Abstract
Purpose
With contemporaneous advances in congenital central hypoventilation syndrome (CCHS), recognition, confirmatory diagnostics with PHOX2B genetic testing, and conservative management to reduce the risk of early morbidity and mortality, the prevalence of identified adolescents and young adults with CCHS and later-onset (LO-) CCHS has increased. Accordingly, there is heightened awareness and need for transitional care of these patients from pediatric medicine into a multidisciplinary adult medical team. Hence, this review summarizes key clinical and management considerations for patients with CCHS and LO-CCHS and emphasizes topics of particular importance for this demographic.
Methods
We performed a systematic review of literature on diagnostics, pathophysiology, and clinical management in CCHS and LO-CCHS, and supplemented the review with anecdotal but extensive experiences from large academic pediatric centers with expertise in CCHS.
Results
We summarized our findings topically for an overview of the medical care in CCHS and LO-CCHS specifically applicable to adolescents and adults. Care topics include genetic and embryologic basis of the disease, clinical presentation, management, variability in autonomic nervous system dysfunction, and clarity regarding transitional care with unique considerations such as living independently, family planning, exposure to anesthesia, and alcohol and drug use.
Conclusions
While a lack of experience and evidence exists in the care of adults with CCHS and LO-CCHS, a review of the relevant literature and expert consensus provides guidance for transitional care areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital central hypoventilation syndrome (CCHS) is a rare disorder that results from a pathogenic variant of the PHOX2B gene located on chromosome 4 and classically manifests in the newborn period with respiratory insufficiency due to chemoreceptor insensitivity [1,2,3]. Since 2003, the confirmation of a CCHS diagnosis by PHOX2B genetic testing has led to an increase in disease recognition, with an estimated 3000 cases worldwide since 1970 [4, 5]. Additionally, the estimated disease prevalence of 1:150,000–200,000 in some populations suggests the potential that ~ 33,000–45,000 CCHS patients worldwide since 1970 remain unidentified [1]. All of these identified, and yet unidentified, patients raise disease prevalence as patients live well into adulthood. Coupled with identification of adults whose diagnosis was “missed” in childhood, it behooves all health care professionals to be familiar with this rare condition so that they might identify the undiagnosed CCHS patient and initiate the process to provide comprehensive medical management throughout the life span of their patients.
CCHS is a neurocristopathy, or a disease of neural crest origin, and the autonomic nervous system (ANS) is derived from the neural crest [6]. Clinical features of CCHS include central hypoventilation and apnea leading to hypercarbia and/or hypoxia due to attenuated or absent peripheral and central chemoreceptor responsiveness. Additionally, features variably include neural crest tumors, Hirschsprung's disease (HSCR), cardiac sinus pauses, and additional symptoms of ANS dysfunction (ANSD) potentially affecting all systems served by the ANS [1]. CCHS is most often diagnosed at less than 1 month of age, but importantly, a subset of patients present with symptoms after this age (later-onset (LO-)CCHS; LO-CCHS) and even a small number of these patients are diagnosed in adulthood [7,8,9,10].
In the opinion of those with expertise in this rare disease, with an increasing number of adolescents with CCHS, transition into adulthood from medical, psychological, and socio-emotional standpoints has become a priority in CCHS care. A heightened awareness of CCHS amongst tertiary and quaternary pediatric care centers, alongside parental advocacy, has helped to optimize outcomes for many of these vulnerable patients with CCHS. However, fundamentally, the care of the adult with CCHS has unique gaps because of the lack of awareness, experience, and understanding of CCHS at adult hospital centers, and the minimal evidence base beyond case reports in adult medicine.
This article consolidates extensive expertise from physicians and physician-scientists at leading academic medical centers with specialized care of pediatric patients with CCHS. Here we aim to review clinical care guidelines in CCHS, including supporting the care of patients with CCHS in transition from pediatric to adult care, those with CCHS diagnosed in adulthood, and adults with a clinical suspicion of LO-CCHS.
PHOX2B genetic variants and testing in CCHS, including mosaicism
Patients with CCHS and LO-CCHS have a heterozygous, typically de novo, pathogenic variant in PHOX2B [4, 5]. The most common disease-causing variant type in PHOX2B is a heterozygous expansion of the polyalanine repeat tract in exon 3 of the gene, causing the wild-type alanine repeat size of 20 (genotype 20/20) to increase to 24–33 repeats (genotypes 20/24-20/33). These are described as PHOX2B polyalanine repeat expansion mutations (PARMs), and the repeat sizes are often correlated with severity of the CCHS phenotypes, with longer expansions associated with more severe disease [5, 9]. These PARMs account for ~ 90% of cases with CCHS [2].
Non-PARMs (NPARMs) are another class of PHOX2B variants associated with CCHS, accounting for ~ 10% of patients [2]. These broadly include missense, nonsense, frameshift, stop codon, and splice site sequence variants in PHOX2B that do not impact the size of the polyalanine repeat tract in exon 3 [11]. As specific NPARMs are reported in few patients with CCHS, their phenotype-specific gene variant correlation has only recently been reported in detail [11]. Zhou, et al., postulated that PHOX2B loss of function sequence variants occurring in exons 1 and 2 of the gene are more likely to trigger nonsense-mediated decay of the aberrant transcripts, in turn causing an association with a milder phenotype in CCHS patients [11]. This might be due to the reduction of aberrant PHOX2B transcripts that can exert a dominant negative effect on regular gene function [12].
Mosaicism for the PARMs has been documented as a source of phenotypic variability in 5–25% of parents who may harbor PHOX2B PARMs, with up to 50% risk of passing the PHOX2B PARM to their offspring [5, 13]. Therefore, accurate molecular identification of PHOX2B PARMs in CCHS patients is crucial for informing patient management, and in their parents to determine reproductive risk [2].
The recommended stepwise testing [2, 8, 14] with molecular methodologies to detect PHOX2B PARMs include Step 1 fragment analysis, also known as PHOX2B targeted variant analysis and PHOX2B screening test. PHOX2B fragment analysis uses electrophoresis to detect all PARMs, NPARMs with nucleotide deletions and duplications that change the length of the coding sequence, and mosaicism. If Step 1 is negative, then Step 2 Sanger sequencing using the same DNA sample can detect all PARMs and all NPARMs, but no low-level mosaicism [1]. If Steps 1 and 2 are negative but clinical suspicion of CCHS remains high, the Step 3 multiplex-ligation dependent probe amplification testing is implemented to identify patients missing the whole PHOX2B gene with or without neighboring genes. Of note, current Next-Generation Sequencing-based technologies do not always detect PHOX2B PARMs, so Sanger sequencing is recommended if fragment analysis is not used as a first step in the testing process.
Fragment size analysis using capillary electrophoresis is the preferred method for detecting PHOX2B PARM mosaicism, as sequencing-based assays will fail to capture low-level mosaicism that would be challenging to identify on sequencing electropherograms [14]. Thus, for patients with CCHS who have been identified with a PHOX2B PARM, or a NPARM 35 or 38 bp deletion, the appropriate follow-up testing for the proband’s parents is the screening test to rule out mosaicism and ascertain reproductive risks. For parents of a patient with other NPARM variants, the PHOX2B sequencing test can be used to assess for higher levels of NPARM mosaicism [14].
Embryologic basis for CCHS
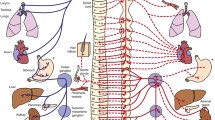
PHOX2B is an important regulator of neuronal development, especially in the early embryology of the ANS (Fig. 1) [15]. Disruptions of PHOX2B function in CCHS result in maldevelopment of key central and peripheral neurons, including those in the brainstem, enteric nervous system (ENS), and autonomic ganglia. PHOX2B is essential for the development of the neuronal systems regulating central chemosensation, including brainstem catecholaminergic neurons, the nucleus of the solitary tract, sympathetic preganglionic neurons, cranial parasympathetic neurons, and the carotid bodies [16,17,18]. Studies employing animal models, limited human autopsy data, and functional MRI (fMRI) have highlighted maldevelopment of key brainstem areas of chemosensation and neuronal integration in CCHS, although these data are impacted by genotype and phenotype variability, and differences in clinical management. Affected regions include the retrotrapezoid nucleus, a primary site of central chemoreception that shows dysgenesis in mouse PARM (20/27) and NPARM (PHOX2B∆8) models [19, 20] and in CCHS patients using fMRI imaging [21]. The locus coeruleus, a primary noradrenergic center on the dorsal pons responsible for regulation of chemosensation and the arousal state, was found to be hypoplastic or aplastic in PARM and NPARM animal models of CCHS [20], as well as in three perinatal autopsy cases [20, 22]. The autopsy cases also documented significant alterations of other PHOX2B-derived structures, including the dorsal median raphe, hindbrain mesencephalic trigeminal nucleus, and dorsal motor of the vagus nerve. Central nervous system involvement in CCHS is undoubtedly more widespread beyond these brainstem regions, as fMRI studies on patients have highlighted abnormalities in other regions known to regulate chemosensation and breathing, including in the hypothalamus, posterior thalamus, cerebellum, and insular and cingulate cortices [21, 23].
