stressful conditions of the recycling process. Concerning the scientific challenges for modern biotechnology, initially, it is necessary to understand the genetic constitution of wild Saccharomyces cerevisiae and yeast strains selected from the industrial alcoholic fermentation process. Industrial yeast strains have a complex genetic constitution, and programs of DNA sequencing, gene expression, proteomics, and metabolomics are necessary tools to understand why these strains are able to survive under different stressing conditions during hundreds of fermentative cycles. The second molecular challenge will be to integrate the knowledge from genetics, physiology, and industrial fermentation processes to improve and introduce new features of industrial interest in yeast strains without affecting the capacity of these new strains to survive successive recycles and stressing conditions. Millions of dollars are being invested worldwide in research and development of new yeast strains without investigating their ability to tolerate stressful conditions similar to those that occur in Brazilian distilleries. Thus, the yeast strains PE2 and CAT1 can be currently considered among the best candidates for expression of foreign genes of industrial importance.
Reduction of vinasse volume
Another scientific challenge that remains is to reduce the volume of vinasse as well as to develop new uses and products from it. Vinasse is the stillage obtained after distillation of the wine and removal of ethanol (Mutton et al. 2010). Vinasse is rich in minerals, such as potassium, calcium, magnesium, nitrogen, and phosphorus (Fig. 1e; Nogueira et al. 2008). Vinasse has been used as a fertilizer in the sugarcane fields, saving millions of liters of water and improving the physical conditions of the soil (van Haandel 2005; Mutton et al. 2010). Moreover, application of vinasse to sugarcane fields allows the recycling of nutrients back to the soil. However, application in the field requires careful procedures to avoid contamination of water tables (Mutton et al. 2010).
Nowadays, Brazilian distilleries produce around 12 l of vinasse for each liter of ethanol. This happens because the alcohol content in the wine at the end of fermentation reaches 8–9%. This concentration of alcohol limits the reduction of vinasse volumes. Increasing alcohol concentrations of the wine reduces the viability of yeast cells. This cellular viability is essential to fermentation processes with yeast cell recycling. These processes depend on maintaining yeast cells alive until the end of the fermentation process because they need to be reused several hundred times during 6 to 8 months (Amorim et al. 2010).
Recently, a new process was developed that reduces vinasse volume by half via doubling the alcohol concentration in the wine to 16% (v/v). This technology reduces vinasse volume to 6 l per liter of ethanol (Cherubin 2011). Furthermore, less water is used in the fermentation. However, because of the reusing of yeast cells and the inhibitory action of ethanol, several parameters of industrial importance need to be changed to maintain the high viability of yeast cells during the recycles. Without solving these scientific challenges, it would be impossible to maintain the yeast cells with high viability and at the same time increase the ethanol concentration aiming to reduce the vinasse volume.
In summary, it has been demonstrated that the development and transference of new technologies in first generation bioethanol needs to be matched with technical, economical, and sustainability proposals. From an industrial point of view, there are several scientific challenges still to be solved in first generation bioethanol.
New feedstocks
It is important to note that the success of the second generation of biofuels depends upon investment in research and the application of the knowledge gained from the development of first generation bioethanol (Regalado 2010; Soccol et al. 2010). The development and transference of new technologies into the bioethanol of first generation needs to be aligned to feedstock availability. Sugarcane has several advantages as a feedstock in relation to other crops such as corn, sugar beet, wheat, and others (Amorim et al. 2009). One of these advantages is that it is a perennial crop, having the capacity to give six or seven (or more) harvestings before being replanted and saving costs in the field (Buckeridge et al. 2010). Sugarcane has been well adapted to the soil and climate in some Brazilian regions. With the advance of sugarcane, several land areas covered by low productivity pastures were replaced with this crop, and new distilleries changed the agrobusiness strategy of several cities. However, the sugarcane harvesting is limited to 6–8 months per year. Sugarcane cannot be stored due to its fast decomposition, whereas corn can be transported long distances and stored for use throughout the year. It means that for 4–6 months per year, the Brazilian distilleries are idle, without producing bioethanol, whereas in the USA, distilleries that utilize corn remain active almost the whole year.