Influence of PHOX2B on neuronal differentiation of the autonomic nervous system, influence on congenital central hypoventilation syndrome, and the related neural crest-derived pathophysiologic conditions. Flowchart is adapted from Bachetti and Ceccherini [15]
Peripheral neuroanatomic manifestations of CCHS reflect the key role of PHOX2B in neural crest cell migration and differentiation to form the ENS and sympathetic ganglia [15]. ENS development requires caudal migration and differentiation of neural crest cells from the dorsal motor vagal region, and a small component migrating cranially from the sacral region, to innervate the intestinal wall and form the ganglion cell neurons of the submucosal and myenteric plexuses, [24] parasympathetic ganglia responsible for regulating relaxation of peristalsis. In HSCR, this process is impaired and portions or, rarely, the entire intestinal tract lacks development of these ganglia, resulting in a bowel that is constricted and fails to support peristalsis, necessitating surgical resection of the affected portion. Histologically, the ganglia are small or absent, and the extrinsic preganglionic nerves innervating these regions become hypertrophied [24]. A zone of transition to the normally innervated bowel is usually present and marked by partially formed or non-circumferential ganglia that lack sufficient functionality. While the etiology is complex, PHOX2B is essential for the regulation of key genes in this process (e.g., RET, SOX10), and is even used as a histologic marker to identify the ganglion cells of the intestinal wall [24].
PHOX2B becomes expressed in neural crest cells of the sympathoadrenal lineage that forms the presynaptic sympathetic neurons, sympathetic neuronal ganglia and Schwann cells, and adrenal medulla (Fig. 1) [15]. Aberrant regulation of this process results from a block to maturation due to diminished PHOX2B function in CCHS and/or persistence of a primitive phenotype from MYCN amplifications of sympathoadrenal neural crest cells that predispose to development of neural crest tumors (NCTs). These embryonal neuroblastic tumors form along the sympathetic chain and adrenal medulla, and demonstrate a range of morphology reflective of the aberrant maturation process [15]. The most aggressive tumors show a persistent primitive phenotype of neuroblasts lacking significant ganglion cell differentiation and a background of neuropil, consisting of unmyelinated axons, dendrites, and glial cells with few cell bodies [25], rather than the mature Schwannian stroma of a ganglion. Once tumors show over 50% maturation of the stromal component into Schwann cells, the prefix ganglio- is added, and depending on maturation of the neuronal cell population, these are further divided into ganglioneuroblastomas containing residual neuroblasts and ganglioneuromas with mature cells [25]. In CCHS, the NCTs associated with NPARMs are more commonly primitive neuroblastomas, and are often multifocal, while uncommon NCTs associated with longer PARMs are typically more differentiated ganglioneuroblastomas or ganglioneuromas with only rare exceptions [26,27,28]. As with HSCR, the etiology of NCTs is complex, and the vast majority (99%) are sporadic tumors lacking PHOX2B alterations, suggesting distinct mechanisms of pathogenesis in CCHS [29].
Overall clinical presentation of CCHS and LO-CCHS
CCHS typically presents in the newborn period with recurrent apnea, cyanosis, and hypoventilation, most apparent during sleep [2, 3]. A subset of patients with CCHS has Hirschsprung's disease and/or NCTs, particularly ganglioneuroma, ganglioneuroblastoma, and neuroblastoma [2, 3, 30] raising suspicion for a diagnosis of CCHS. Additionally, a characteristic facial phenotype in CCHS includes a broad, flattened profile with a boxy configuration and inferior inflection of the lateral vermillion borders of the upper lip that is neural crest in origin [2].
Patients with LO-CCHS present over 1 month of age with unexplained hypoventilation, apnea, cyanosis, seizures, signs of right heart failure, increased erythropoiesis, or elevated serum bicarbonate, as well as unexplained neurocognitive delay [7]. Some patients present with signs of respiratory insufficiency in response to physiologic stressors, including general anesthesia or moderate sedation for surgical and dental procedures, respiratory depressants, severe respiratory infections, pregnancy, and labor and delivery [31,32,33]. Thus, any patient with a delay in waking or achieving adequate ventilation following general anesthesia or following a severe respiratory illness should raise a suspicion of having LO-CCHS. Additionally, the diagnosis of LO-CCHS, typically PHOX2B 20/24 and 20/25 genotypes, may be identified in parents of a proband with newly diagnosed CCHS.
Management of CCHS: ventilatory management and ANSD screening
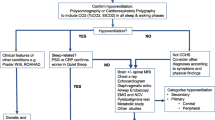
The critical pillars in the management of patients with CCHS include (1) ensuring optimal oxygenation and ventilation during both sleep and wakefulness and (2) surveillance and timely treatment of associated ANSD with comprehensive physiologic evaluations every 6 months until age 3 years, then every 12 months thereafter [2, 3]. CCHS is a generalized disorder of the ANS and affects more than just control of breathing. Table 1 summarizes the recommendations for testing in CCHS and screening of ANSD-related conditions [2, 3]. All patients with CCHS require lifelong comprehensive follow-up, and this can be provided by a multidisciplinary approach [34, 35]. Adherence to up-to-date guidelines in their evaluation, compliance in use of artificial ventilation, and management balanced with available new information and patient and family goals offer optimized care.
Ventilatory management
Artificial ventilation is critical in CCHS management as a means of life support due to hypoventilation and attenuated to absent central and peripheral chemoreceptor responsiveness to hypercarbia and hypoxia (Fig. 2) [2, 3]. Varied options for ventilatory support are available, and it is necessary for the mode of artificial ventilation to be individualized to meet each patient’s needs, goals of treatment, and quality of life. The aim of ventilatory support is to maintain SpO2 ≥ 95% and end-tidal carbon dioxide < 45 mmHg [2, 3]. As patients with CCHS generally have no lung disease, several options are available, including positive pressure ventilation (PPV) via tracheostomy, non-invasive positive pressure ventilation (NPPV), phrenic nerve-diaphragm pacing, and negative pressure ventilation [2, 3]. In infants and patients who require continuous ventilatory support, PPV is preferred as the most stable and conservative management [2]. Patients aged 2 years and older on 24-h per day ventilatory support may benefit from use of phrenic nerve-diaphragm pacing during the day, and PPV via tracheostomy during all sleep time [2, 3]. School-age patients through adulthood who require ventilatory support during sleep only, [2] and whose awake breathing is adequate without hypoxemia and hypercarbia during varied activities of daily living, including strenuous activity, may be candidates for NPPV [3]. Phrenic nerve-diaphragm pacing during sleep necessitates a stable and patent airway because of pacer-induced negative inspiratory pressure ventilation. The role of the ANS in maintaining a patent airway during sleep is undefined in both the general population and those with CCHS [36]. Rarely, teenagers and adults may be successfully paced without a tracheostomy while asleep, but this requires prior sleep endoscopy and continued oximetry and capnography during all sleep time due to anticipated partial, and potentially severe, airway obstruction [2, 3]. Supplemental oxygen without artificial ventilation does not constitute proper or adequate treatment of the hypoventilation [2].
An example from a patient with congenital central hypoventilation syndrome (CCHS) and the characteristic attenuation of chemosensation during a protocolized exogenous ventilatory challenge at baseline, during the exposure to combined 7% fractional inspired carbon dioxide and 14% fractional inspired oxygen during awake spontaneous breathing, and into recovery. During the gas exposure challenge as shown in the flow signal in shaded grey (a), the patient lacks an increase in inspired volumes (as shown in the top dotted line) and an increase in rate (shown as the inferior solid line). As a result (b), this muted response leads to a decline in oxygen saturation (SpO2) to as low as 90% and end-tidal (ETCO2) as high as 66 mmHg. The additional columns (b) demonstrate a cardiovascular response to the exposure with an increase in blood pressure (BP) and a delayed increase in heart rate (HR). Since patients with CCHS lack a fully intact inherent response to hypercarbia/hypoxia, an approach to respiratory management is a custom ventilator “ladder” that varies respiratory rate by mechanical ventilation using measured end-tidal capnography to guide ventilator setting changes (c)
Patients with CCHS lack the perception of dyspnea and thus do not manifest respiratory distress [2, 3]. The use of continuous monitoring with oxygen saturation by pulse oximetry and carbon dioxide monitoring by end-tidal capnography during sleep and intermittently during wakefulness is essential for their optimized management [2]. The American Thoracic Society recommends 24 h per day in-home care with a highly trained registered nurse experienced in artificial ventilation, and continuous monitoring with pulse oximetry and capnography [2]. By the authors’ consensus, the pulse oximeter alarm setting is patient-specific but is generally set to alarm at SpO2 of 90 to 92% and, after 1 year of age, pulse rate at 50 beats per minute. The end-tidal carbon dioxide level is set to alarm at a high of 55 mmHg and a low of 25 mmHg. These alarm settings allow for reduced nuisance alarms but adequate time for caregivers to respond to potential emergencies. Apnea and bradycardia monitoring with a transthoracic impedance monitor will not identify an obstructed airway and does not provide a measure of hypoventilation [2]. Patients should undergo awake and asleep physiologic recording over a several day inpatient comprehensive physiologic evaluation every 6 months under age 3 years, and annually over age 3 years [2, 3]. The collective goal of these evaluations is to optimize management and identify changes with advancing age to ensure adequate ventilatory support, and to evaluate awake spontaneous breathing in varied age-appropriate activities of daily living [2, 3, 37]. By the authors’ experience, those with tracheostomy require evaluation by an otolaryngologist at least annually.
Positive pressure ventilation via tracheostomy
PPV via tracheostomy is the most common form of ventilatory support in patients with CCHS [2, 3, 30] and the primary mode of ventilation in infants and young children because of frequent rapid transitions between wakefulness and sleep in this age group. Furthermore, the immaturity of their respiratory system both physiologically and mechanically predisposes them to significant respiratory instability [38], which can be exacerbated with even minor intercurrent infections. Lastly, because they have longer sleep periods, and therefore require more hours of ventilatory support per day, tracheostomy with PPV provides a stable and consistent option for respiratory support during this developmentally vulnerable age [2, 3]. The assist control/pressure control (AC/PC) mode or synchronized intermittent mandatory ventilation pressure control (SIMV-PC) with an appropriate rate for metabolic needs are the typically recommended modes [2], because patients with CCHS do not appropriately increase their breathing tidal volume or respiratory rate in response to hypercapnia and hypoxemia experienced in different sleep states and varied age-appropriate activities of daily living. These modes provide a predictable mandatory breath rate at intervals that most closely match the intrinsic higher respiratory rates of young children. Toddlers, older children, adolescents, and adults who require 24-h per day ventilation, can be transitioned to phrenic nerve-diaphragm pacing for use when awake while continuing with PPV via tracheostomy or NPPV during all sleep time [3].
Non-invasive positive pressure ventilation
NPPV via nasal or face mask using bi-level positive airway pressure is an option for stable children at least 6 years of age who have hypoventilation only during sleep, and developmentally can secure their mask or are supervised overnight by an awake caregiver [2, 3, 34]. NPPV initiated at younger ages, including in infancy in some centers, has encountered adaptation difficulties in these patients [34]. For instance, the use of long-term non-invasive ventilation can lead to the development of midface hypoplasia and dental malocclusion, which may be mitigated by alternating between different interfaces and close follow-up by a craniofacial team monitoring facial growth and potential deformation [2, 3]. This assisted ventilation technique allows stable older children who hypoventilate during sleep only to be transitioned from PPV to decannulation [34]. For patients ventilated by NPPV, only spontaneous/timed or timed modes with mandatory backup rates guarantee breath delivery.
Phrenic nerve-diaphragm pacing
Phrenic nerve-diaphragm pacing is primarily considered for daytime ventilatory support in patients who are continuously dependent on artificial ventilation [2, 3]. Diaphragm pacing provides an option from the home ventilator during varied activities of daily living, although it requires surgically implanted pacer components and external antennae connected to a pacer transmitter [39]. Although rare, diaphragm pacing can theoretically be the sole ventilatory support for adolescents and adults who are ventilator-dependent during sleep only [2, 3]. With this option, potential for tracheal decannulation can be considered, but should include careful consideration of risks for airway obstruction [40]. Ideal candidates for diaphragm pacing have normal and symmetrically functioning diaphragms as determined by fluoroscopy, bilateral intact phrenic nerve-diaphragm axes, no lung disease, an anatomically normal airway, and healthy weight for age and height. Patients who are overweight or obese may not be adequately ventilated by diaphragm pacing because the adipose tissue adds distance between the external antennae and the subcutaneously implanted receivers, compromising the signal inputs to the receivers. Additionally, the loaded breathing related to abdominal obesity may exceed the capacity from the pacer stimulated diaphragm contractions [40].
Before phrenic nerve-diaphragm pacer implantation is considered, parents and patients need to be fully aware that use of diaphragm pacers does not guarantee safe removal of the tracheostomy. Especially in young children less than 6 years old, paced breaths asleep will result in obstruction at various levels of the airway, and can be observed even awake after decannulation [36]. In older children greater than 6 years old and into adolescent age, pacing will still potentially induce obstructed breaths [40]. When the goals for diaphragm pacing are decannulation and diaphragm pacing without tracheostomy for use during sleep, the following requirements must be met: (1) ventilator dependence only while asleep as determined after extensive physiologic testing during wakefulness while breathing spontaneously in a broad array of age-appropriate activities of daily living, including exertion, (2) stable medical course with rare hospitalizations, (3) not requiring continuous ventilatory support during intercurrent illnesses, (4) acceptance that phrenic nerve-diaphragm pacing without tracheostomy is not as secure a method of life-support as mechanical ventilation via tracheostomy, primarily due to limitations in pressure/volume delivered by pacing compared to the ventilator, as well as the ready availability of replacement equipment for the ventilator compared to diaphragm pacing and particularly, the internal components, (5) acceptance that intubation may be necessary for serious illnesses, sedation, and with pacer equipment issues, and (6) normal sleep endoscopy documenting mature, stable airway integrity during pacer-induced negative pressure ventilation [40]. Diaphragm pacing without tracheostomy can predispose to obstructive sleep apnea and upper airway obstruction, due to diaphragmatic contraction without synchronous upper airway dilation [36].
Obstructive sleep apnea due to diaphragmatic pacing may possibly be alleviated by decreasing the electrical current delivered via the diaphragm action potential amplitude, leading to decreased tidal volumes during inspiration with each diaphragm contraction [41]. However, this approach is only effective if the patient remains well oxygenated and ventilated with these reduced settings. Other strategies to decrease tidal volume include decreasing the inspiratory time, widening the interpulse interval, and decreasing the pulse width. At present, diaphragm pacing without tracheostomy is not a viable mode of ventilatory support in children before adolescence, especially those aged 5 years and younger, primarily due to immaturity of airway dynamics [36]. Diaphragm pacing is typically used for a maximum of 12–15 h per day, with limitation due to theoretical risk of diaphragm fatigue [2, 3, 39]. During an acute illness or when additional time on assisted ventilation is necessary, a decannulated patient with CCHS must have an alternative form of ventilatory support, such as NPPV or a home ventilator, and may require hospitalization and intubation to receive continuous ventilation.
For children, diaphragm pacers should be evaluated electrophysiologically by digital oscilloscope and surface electromyography every 6 months for the first 2 years after implantation, then annually thereafter [2, 3]. For adults with diaphragm pacers, comprehensive electrophysiologic evaluation of phrenic nerve-diaphragm pacers should be completed every 1–1.5 years unless there is a significant increase or decrease in weight, in which case the interval to evaluate should be decreased [2, 3, 40]. For optimal diaphragm pacer implantation, an experienced surgeon using a minimally invasive surgical approach is necessary [39]. The authors also recommend implantation involving a medical team with expert-level familiarity with CCHS and electrophysiological programming of the pacers. Ideally, diaphragm pacers will be evaluated with a digital oscilloscope and surface electromyogram electrodes at the costal margin to obtain valid measures to trend over time, particularly of nerve conduction time, which is a marker of nerve function, and to optimize diaphragmatic action potential amplitude to preserve nerve function [2]. Even after electrophysiologic programming of the pacer settings, fine adjustments of the pacer settings in varied age-appropriate activities of daily living are needed to confirm optimal oxygenation and ventilation [2, 3].
Autonomic nervous system dysfunction and screening in CCHS
Cardiovascular
Patients with CCHS are at risk for life-threatening prolonged abrupt cardiac asystoles [2, 42, 43]. In patients with prolonged abrupt sinus pauses, patients are most often asymptomatic, although they may rarely report syncope or “staring” spells [3, 43]. Thus far, and by author experience, sinus pauses are only noted to occur during wakefulness. Patients require Holter monitoring for 72 h and electrocardiogram every 6 months until age three and then annually thereafter to identify such sinus pauses [2]. If a sinus pause is captured, is ≥ 3 s, and is confirmed to be non-physiologic, the patient needs prompt referral to the electrophysiology service for bipolar cardiac pacemaker placement [2, 3]. The pacemaker must be regularly interrogated and will need to be replaced on average every 5–7 years.
Patients with CCHS are at risk for cor pulmonale, necessitating surveillance echocardiography assessing for pulmonary hypertension and right ventricular hypertrophy every 6 months in patients less than 3 years old [2] and annually thereafter into adulthood [2, 3]. When pulmonary hypertension is present, it must be assumed that it is due to underventilation until proven otherwise. The development of pulmonary hypertension when ventilated adequately during sleep suggests hypoventilation during wakefulness with need for daytime ventilatory support.