There are oscillations in the market due to the bioethanol season production. Recently, several efforts have been made to introduce new feedstocks aiming to extend the production time of distilleries. One of the most promising crops is sweet sorghum (Amorim et al. 2009). Nowadays, some distilleries have tried to use sweet sorghum stalks to augment the fermentation process and ethanol production. Sorghum has several advantages over other cultures such as less demand for water and nutrients, high productivity, faster harvesting, flexibility to plan the use of land, use of seeds instead of stalks, and others (Swayze 2009). The challenges include the breeding of new sorghum varieties, planting techniques, harvesting time (especially during the rainy season), crushing and fermentation, as well as reduction of compounds that affect industrial processes such as aconitic acid, phenolic compounds, and starch.
Sustainability of bioethanol production
Nowadays, efforts are directed at improving the sustainability of biofuel production by Brazilian distilleries, to save natural resources (water, land, etc.) and to reduce the burning of sugarcane and the release of greenhouse gas (GHG) (Leal and Walter 2010). The use of different feedstocks such as sorghum bagasses, corn stover, grass, algae, etc. has been evaluated aiming to help mitigate GHG emissions worldwide (Amorim et al. 2010). However, the GHG emissions from Brazilian processes of bioethanol production based on sugarcane have been considered the best among the main substrates currently used for bioethanol production (Walter et al. 2008). The challenge to these distilleries would be to find appropriate substrates more adapted to local conditions for each region in Brazil.
Considering the complete cycle of bioethanol production and its use as a biofuel, there is a reduction of GHG emissions of 90% in comparison with gasoline while for corn and beet, this reduction is about 30% and 45%, respectively (Macedo et al. 2008). In addition, the most significant emissions of GHG are due to soil emissions, followed by agricultural operations and transport, burning of the sugarcane fields before manual harvesting, use of fertilizers, and ethanol distribution (Macedo et al. 2008). New challenges could be focused to improve crop practices, transport and use part of sugarcane trash instead of burning the fields or leaving the residue for decomposition. It may increase the offer of plant biomass for different purposes such as biosteam production, bioelectricity, or second generation bioethanol.
Biosteam, bioelectricity, or second generation bioethanol
Nowadays, around 90% of Brazilian distilleries are linked to sugar factories. These industries consume energy to carry out their processes from sugarcane milling, juice clarification, evaporation, and distillation. Many steps of the sugar and alcohol production process demand tons of steam. Distilleries use sugarcane bagasse to produce the steam required to run a factory. The challenge has been to improve the efficiency of steam use by distilleries. As more steam is saved, more bagasse may be used for other purposes. This is currently the chief use of sugarcane bagasse by distilleries. On the other hand, the development of high-efficiency boilers permits the burning of less bagasse. Then, the excess of bagasse may be used for different purposes such as bioelectricity (Fig. 1b) and second generation bioethanol. When the world energy matrix is compared with the Brazilian energy matrix, it is possible to see a remarkable difference. While the world uses just 12% of renewable energy, Brazil has used more than 45% of renewable energies (Goldemberg 2007). Part of this energy comes from distilleries. In 2009, the electricity produced from bagasse combustion contributed with 3% of the total electricity generated in the country, opening new opportunities to the distilleries (for a review, see Cortez 2010; dos Santos et al. 2010).
A total of 100 Brazilian distilleries are able to produce and export bioelectricity derived from sugarcane bagasse (Roberto 2010). It represents a viable alternative for industries to process surplus bagasse for cogeneration of electricity. It is quite interesting to distilleries that sugarcane harvesting season coincides with the dry season in many regions in Brazil. In the last years, the water reservoirs have reached critical levels a few times, at the same time as bioelectricity from sugarcane achieved its peak. This balance of energy supply is very interesting for some regions and industries considering that the development of several industries depends on electric power.