Patients with CCHS have additional cardiovascular features related to ANSD. First, patients have reduced heart rate variability [42, 44]. Second, patients often have attenuated changes in blood pressure during wakefulness and sleep. Nocturnal non-dipping of blood pressure can be postulated to be nocturnal hypertension and is a risk factor for chronic hypertension in adulthood [44, 45]. While blood pressure abnormalities are a risk for all patients with CCHS, Dudoignon, et al., suggest non-nocturnal non-dipping may be more prevalent in patients with longer PARM genotypes (20/27 and higher) compared to shorter PARM genotypes and NPARMs [44]. The management of blood pressure in CCHS is ill-defined at present. In pediatric centers, observation and treatment options for hypertension are typically overseen by a pediatric cardiologist or nephrologist. During the transition to adult care for those with CCHS, the primary care doctor, internist, or cardiologist typically resumes management. A third cardiovascular feature in CCHS is noted during orthostatic assessments with head-up tilt testing, where an exaggerated decrement in blood pressure with inadequate increase in heart rate leads to a greater period of time with abnormal cerebral autoregulation in those with CCHS compared to pediatric controls [46, 47]. The cardiovascular risk profiles of patients with CCHS, especially into adulthood, are an important area of further research.
Gastrointestinal motility and Hirschsprung's disease
HSCR is, by definition, the absence of enteric ganglia for variable length in the distal intestinal tract, thus giving rise to short HSCR, long HSCR, or total colonic aganglionosis [48, 49]. HSCR affects 1:5000 births, and occurs either in isolation or in association with other neurocristopathies, including neuroblastoma and CCHS [6, 48, 49]. HSCR is seen in 20–30% of patients with CCHS, particularly in those with longer PARMs (20/26 or higher) and NPARMs with a missense, nonsense, or frameshift mutation [2, 3, 34, 50].
HSCR is a complex genetic disease, resulting from a non-Mendelian mode of inheritance with a polygenic and multifactorial susceptibility involved in the enteric neural crest cell fate. The major genetic risk factors for HSCR are both rare variants of the coding region of the RET gene and common polymorphisms within cis-regulatory elements of the same gene, which reduce its expression during ENS development [48, 49, 51]. PHOX2B also influences neural crest migration (Fig. 1), a downstream effect of the development of the ENS. CCHS causative PHOX2B variants have been identified in patients with HSCR and CCHS and/or neuroblastoma, belonging to all genotypic classes and occurring in any of the three exons [2, 3, 28, 52]. Furthermore, it has been observed that the regulation of RET expression is PHOX2B-dependent [53], including in human cell lines in which the RET promoter has been demonstrated as a PHOX2B transcriptional target [54] so that PHOX2B alterations in CCHS may cause or predispose to HSCR. Based on what is already known about RET haploinsufficiency in HSCR, the susceptibility to develop HSCR in CCHS is likely due to the over-representation of the so-called “RET + 3” allele that is associated with the downregulation of RET expression [55, 56]. Reduced RET levels would be caused by the additive effect of large PARMs in the PHOX2B gene, impairing the transactivation of the RET promoter and the induction of RET [57]. Similarly, severe NPARMs, resulting from a gain of one nucleotide or a loss of two nucleotides and known as “frame2”, could abolish the induction of PHOX2B-mediated RET expression, thus explaining their association with syndromic CCHS [52].
HSCR most often clinically presents in the neonatal period with delayed passage of meconium, abdominal distention, emesis, or feeding intolerance. The primary manifestation of undiagnosed Hirschsprung's disease after the first few days of life through age 3 years old is failure to thrive with constipation and similar gastrointestinal manifestations. The diagnosis of HSCR is very rare for patients with CCHS beyond early childhood, since symptoms are nearly all present since birth [58, 59]. The gold standard for diagnosis is a punch or full thickness rectal biopsy with sufficient tissue demonstrating the absence of ganglion cells. Patients can also be screened based on level of suspicion with an abdominal radiograph, barium enema, and anorectal manometry [58]. The treatment for HSCR is surgical resection with leveling enterostomy or colostomy, and ultimately, a pull-through surgery to prioritize preservation of the internal anal sphincter function.
After surgery, many patients achieve normal or near-normal bowel function. The most common long-term problems of those with HSCR into adulthood are related to impaired bowel function and motility, including bowel emptying problems, constipation, and fecal incontinence [60]. Associated complications in HSCR include intestinal failure with long segment and total colonic HSCR, HSCR entercolitis, toxic megacolon, and volvulus, but are primarily issues for children younger than 5 years old [59]. Ongoing medical management of constipation, fecal incontinence, and diarrhea due to HSCR consists of diet modifications, bulking agents, including fiber supplementation, anti- and pro-motility agents, and injection of botulinum toxin to the external anal sphincter. For those with poor anorectal sensation or sphincter abnormalities, more invasive options can be offered, including a myotomy or diverting enterostomy or colostomy. Large quaternary pediatric hospital centers have dedicated HSCR surgical teams with expertise to manage patients throughout childhood, and occasionally follow active patient cases into young adulthood. The transition of medical care in those with HSCR is important and has been identified as a common need in this patient group [60]. Patients with CCHS and HSCR require long-term follow-up by a gastroenterologist. Furthermore, a proposed model of transitional care for those with HSCR disease also includes a colorectal surgeon, continence nurse, and gynecologist [60, 61]. Into adolescence and young adulthood, referral for pelvic floor therapy can also be important.
Additional features of gastrointestinal dysfunction in those with CCHS commonly include esophageal dysmotility, dysphagia, and delayed oral motor skills and speech. These issues tend to improve in the first years of life with normalized function, although dysarthria may remain into adolescence [50]. Management may include a gastrostomy tube for a limited duration, and active Speech Therapy. Early evaluation for these issues is indicated, and patients should be screened for motility-related gastrointestinal issues at each visit [3].
Neural crest tumors
Tumors of neural crest origin, including neuroblastoma, ganglioneuroma, and ganglioneuroblastoma (Fig. 1), are present in up to 5% of patients with CCHS, compared to 1: 10,000 in the general population [28]. NCTs are rare in patients with PARMs, occurring in < 2% of those with genotypes 20/30–20/33, and more common in those with NPARMs, occurring in > 45% of patients [1, 62]. NCTs in PARMs are usually ganglioneuroma and ganglioneuroblastoma, except for one known pediatric patient with genotype 20/33 and metastatic neuroblastoma [1, 26,27,28]. Tumors among patients with NPARMs are most typically neuroblastoma, although a subset will present with benign NCTs [1].
NCTs can occur anywhere along the sympathetic chain from neck to pelvis, with the most common location in the adrenal medulla [15]. The median age of presentation of CCHS-related NCTs mirrors the age of presentation in non-CCHS patients with sporadic tumors [62] with median age of presentation at 20 months. In a Children’s Oncology Group analysis of 3666 patients with neuroblastoma that presented clinically, 80% of patients were identified by the age of 6 years and 98% were diagnosed by 10 years of age [63].
NCTs that present in patients with CCHS overall have variable clinical behavior, though are generally less aggressive than sporadic neuroblastomas [26, 27]. Treatment may require surgery alone if the tumor is localized [1]. If metastatic or more aggressive, based on histologic or molecular features, chemotherapy as well as radiotherapy, autologous stem cell transplantation, or immunotherapy may be required.
With the large variation in tumor prevalence by PHOX2B genotype, CCHS-based guidelines recommend a screening approach based on PHOX2B variant [1,2,3]. Specifically, surveillance for NCT has been recommended for all patients with NPARM variants and PARMs with 28 polyalanine repeats or longer [1,2,3]. Surveillance testing is performed with abdominal ultrasound, chest X-ray, and urine catecholamines, including homovanillic acid and vanillylmandelic acid [62]. Screening recommendations vary, but the overarching approach is based on age of disease presentation, such as screening every 3–6 months until age 6 years, and then every 6 months until age 10 years [2, 3, 34, 62]. After age 7 years, frequency and type of imaging and surveillance should be performed in collaboration with a pediatric oncology team. Most often, the likelihood of developing a neural crest tumor after age 10 years is very low, so no additional surveillance is recommended at this time [63].
Ophthalmologic
Pupillary reactivity to light may be altered in patients with CCHS. While those with PHOX2B 20/25 genotype have responses similar to controls, those with 20/26 and 20/27 genotypes have decreased pupillary responsiveness [64]. Reactivity to light stimulus also becomes more pronounced with increases in age [65]. Additional common ophthalmological abnormalities in CCHS are strabismus and ptosis [3, 64]. Comprehensive ophthalmologic evaluation is recommended at the time of diagnosis and often needed annually [3].
Neurodevelopment
Neurocognitive testing is an important aspect of management in patients with CCHS, since they are at risk for deficits. Developmental delay may occur as early as preschool age [66, 67]. Those with CCHS have particular vulnerabilities in fluid reasoning and, compared to a normative population, have lower processing speed and working memory, as well as perceptual motor and visuographic skills [67, 68]. The mechanism for at-risk cognitive outcomes in those with CCHS is likely multifactorial and may include repeated intermittent hypoxia events, abnormal central neuronal differentiation, altered cerebral autoregulation, and atypical ANS responses to physiologic stressors, including orthostatic challenges and exercise [2, 19, 20, 46, 66].
Currently, there are insufficient data to conclude a PHOX2B genotype association with neurocognitive outcome, at least in middle-school age children with CCHS [67, 68]. It is likely that phenotype severity and the relationship to neurocognitive performance is confounded by PHOX2B genotype, age at presentation, age at diagnosis, time to intervention, compliance with ventilator management, and rigor of management [69, 70]. It appears that patients with CCHS and LO-CCHS, including adult diagnoses, who receive early and adequate respiratory support have higher cognitive testing scores, although studies are limited by sample size [10, 70, 71]. Therefore, prompt identification of CCHS and ventilator intervention with conservative respiratory management, particularly in the newborn period, and prioritizing adequate gas exchange into and throughout adulthood, likely optimizes long-term outcomes.
Consequently, formal neurocognitive testing is essential every 6 months before 3 years of age, and then annually thereafter. Serial neurocognitive testing allows the provider to make prompt referrals for therapy, services, and specifications for support, including an individualized learning plan in school. Testing results may serve as a proxy of ventilator compliance and management [2, 3].
PHOX2B genotype–phenotype heterogeneity
The relationship between the genetic basis of PHOX2B and the CCHS phenotype is becoming clearer [2, 9, 12]. This is especially true with regard to PARM variants, although significant improvements have recently been made to reveal the genotype–phenotype correlation for NPARMs [15, 52]. There are notable complications, too, when considering the possible clinical effects of PHOX2B variants, as both reduced penetrance and variable expressivity have been reported [15, 35]. In particular, reduced penetrance refers to the case in which a causative variant is found in both affected and unaffected individuals, with full penetrant variants present only in affected individuals and never found in unaffected ones. On the other hand, regardless of the level of penetrance, affected individuals in a family with recurrence of a PHOX2B variant may have different symptoms, varying degrees of symptom severity, and variable symptom expressivity. Finally, there are PHOX2B variants that show phenotypic variability, e.g., the same variant can induce different phenotypes, sometimes in different individuals and with different degrees of penetrance [72, 73]. What contributes to the reduced penetrance and variable expressivity of PHOX2B variants is unknown. Several cases are described here, exemplifying the complexities of the role of PHOX2B and the genotype–phenotype relationship.
LO-CCHS is the most frequent manifestation of clinical variability in CCHS, mainly with genotypes 20/24 and 20/25, and occasionally NPARMs [2, 3, 35]. Additionally, copy number variations at the PHOX2B locus, other variants in the genetic background, and environmental cofactors have been proposed to underlie the late and highly variable manifestations of CCHS [2, 3, 35].
LO-CCHS manifests later in life, often with a dramatic and unexpected presentation of respiratory failure, possibly accompanied by a history of breath-holding spells, constipation, and neurocognitive delays [74,75,76,77]. A case report describes three generations of a pathogenic PHOX2B familial variant where the proband presented at age 4 years old with cyanosis in the setting of severe pneumonia, and was diagnosed with a PARM 20/25. The patient’s father was subsequently diagnosed with the PARM variant, and retrospectively was found to have severe headaches and excessive daytime sleepiness. Likewise, the proband's grandmother died of respiratory failure after benzodiazepine administration at age 50, and she was highly suspected of having CCHS [78]. Patients with PHOX2B NPARMs can have particularly highly variable phenotypes, too, ranging from severe respiratory conditions requiring continuous assisted ventilation, HSCR, increased risk of NCT, and subclinical manifestations that may eventually become evident with an additional stressor, such as airway infections or anesthesia [79].
Although most CCHS-associated PHOX2B variants occur as de novo events, the PHOX2B causative variant of mild phenotypes can be transmitted from asymptomatic parents, possibly manifesting symptoms later in life or remaining asymptomatic [3, 80]. An example of high phenotypic variability was presented in a case report across three generations with PHOX2B NPARM, c.245C > T (p.P82L). Of the four individuals described, two family members had ventilator dependence during sleep without other complications, another developed systemic hypertension during adulthood, and the last remained asymptomatic [80]. In addition, a three-generation family has also been reported in which four individuals were variably affected due to a PHOX2B NPARM (c.691_698dup p.Gly234Alafs*78). The proband exhibited hypoventilation at 4 weeks of life and had HSCR. His mother denied any symptoms other than occasional headaches, although she was a carrier of the same pathogenic variant. A maternal uncle also had HSCR, and died at age 4 from neuroblastoma. Finally, the proband’s maternal grandfather, also a carrier of the c.691_698dup variant, showed subtle abnormalities with short central apneas on a polysomnogram, although he maintained normoxia [75]. Lastly, a three-generation family heterozygous for an NPARM variant (c.241 + 2delT) has been reported with similar variability in clinical expression, including recurrent episodes of respiratory insufficiency associated with nocturnal hypoventilation and hypoxemia, chronic constipation, and HSCR [81].
As a further explanation, somatic mosaicism is most likely the reason for variability in phenotypes observed in families with a pathogenic PHOX2B variant [82,83,84]. Specifically, up to 25% of affected individuals with CCHS and a pathogenic PHOX2B variant have an asymptomatic parent who carries the PHOX2B familial pathogenic variant. A proportion of these parents remain asymptomatic as the PHOX2B variant exhibits low penetrance, e.g., PARMs with smaller expansions up to 25 alanines. Conversely, others carry a penetrant PHOX2B pathogenic variant in mosaic form, though this is difficult to quantify and genetically confirm, as specific tissue types may genetically differ [71, 84]. As such, it is assumed that asymptomatic parents with larger PHOX2B polyalanine expansions likely have mosaicism as these variants are fully penetrant, e.g., PARMs with more than 25 alanines on the affected allele [82, 83].
Transitional care in CCHS
Practical aspects of living independently in CCHS
There are various paradigms of living independently with chronic illness and the “health care transition,” or the planned and purposeful movement of adolescents and young adults from child-centered to adult-centered care (e.g., the AAP Clinical Report “Supporting the Health Care Transition From Adolescence to Adulthood in the Medical Home” and the Six Core Elements of Health Care Transition at gottransition.org) [85,86,87]. Across all ages and capabilities, the common starting point along this developmental trajectory is self-care and self-knowledge. Therefore, it is crucial for a pediatric patient with CCHS to have ample opportunity to provide self-care in both general and condition-specific ways for themselves. Parents and caregivers of those with CCHS must evaluate their child’s healthcare plan with each developmental and practical transition. For example, transitions in childhood and through adolescence routinely involve changes in classes, seasonality with the school year or summer, and weekday versus weekend routines. During these transitions, the adolescent is encouraged to gradually administer self-care with both medical knowledge and medical skills, to practice conversations in medical planning with family and friends to participate in care, and to contribute to emergency plans. Ultimately, clear communication of their medical condition and care plans by adults with CCHS to other responsible adults is critical to the maintenance and success of their independence and health. An example of a similar three-prong approach, ‘the “Pathway to Independence” tool’, is illustrated in the supplement (Online Resource 1).The use of technology also assists in disease management, including the use of pre-set reminders on a smart phone and watch to remind the patient to obtain pulse oximetry and capnography measurements throughout the day. With newer technology, wearable options to detect oxygen saturation and heart rate are available. Lastly, the introduction of smaller ventilators allows for mobility. Ventilator add-ons enable the integration of pulse oximetry and end-tidal capnography, which decreases equipment needs.
Along these lines, it is recommended to have a Portable Medical Health Summary readily available on mobile devices and stored electronically to be easily accessed and shared with others, including Emergency Medical Services. It is also advisable for each patient with CCHS to carry critical medical information available on a smart watch, medical bracelet or necklace, or card located on a phone cover, for example.
Additional facets of health care transition involve transfer of a multi-disciplinary medical care team from pediatric physicians to adult providers, including an adult primary care physician, pulmonologist specializing in ventilators, cardiologist, gastroenterologist, and sleep medicine physician [35]. If care is established during adolescence, psychologists often will continue to follow patients into adulthood. This an important area in the patient healthcare plan and can greatly contribute to self-care and self-knowledge. Additionally, patients will need access to health insurance coverage, knowledge of accepted providers and hospitals and programs, skills to identify and advocate for accommodations needed for successful functioning at school and work, and access to appropriate adult community resources based on individual needs. Young adults with CCHS, as with other chronic medical conditions, may be referred to a “Transition to Adult Healthcare” program often found in pediatric hospital centers. Appointments can be made with these dedicated teams that include physicians, practitioners, case managers, and social workers for individual recommendations and management. This team can also be utilized for referral towards additional programs and resources, particularly for those who qualify for publicly available services.
Family planning and childbearing in CCHS
The influence of CCHS on sexual function and complications in patients is undefined, although anecdotally the condition does not appear to be an impediment to achieving pregnancy. Likewise, the influence of the menstrual cycle may also contribute to subtle variations in gas exchange requiring small adjustments to respiratory support, but this, too, is based on anecdotal reports and is an area that needs further research.