Moreover, the new scientific and technological challenges are focused on developing and improving industrial processes that can save more energy. In this way, Brazilian distilleries will become more efficient with good energy balance in comparison with other countries. A higher ratio between renewable energy products and fossil fuels used in the production process can be obtained. The balance for bioethanol from sugarcane is 9.3:1 while ethanol from corn has a ratio of 1.3:1 (Goldemberg 2007). In other words, this means that 9.3 energy units (ethanol) were produced from one unit of fossil-fuel energy. This means the proportion of energy from fossil fuel in relation to bioethanol energy produced by distilleries is higher using sugarcane feedstocks. In terms of energetic balance, the scientific challenge for the next years will be to increase the output of renewable energy through the improved use of sugarcane bagasse and vegetal trash.
This highly positive ratio for sugarcane is due to the fact that bagasse is used as a source to generate biosteam and bioelectricity in the production of bioethanol, including crushing, evaporation, and distillation (Doerfler and Amorim 2007). One third of all sugarcane produced by Brazilian mills (600 million tons) was bagasse with 50% moisture. In the future, distilleries will produce steam and bioelectricity not only from bagasse but also from sugarcane trash (Eggleston 2010). In this case, the scientific challenge is to develop new technologies to harvest, transport, and process trash with technical and economical efficiency, as well as to reduce the dependency on fossil fuels.
The third usage for sugarcane biomass will be the production of second generation ethanol. The solution is likely to come from multidisciplinary teams who understand all features of the current processes involved in the production of first generation bioethanol, acting with a holistic view and selecting new yeast strains and new sugarcane varieties, improving bagasse hydrolysis, and establishing more efficient processes of alcoholic fermentation. It is not difficult to predict the future when we learn from the past and present. Second generation bioethanol needs intensive efforts of scientific investigation over the next decades. However, there are no viable industrial processes to date. Thus, one can deduce that the technology of second generation ethanol will arrive to distilleries only if it proves its technical and economical feasibility.
In order to get protection against market oscillations, the bioethanol industry needs the diversification of the sugar biomass, dry yeast production, and bioelectricity generation. In addition, there are new investors coming to Brazil interested to transform mills into biorefineries. However, it is necessary to invest in research, transfer of new technologies, and training of technical staff. Without an integrated vision about technical, cultural, legal, environmental, and economical perspectives, it will be very difficult to introduce new technologies. Then, in the future, distilleries will decide how much biomass will be used for biosteam, bioelectricity, or second generation bioethanol. The greatest scientific challenge will be to collaborate with the distilleries. The industries and research institutes need to work in the same direction. It will be essential to align the vision of the academics with the needs of the industry.
References
Amorim HV, Fernandes EAN, Nepomuceno MF, Trevizan AB (1998) From potential to reality: yeasts derived from ethanol production for animal nutrition. J Radioanal Nucl Chem 234:113–118
Amorim HV, Basso LC, Oliveira AJ, Godoy A, Cherubin RA, Lopes ML (2004) Identification and selection of yeast strains from alcoholic fermentations in Brazil by electrophoretic karyotyping. In: Eleventh International Congress on Yeasts in Science and Technology, 2004, Rio de Janeiro, vol. 1, p 51–51
Amorim HV, Lopes ML (2004) Os principais processos de fermentação para álcool combustível no mundo. Guia Internacional do Álcool 1:74–77
Amorim HV, Leão RM (2005) In: Amorim HV (ed) Fermentação alcoólica: Ciência e Tecnologia. Fermentec Editora, Piracicaba, SP, Brazil, pp 190–191
Amorim HV, Lopes ML (2005) Ethanol production in a petroleum dependent world: the Brazilian experience. Sugar J 67:11–14
Amorim HV (2006) Ethanol production in Brazil: a successful history. Proceedings of the Sugar Processing Research Conference 1:44–47
Amorim HV, Basso LC, Lopes ML (2009) Sugar cane juice and molasses, beet molasses and sweet sorghum: composition and usage. In: Ingledew WM, Kelsall DR, Austin GD, Kluhspies C (eds) The alcohol textbook: a reference for the beverage, fuel, and industrial alcohol industries, vol. 1. Nottingham University Press, Nottingham, pp 39–46
Amorim HV, Gryschek M, Lopes ML (2010) The success and sustainability of the Brazilian sugarcane−fuel ethanol industry. In: Eggleston G (ed) Sustainability of the sugar and sugar ethanol industries, ACS Symposium Series. American Chemical Society, Washington, DC, pp 73–82, ACS Symposium Series, Vol. 1058, Chapter 5
Argueso JL, Carazzolle MF, Mieczkowski PA, Duarte FM, Netto OVC, Missawa SK, Galzerani F, Costa GGL, Vidal RO, Noronha MF, Dominska M, Andrietta MGS, Andrietta SR, Cunha AF, Gomes LH, Tavares FCA, Alcarde AR, Dietrich FS, McCusker JH, Petes TD, Pereira GAG (2009) Genome structure of a Saccharomyces cerevisiae strain widely used in bioethanol production. Genome Res 19:2258–2270
Basílio AC, de Araújo PR, de Morais JO, da Silva Filho EA, de Morais MA, DA Jr S (2008) Detection and identification of wild yeast contaminants of the industrial fuel ethanol fermentation process. Curr Microbiol 56:322–326
Basso LC, Oliveira AJ, Orelli VFDM, Campos AA, Gallo CR, Amorim HV (1993) Dominância das leveduras contaminantes sobre as linhagens industriais avaliada pela técnica da cariotipagem. Anais Congresso Nacional da STAB 5:246–250
Basso LC, Amorim HV, Oliveira AJ, Lopes ML (2008) Yeast selection for fuel ethanol production in Brazil. FEMS Yeast Res 8:1155–1163
BNDES, CGEE (2008) Cana-de-açúcar: energia para o desenvolvimento sustentável. BNDES, Rio de Janeiro, p 316
Buckeridge MS, Dos Santos WD, De Souza AP (2010) Routes for cellulosic ethanol in Brazil In: sugarcane bioethanol: R&D for productivity and sustainability (ed Cortez LAB), pp 365–380. Edgard Blucher, São Paulo
Cabrini KT, Gallo CR (1999) Identificação de leveduras no processo de fermentação alcoólica em usina do estado de São Paulo, Brasil. Scientia Agricola [online] vol.56(n.1): pp. 207–216. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-90161999000100028&lng=en&nrm=iso. Accessed 28 March 2011
Chaddad FR (2010) UNICA: challenges to deliver sustainability in the Brazilian sugarcane industry. International Food and Agribusiness Management Review 13:173–192
Cherubin N (2011) Novas tecnologias em fermentação: garantia de eficiência e ganhos na produção de etanol. Revista Idea News 122:30–34