CCHS is a disorder transmitted in an autosomal dominant manner where an affected individual with a PHOX2B pathogenic variant has a 50% risk of producing an affected child [1]. With this information, reproductive education to review options for family planning is very important for young adults. Pre-implantation genetics can identify PHOX2B abnormalities in embryos in which one biologic parent has a known PHOX2B pathogenic variant. Fertility options include eliminating the chance of inheritance through intra-uterine insemination (IUI) or in vitro fertilization (IVF) using non-affected sperm donors with IUI or IVF, egg donors with IVF, or embryos with IVF. Adoption is also an option.
Once pregnant, determining whether a fetus has CCHS can be done via chorionic villus sampling (CVS) or amniocentesis [88]. CVS can be done as early as 11–14 weeks and amniocentesis is generally performed between 15 and 20 weeks.
Medical care of the expectant mother with CCHS throughout the pregnancy requires vigilant attention and meticulous management. The mother’s care should be coordinated amongst a multidisciplinary team, including maternal–fetal medicine, pulmonary, sleep medicine, and experts in CCHS. Generally, sleep-disordered breathing worsens over the course of a pregnancy, and is associated with adverse maternal and fetal outcomes [89, 90]. Maternal ventilatory support may need to be adjusted, given the changing respiratory mechanics of pregnancy offsetting what potential benefit progesterone may have on ventilatory drive. Sleep studies can be used to adjust ventilator settings and evaluate for adequacy of gas exchange, though clinical titrations during continuous oximetry and capnography may be sufficient should polysomnography not be readily available.
Fetal outcomes are somewhat undefined in infants born to a mother with CCHS, but a recent case-series reported reassuring neonatal outcomes [90]. Of note, fetal breathing in those with CCHS prenatally determined is thought to be similar to respirations in a fetus without CCHS, apart from a lack of responsiveness to moderate hypercarbia, in which the normal fetal response is an increase in continuous breathing [88]. In one neonatal case with postnatally confirmed CCHS with an NPARM variant and HSCR, a prenatal description of polyhydramnios, decreased fetal heart rate variability, and decreased fetal movement was reported [91].
For expectant mothers with CCHS, labor and delivery are recommended at a tertiary or quaternary center with a level III or IV neonatal intensive care unit. Additional considerations for expectant mothers with CCHS and those with antenatal fetal diagnoses of CCHS are described in Table 2. For both, a multidisciplinary team should be present, including providers from maternal–fetal medicine, adult and pediatric pulmonology, critical care, obstetric anesthesia, and neonatology to support the high-risk delivery (Table 3) for the mother and to ensure a smooth transition to extrauterine life for the newborn [92].
Risks of anesthesia with procedures in CCHS
Procedures requiring anesthesia deserve special preparation and considerations for patients with CCHS. It is recommended that an anesthesia team be educated toward an understanding of the innate features of CCHS and prepared for the most likely complications [93]. Plans for the case should involve the surgeon, a pain medicine specialist, critical care physician, pulmonologist, and experts in the management of CCHS. In general, the goal of any procedure involving anesthesia is to avoid unnecessary risk of cardiopulmonary depression. Those with CCHS are at particularly high risk of worsened hypoventilation and acute respiratory failure with exposure to anesthesia [93, 94]. Additionally, in the setting of anesthesia, those with CCHS are susceptible to bradyarrhythmia, hemodynamic instability, and hypothermia [42, 43, 93, 94].
With preparation, most anesthetic agents can be safely used in patients with CCHS [93]. Largely, the most important aspects of administered anesthesia in those with CCHS involve monitoring throughout and into recovery. Standard monitoring recommendations include continuous pulse oximetry, non-invasive blood pressure, temperature, end-tidal capnography, and ECG [95]. Capnography is required for all procedures with sedation because patients with CCHS may have profound respiratory depression with even minimally sedating medications. Cerebral near-infrared spectroscopy may also be helpful in predicting regional brain perfusion and oxygenation.
Ambulatory dental procedures requiring anesthesia should have similar considerations for those with CCHS. Successful cases have involved the use of rapid-reversal inhaled anesthetic (e.g., sevoflurane), local or regional blocks, and behavioral modifications, including tell-show-do positive reinforcement and distraction [94, 96]. Depending on the nature of the dental procedure, use of masks for NIPPV may be painful, and so preemptive selection of a mask able to be used comfortably during postoperative recovery is recommended. Most important is ensuring that the patient receives appropriate artificial ventilation throughout the procedure and during recovery. Vigilant monitoring of oxygenation and ventilation is accomplished by using pulse oximetry and end-tidal capnography.
Risks of medications, alcohol, and drugs in CCHS
In general, over the counter (OTC) medications, prescription medications, and the use of drugs and alcohol have not been formally studied in individuals with CCHS, but potential risk exists with use. OTC medications with an adverse effect of drowsiness, including antihistamines, melatonin, and other sleep-aids, could potentially compromise both levels of alertness and inherent ventilatory drive. The use of prescription medication known to have sedating effects, including anxiolytics, opioid pain medications, antiemetics, and antiepileptic medications, pose similar risks. With initial use of these medications, the authors recommend monitoring the patient’s gas exchange closely over the initial weeks of treatment. Likewise, alcohol, many illicit drugs, and marijuana can depress respiratory drive and level of alertness, which places the patient at risk for respiratory insufficiency requiring higher levels of respiratory support. There is risk for death if the patient is left without artificial ventilation [97]. The authors suggest preemptive substance abuse counseling starting in early adolescence. Conventionally, for an adolescent with CCHS, this would focus on total abstinence. However, this strategy may not prove realistic with the societal behaviors and perceived norms involving alcohol and drugs. Therefore, thorough education regarding the effects of drugs and alcohol on control of breathing is crucial for all adolescents and adults with CCHS.
Acute illness and emergency management in CCHS
Patients with CCHS are at-risk for life-threatening events related to hypoventilation, airway emergencies, features of ANSD (e.g., cardiac sinus pauses), and complications with intercurrent illness [2, 3]. Those with CCHS have reported a decreased sense of anxiety with abnormal activity in the amygdala and limbic system by fMRI, [1, 98] and, perhaps, because of this, they are not aware of physiologic compromise. Thus, it is recommended to have a low threshold to evaluate for an intercurrent illness. With common infections, such as an upper respiratory viral infection, independent of airway or parenchymal lung involvement, patients tend to need additional and, even continuous, respiratory support, [1,2,3] likely secondary to the physiologic burden of illness and inflammatory responses. It is imperative that end-tidal capnography and pulse oximeter measurements be obtained hourly when awake, at a minimum, and continuously during sleep, since these are sometimes the only indicator of an early intercurrent illness [2, 3]. With abnormal gas measurements, the authors recommend consideration of a chest radiograph to assess for an indolent pneumonia.
Monitoring for indirect signs of illness is also advisable, such as a mild elevation in temperature, since patients with CCHS typically have attenuated temperatures and circadian patterning of temperatures. Even with severe illness, patients with CCHS have a reduced ability to mount a febrile response [99]. Likewise, anecdotally, even with severe illness such as hypovolemic shock with end-organ injury, patients may have abnormal cardiovascular responsiveness and demonstrate an inability to appropriately increase resting heart rate [37, 46]. Therefore, the authors recommend establishing detailed knowledge of a patient’s responses to mild, moderate, and severe illness or physical stressors for anticipatory care.
Another aspect of managing acute and severe illness in those with CCHS includes providing anticipatory guidance in situations requiring Emergency Medical Services and Emergency Room care. Cardiopulmonary resuscitation is a standardized emergency procedure, and the initial patient assessment may not adequately address even basic life support for those with CCHS. For instance, providers may assess the patient to be breathing regularly, although covert hypoventilation is present, placing the patient at risk of a cardiac arrest due to acute respiratory insufficiency. It is imperative the patient and family be well-versed at informing medical providers in an emergency that the patient has a rare disease without sufficient breathing, and must have manual breaths delivered until established on continuous monitors with capnography and pulse oximetry.
Conclusions
-
CCHS is a condition due to a pathogenic variant in the PHOX2B gene that results in presentation with hypoventilation and apnea, risk for HSCR and NCT, and ANSD, including cardiovascular, gastrointestinal, and ophthalmologic abnormalities.
-
The transition from pediatric to adult medical care requires a thorough understanding of CCHS by a multi-disciplinary team with subspecialities based on the patient’s needs, and heavily influenced by the PHOX2B genotype–phenotype relationship.
-
CCHS is classically confirmed with PHOX2B genetic testing within the first month of life, but patients can also present after this time, and even into adulthood, with LO-CCHS. While a genotype–phenotype relationship is recognized in CCHS and LO-CCHS, there is also phenotypic heterogeneity, and, in a subset of patients, reduced penetrance and variable expressivity.
-
Topics of transitional care for adolescent and adult patients with CCHS include family planning, anesthesia, alcohol and drugs, and acute illness and emergencies. Counseling is the most important aspect of these topics to lay the foundation for self-care and independence, the hallmark of the transition to adult medical care from pediatric medicine.
-
As CCHS increases in disease prevalence due to clinical recognition and increased survivorship, the medical care of those with CCHS in the transition from pediatric to adult medicine is a promising area of clinical and research development.
Data availability
This is a review article and data are available in a public (institutional, general or subject specific) repository that issues datasets with DOIs (non-mandated deposition).
References
Weese-Mayer DE, Rand CM, Khaytin I et al (2021) Congenital central hypoventilation syndrome. In: Adam MP, Mirzaa GM, Pagon RA et al (eds) GeneReviews. University of Washington, Seattle
Weese-Mayer DE, Berry-Kravis EM, Ceccherini I et al (2010) An official ATS clinical policy statement: congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med 181(6):626–644
Trang H, Samuels M, Ceccherini I et al (2020) Guidelines for diagnosis and management of congenital central hypoventilation syndrome. Orphanet J Rare Dis 15(1):252–252
Amiel J, Laudier B, Attie-Bitach T et al (2003) Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet 33(4):459–461
Weese-Mayer DE, Berry-Kravis EM, Zhou L et al (2003) Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am J Med Genet A 123A(3):267–278
Bolande RP (1997) Neurocristopathy: its growth and development in 20 years. Pediatr Pathol Lab Med 17(1):1–25
Antic NA, Malow BA, Lange N et al (2006) PHOX2B mutation-confirmed congenital central hypoventilation syndrome: presentation in adulthood. Am J Respir Crit Care Med 174(8):923–927
Jennings LJ, Yu M, Rand CM et al (2012) Variable human phenotype associated with novel deletions of the PHOX2B gene. Pediatr Pulmonol 47(2):153–161
Matera I, Bachetti T, Puppo F et al (2004) PHOX2B mutations and polyalanine expansions correlate with the severity of the respiratory phenotype and associated symptoms in both congenital and late onset central hypoventilation syndrome. J Med Genet 41(5):373–380
Hino A, Terada J, Kasai H et al (2020) Adult cases of late-onset congenital central hypoventilation syndrome and paired-like homeobox 2B-mutation carriers: an additional case report and pooled analysis. J Clin Sleep Med 16(11):1891–1900
Zhou A, Rand CM, Hockney SM et al (2021) Paired-like homeobox gene (PHOX2B) nonpolyalanine repeat expansion mutations (NPARMs): genotype–phenotype correlation in congenital central hypoventilation syndrome (CCHS). Genet Med 23(9):1656–1663
Berry-Kravis EM, Zhou L, Rand CM, Weese-Mayer DE (2006) Congenital central hypoventilation syndrome: PHOX2B mutations and phenotype. Am J Respir Crit Care Med 174(10):1139–1144
Bachetti T, Di Duca M, Monica MD, Grappone L, Scarano G, Ceccherini I (2014) Recurrence of CCHS associated PHOX2B poly-alanine expansion mutation due to maternal mosaicism. Pediatr Pulmonol 49(3):E45–E47
Jennings LJ, Yu M, Zhou L, Rand CM, Berry-Kravis EM, Weese-Mayer DE (2010) Comparison of PHOX2B testing methods in the diagnosis of congenital central hypoventilation syndrome and mosaic carriers. Diagn Mol Patho 19(4):224
Bachetti T, Ceccherini I (2020) Causative and common PHOX2B variants define a broad phenotypic spectrum. Clin Genet 97(1):103–113
Ikeda K, Takahashi M, Sato S et al (2015) A Phox2b BAC transgenic rat line useful for understanding respiratory rhythm generator neural circuitry. PLoS ONE 10(7):e0132475
Moreira TS, Takakura AC, Czeisler C, Otero JJ (2016) Respiratory and autonomic dysfunction in congenital central hypoventilation syndrome. J Neurophysiol 116(2):742–752
Ruffault PL, D’Autréaux F, Hayes JA et al (2015) The retrotrapezoid nucleus neurons expressing Atoh1 and Phox2b are essential for the respiratory response to CO2. Elife. https://doi.org/10.7554/eLife.07051
Dubreuil V, Ramanantsoa N, Trochet D et al (2008) A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci U S A 105(3):1067–1072
Nobuta H, Cilio MR, Danhaive O et al (2015) Dysregulation of locus coeruleus development in congenital central hypoventilation syndrome. Acta Neuropathol 130(2):171–183
Kumar R, Macey PM, Woo MA, Alger JR, Harper RM (2008) Diffusion tensor imaging demonstrates brainstem and cerebellar abnormalities in congenital central hypoventilation syndrome. Pediatr Res 64(3):275–280
Tomycz ND, Haynes RL, Schmidt EF, Ackerson K, Kinney HC (2010) Novel neuropathologic findings in the Haddad syndrome. Acta Neuropathol 119(2):261–269
Kumar R, Macey PM, Woo MA, Harper RM (2010) Rostral brain axonal injury in congenital central hypoventilation syndrome. J Neurosci Res 88(10):2146–2154
Alturkustani M, Shillingford N, Zhou S, Wang L, Warren M (2021) Phox2b immunohistochemical staining in detecting enteric neural crest cells in Hirschsprung disease. Pediatr Dev Pathol 24(1):19–26
Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B (1999) Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer 86(2):349–363
Armstrong AE, Weese-Mayer DE, Mian A et al (2015) Treatment of neuroblastoma in congenital central hypoventilation syndrome with a PHOX2B polyalanine repeat expansion mutation: new twist on a neurocristopathy syndrome. Pediatr Blood Cancer 62(11):2007–2010
Heide S, Masliah-Planchon J, Isidor B et al (2016) Oncologic phenotype of peripheral neuroblastic tumors associated with PHOX2B non-polyalanine repeat expansion mutations. Pediatr Blood Cancer 63(1):71–77
Trochet D, Bourdeaut F, Janoueix-Lerosey I et al (2004) Germline mutations of the paired-like homeobox 2B ( PHOX2B) gene in neuroblastoma. Am J Hum Genet 74(4):761–764
Raabe EH, Laudenslager M, Winter C et al (2008) Prevalence and functional consequence of PHOX2B mutations in neuroblastoma. Oncogene 27(4):469–476
Vanderlaan M, Holbrook CR, Wang M, Tuell A, Gozal D (2004) Epidemiologic survey of 196 patients with congenital central hypoventilation syndrome. Pediatr Pulmonol 37(3):217–229
Magalhães J, Madureira N, Medeiros R et al (2014) Late-onset congenital central hypoventilation syndrome and a rare PHOX2B gene mutation. Sleep Breath 19(1):55–60
Mahfouz AKM, Rashid M, Khan MS, Reddy P (2011) Late onset congenital central hypoventilation syndrome after exposure to general anesthesia. Can J Anesth 58(12):1105–1109
Doherty LS, Kiely JL, Deegan PC et al (2007) Late-onset central hypoventilation syndrome: a family genetic study. Eur Respir J 29(2):312–316
Porcaro F, Paglietti MG, Cherchi C, Schiavino A, Chiarini Testa MB, Cutrera R (2021) How the management of children with congenital central hypoventilation syndrome has changed over time: two decades of experience from an Italian center. Front Pediatr 9:648927–648927
Kasi AS, Li H, Harford K-L et al (2022) Congenital central hypoventilation syndrome: optimizing care with a multidisciplinary approach. J Multidiscip Healthc 15:455–469
Valika T, Chin AC, Thompson DM et al (2019) Airway obstruction during sleep due to diaphragm pacing precludes decannulation in young children with CCHS. Respiration 98(3):263–267
Ghosh RN, Guglani L, Westbrook AL et al (2022) Impaired ventilation during 6-min walk test in congenital central hypoventilation syndrome. Pediatr Pulmonol 57(7):1660–1667
Alvaro RE, Rigatto H (2017) Control of breathing in fetal life and onset and control of breathing in the neonate, 5th edn. Elsevier Inc, New York, pp 737–747.e733
Chin AC, Shaul DB, Patwari PP, Keens TG, Kenny AS, Weese-Mayer DE (2012) Diaphragmatic pacing in infants and children with congenital central hypoventilation syndrome. In: Kheirandish-Gozal L, Gozal D (eds) Sleep disordered breathing in children: a comprehensive clinical guide to evaluation and treatment. Humana Press, Totowa, pp 553–573
Diep B, Wang A, Kun S et al (2015) Diaphragm pacing without tracheostomy in congenital central hypoventilation syndrome patients. Respiration 89(6):534–538
Wang A, Kun S, Diep B, Davidson Ward SL, Keens TG, Perez IA (2018) Obstructive sleep apnea in patients with congenital central hypoventilation syndrome ventilated by diaphragm pacing without tracheostomy. J Clin Sleep Med 14(2):261–264
Silvestri JM, Hanna BD, Volgman AS, Jones PJ, Barnes SD, Weese-Mayer DE (2000) Cardiac rhythm disturbances among children with idiopathic congenital central hypoventilation syndrome. Pediatr Pulmonol 29(5):351–358