Cortez LAB (2010) Routes for cellulosic ethanol in Brazil. In: Cortez LAB (ed) Sugarcane bioethanol: R&D for productivity and sustainability. Edgard Blucher, São Paulo, p 954
de Souza Liberal AT, da Silva Filho EA, de Morais JO, Simões DA, de Morais MA Jr (2005) Contaminant yeast detection in industrial ethanol fermentation must by rDNA-PCR. Lett Appl Microbiol 40:19–23
Doerfler J, Amorim HV (2007) Applied bioethanol technology in Brazil. Sugar Industry 132:694–697
dos Santos WD, Buckeridge MS, de Souza AP (2010) Routes for cellulosic ethanol in Brazil. In: Cortez LAB (ed) Sugarcane bioethanol: R&D for productivity and sustainability. Edgard Blucher, São Paulo, pp 365–380
Eggleston G (2010) Future sustainability of the sugar and sugar−ethanol industries. In: Eggleston G (ed) Sustainability of the sugar and sugarethanol industries. ACS Symposium Series; American Chemical Society, Washington, DC, pp 1–19, Chapter 1, ACS Symposium Series, vol. 1058
Gallo CR (1990) Determinação da microbiota bacteriana de mosto e de dornas de fermentação alcoólica. 388 p. Tese de Doutorado—Faculdade de Engenharia de Alimentos, Universidade Estadual de Campinas (UNICAMP), Campinas, SP. Available from: http://cutter.unicamp.br/document/?code=000038046&fd=y
Godoy A, Amorim HV, Lopes ML, Oliveira AJ (2008) Continuous and batch fermentation processes: advantages and disadvantages of these processes in the Brazilian ethanol production. Int Sugar J 110:175–181
Goldemberg J (2007) Ethanol for a sustainable energy future. Science 315:808–810
Goldemberg J (2008) The Brazilian biofuels industry. Biothecnol Biofuels 1:6
Ibeto CN, Ofoefule AU, Agbo KE (2011) A global overview of biomass potentials for bioethanol production: a renewable alternative fuel. Trends Appl Sci Res 6:410–425
Leal MRLV, Walter AS (2010) Sustainability of the production of ethanol from sugarcane: the Brazilian experience. Int Sugar J112:390–396
Leite RCC, Leal MRLV, Cortez LAB, Griffin WM, Scandiffio MIG (2009) Can Brazil replace 5% of the 2025 gasoline world demand with ethanol? Energy 34:655–661
Lopes ML (2000) Estudo do polimorfismo cromossômico em Saccharomyces cerevisiae (linhagem PE2) utilizada no processo industrial de produção de etanol. PhD thesis, Instituto de Biociências de Rio Claro, Universidade Estadual Paulista “Julio de Mesquita Filho”, Rio Claro, SP
Lopes ML, Basso LC, Amorim HV (2002) Chromosomal polymorphism in Saccharomyces cerevisiae (strain PE-2) used in industrial fermentation for ethanol production. In: 2002 Yeast Genetics and Molecular Biology Meeting, University of Wisconsin, Madison, WI. 2002 Yeast Genetics & Molecular Biology, p. 159 (abstract 348). Available from: http://www.yeastgenome.org/community/meetings/yeast02/abshtml/348.html
Lopes ML, Amorim HV, Godoy A, Oliveira AJ, Cherubin RA, Basso LC (2004) Interaction between yeast and acetic acid bacteria in industrial fermentation for ethanol production: a case study. In: Eleventh International Congress on Yeast-Yeasts in Science and Technology, 2004, Rio de Janeiro. Eleventh International Congress on Yeast–Yeasts in Science and Technology 1:183–183
Lopes, ML (2010) 30 years of fuel ethanol production in Brazil. Identification and selection of dominant industrial yeast strains. In: 12th International Conference on Culture Collections. Biological resources centers: gateway to biodiversity and services for innovation in biotechnology Florianópolis, SC. Available from: http://www.iccc12.info/presentations/mlopes.pdf
Lucena BT, dos Santos BM, Moreira JL, Moreira AP, Nunes AC, Azevedo V, Miyoshi A, Thompson FL, de Morais MA Jr (2010) Diversity of lactic acid bacteria of the bioethanol process. BMC Microbiol 10:298
Macedo IC, Seabra JEA, Silva JE (2008) Green house gases emissions in the production and use of ethanol from sugarcane in Brazil: the 2005/2006 averages and a prediction for 2020. Biomass and Bioenergy 32:582–595
Mutton MA, Rossetto R, Mutton MJR (2010) Utilização agrícola da vinhaça. In: Cortez LAB (ed) Sugarcane bioethanol: R&D for productivity and sustainability. Edgard Blucher, São Paulo, pp 423–440
Nogueira LAH, Seabra JEA, Best G, Leal MRLV, Poppe MK (2008) Bioetanol de cana-de-açúcar: energia para o desenvolvimento sustentável. BNDES, Rio de Janeiro, p 316, v.1
Porto SI, Silva ACP, Bestetti CR, Bressan Filho A, Oliveira CC, Oliviera JB, Negreiros JC, Almeida MBA, Andrade RA (2009) Brazilian crop assessment: sugarcane 2009/2010. Third estimate. 17p. CONAB, Brasilia, DF. Available from: http://www.conab.gov.br/OlalaCMS/uploads/arquivos/fcff266126165d8ec2e9915c5d77d322..pdf
Porto SI, Silva ACP, Bestétti CR, Bressan Filho A, Oliveira EP, Costa FC, Negreiros JC, Oliveira JB, Almeida MBA, Andrade RA (2011) Acompanhamento da safra brasileira. Cana-de-açúcar. Safra 2010/2011, terceiro levantamento. 19p. CONAB, Brasilia, DF. Available from: http://www.conab.gov.br/OlalaCMS/uploads/arquivos/11_01_06_09_14_50_boletim_cana_3o_lev_safra_2010_2011..pdf
Regalado A (2010) Brazil. Race for cellulosic fuels spurs Brazilian research program. Science 327:928–929
Roberto C (2010) Cogerar: um verbo cada vez mais conjugado pelo setor sucroalcooleiro. Idea News 116:20–26
Soccol CR, Vandenberghe LP, Medeiros AB, Karp SG, Buckeridge M, Ramos LP, Pitarelo AP, Ferreira-Leitão V, Gottschalk LM, Ferrara MA, da Silva Bon EP, de Moraes LM, Araújo Jde A, Torres FA (2010) Bioethanol from lignocelluloses: status and perspectives in Brazil. Bioresour Technol 101:4820–4825
Stambuk BU, Dunn B, Alves Junior SL, Duval EH, Sherlock G (2009) Industrial fuel ethanol yeasts contain adaptive copy number changes in genes involved in vitamin B1 and B6 biosynthesis. Genome Res 19:2271–2278
Swayze S (2009) The sweet sorghum opportunity: a complementary source of low-cost fermentable sugars for biofuel. Int Sugar J 111:691–695
van Haandel AC (2005) Integrated energy production and reduction of the environmental impact at alcohol distillery plants. Water Sci Technol 52:49–57
Walter A, Dolzan P, Quilodrán O, Garcia J, Silva C, Piacente F, Segerstedt A (2008) A sustainability analysis of the Brazilian ethanol. Universidade Estadual de Campinas—UNICAMP, Campinas, SP. 167 p. Available from: http://www.globalbioenergy.org/uploads/media/0811_Unicamp_-_A_sustainability_analysis_of_the_Brazilian_ethanol.pdf
Wheals A, Basso LC, Alves DMG, Amorim HV (1999) Fuel ethanol after 25 years. Trends Biotechnol 17:482–487
Zanin GM, Santana CC, Bon EP, Giordano RC, de Moraes FF, Andrietta SR, de Carvalho Neto CC, Macedo IC, Fo DL, Ramos LP, Fontana JD (2000) Brazilian bioethanol program. Appl Biochem Biotechnol 84–86:1147–1161
Acknowledgments
We would like to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Instituto Nacional de Ciência e Tecnologia do Bioetanol, Ministério da Ciência e Tecnologia, all from Brazil, for financial support, the two anonymous reviewers for their comments, and Dr. Neil Andrew Brown for critical reading of the manuscript.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amorim, H.V., Lopes, M.L., de Castro Oliveira, J.V. et al. Scientific challenges of bioethanol production in Brazil. Appl Microbiol Biotechnol 91, 1267–1275 (2011). https://doi.org/10.1007/s00253-011-3437-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3437-6