Gronli JO, Santucci BA, Leurgans SE, Berry-Kravis EM, Weese-Mayer DE (2008) Congenital central hypoventilation syndrome: PHOX2B genotype determines risk for sudden death. Pediatr Pulmonol 43(1):77–86
Dudoignon B, Denjoy I, Patout M et al (2022) Heart rate variability in congenital central hypoventilation syndrome: relationships with hypertension and sinus pauses. Pediatr Res. https://doi.org/10.1038/s41390-022-02215-4
Trang H, Boureghda S, Denjoy I, Alia M, Kabaker M (2003) 24-Hour BP in children with congenital central hypoventilation syndrome. Chest 124(4):1393–1399. https://doi.org/10.1378/chest.124.4.1393
Vu EL, Dunne EC, Bradley A et al (2021) Cerebral autoregulation during orthostatic challenge in congenital central hypoventilation syndrome. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202103-0732OC
Weese-Mayer DE, Silvestri JM, Huffman AD et al (2001) Case/control family study of autonomic nervous system dysfunction in idiopathic congenital central hypoventilation syndrome. Am J Med Genet 100(3):237–245
Amiel J, Sproat-Emison E, Garcia-Barcelo M et al (2008) Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet 45(1):1–14
Alves MM, Sribudiani Y, Brouwer RWW et al (2013) Contribution of rare and common variants determine complex diseases—Hirschsprung disease as a model. Dev Biol 382(1):320–329
Balakrishnan K, Perez IA, Keens TG, Sicolo A, Punati J, Danialifar T (2020) Hirschsprung disease and other gastrointestinal motility disorders in patients with CCHS. Eur J Pediatr 180(2):469–473
Chatterjee S, Karasaki KM, Fries LE, Kapoor A, Chakravarti A (2021) A multi-enhancer RET regulatory code is disrupted in Hirschsprung disease. Genome Res 31(12):2199–2208
Di Lascio S, Benfante R, Di Zanni E et al (2018) Structural and functional differences in PHOX2B frameshift mutations underlie isolated or syndromic congenital central hypoventilation syndrome. Hum Mutat 39(2):219–236
Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF (1999) The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399(6734):366–370
Bachetti T, Borghini S, Ravazzolo R, Ceccherini I (2005) An in vitro approach to test the possible role of candidate factors in the transcriptional regulation of the RET proto-oncogene. Gene Expr 12(3):137–149
Emison ES, Garcia-Barcelo M, Grice EA et al (2010) Differential contributions of rare and common, coding and noncoding ret mutations to multifactorial Hirschsprung disease liability. Am J Hum Genet 87(1):60–74
Fitze G, König IR, Paditz E et al (2008) Compound effect of PHOX2B and RET gene variants in congenital central hypoventilation syndrome combined with Hirschsprung disease. Am J Med Genet A 146A(11):1486–1489
Di Zanni E, Adamo A, Belligni E et al (2017) Common PHOX2B poly-alanine contractions impair RET gene transcription, predisposing to Hirschsprung disease. Biochim Biophys Acta 1863(7):1770–1777
Arshad A, Powell C, Tighe MP (2012) Hirschsprung’s disease. BMJ 345(oct012):e5521–e5521
Doodnath R, Puri P (2010) A systematic review and meta-analysis of Hirschsprung’s disease presenting after childhood. Pediatr Surg Int 26(11):1107–1110
Hoel AT, Tofft L, Bjørnland K et al (2021) Reaching adulthood with Hirschsprung’s disease: patient experiences and recommendations for transitional care. J Pediatr Surg 56(2):257–262
Giuliani S, Grano C, Aminoff D et al (2017) Transition of care in patients with anorectal malformations: consensus by the ARM-net consortium. J Pediatr Surg 52(11):1866–1872
Kamihara J, Bourdeaut F, Foulkes WD et al (2017) Retinoblastoma and neuroblastoma predisposition and surveillance. Clin Cancer Res 23(13):e98–e106
London WB, Castleberry RP, Matthay KK et al (2005) Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J Clin Oncol 23(27):6459–6465
Patwari PP, Stewart TM, Rand CM et al (2012) Pupillometry in congenital central hypoventilation syndrome (CCHS): quantitative evidence of autonomic nervous system dysregulation. Pediatr Res 71(3):280–285
Fadl-Alla A, Winston M, Rand C, et al. (2022) Pupillary Parasympathetic and Sympathetic Dysfunction in a Longitudinal Congenital Central Hypoventilation Syndrome (CCHS) Cohort from Infancy to Young Adulthood: Potential Biomarker for Intervention Trials. In: C110 Bridging the gaps: sleep, NIV, pulmonary disease, and comorbidities ed: American Thoracic Society International Conference Abstracts A4997-A4997 261.
Charnay AJ, Antisdel-Lomaglio JE, Zelko FA et al (2016) Congenital central hypoventilation syndrome: neurocognition already reduced in preschool-aged children. Chest 149(3):809–815
Zelko FA, Nelson MN, Leurgans SE, Berry-Kravis EM, Weese-Mayer DE (2010) Congenital central hypoventilation syndrome: neurocognitive functioning in school age children. Pediatr Pulmonol 45(1):92–98
Zelko FA, Stewart TM, Brogadir CD, Rand CM, Weese-Mayer DE (2018) Congenital central hypoventilation syndrome: Broader cognitive deficits revealed by parent controls. Pediatr Pulmonol 53(4):492–497
Trang H, Bourgeois P, Cheliout-Heraut F (2020) Neurocognition in congenital central hypoventilation syndrome: influence of genotype and ventilation method. Orphanet J Rare Dis 15(1):322
Ogata T, Muramatsu K, Miyana K, Ozawa H, Iwasaki M, Arakawa H (2020) Neurodevelopmental outcome and respiratory management of congenital central hypoventilation syndrome: a retrospective study. BMC Pediatr 20(1):342–342
Shimokaze T, Sasaki A, Meguro T et al (2015) Genotype–phenotype relationship in Japanese patients with congenital central hypoventilation syndrome. J Hum Genet 60(9):473–477
Cooper DN, Krawczak M, Polychronakos C, Tyler-Smith C, Kehrer-Sawatzki H (2013) Where genotype is not predictive of phenotype: towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum Genet 132(10):1077–1130
Kingdom R, Tuke M, Wood A et al (2022) Rare genetic variants in genes and loci linked to dominant monogenic developmental disorders cause milder related phenotypes in the general population. Am J Hum Genet 109(7):1308
Chuen-im P, Marwan S, Carter J, Kemp J, Rivera-Spoljaric K (2014) Heterozygous 24-polyalanine repeats in the PHOX2B gene with different manifestations across three generations: heterozygous 24-polyalanine repeats. Pediatr Pulmonol 49(2):E13–E16
Low KJ, Turnbull AR, Smith KR et al (2014) A case of congenital central hypoventilation syndrome in a three-generation family with non-polyalanine repeat PHOX2B mutation. Pediatr Pulmonol 49(10):E140–E143
Sivan Y, Zhou A, Jennings LJ et al (2019) Congenital central hypoventilation syndrome: severe disease caused by co-occurrence of two PHOX2B variants inherited separately from asymptomatic family members. Am J Med Genet A 179(3):503–506
Trivedi A, Waters K, Suresh S, Nair R (2010) Congenital central hypoventilation syndrome: four families. Sleep Breath 15(4):785–789
Klaskova E, Drabek J, Hobzova M et al (2016) Significant phenotype variability of congenital central hypoventilation syndrome in a family with polyalanine expansion mutation of the PHOX2B gene. Biomed Pap 160(4):495–498
Kasi AS, Li H, Jurgensen TJ, Guglani L, Keens TG, Perez IA (2021) Variable phenotypes in congenital central hypoventilation syndrome with PHOX2B nonpolyalanine repeat mutations. J Clin Sleep Med 17(10):2049–2055
Kasi AS, Jurgensen TJ, Yen S, Kun SS, Keens TG, Perez IA (2017) Three-generation family with congenital central hypoventilation syndrome and novel PHOX2B gene non-polyalanine repeat mutation. J Clin Sleep Med 13(7):925–927
Pace NP, Pace Bardon M, Borg I (2020) A respiratory/Hirschsprung phenotype in a three-generation family associated with a novel pathogenic PHOX2B splice donor mutation. Mol Genet Genom Med. 8(12):e1528
Bachetti T, Parodi S, Di Duca M, Santamaria G, Ravazzolo R, Ceccherini I (2011) Low amounts of PHOX2B expanded alleles in asymptomatic parents suggest unsuspected recurrence risk in congenital central hypoventilation syndrome. J Mol Med 89(5):505–513
Meguro T, Yoshida Y, Hayashi M et al (2012) Inheritance of polyalanine expansion mutation of PHOX2B in congenital central hypoventilation syndrome. J Hum Genet 57(5):335–337
Parodi S, Vollono C, Baglietto MP et al (2010) Congenital central hypoventilation syndrome: genotype–phenotype correlation in parents of affected children carrying a PHOX2B expansion mutation. Clin Genet 78(3):289–293
Six Core Elements of Health Care Transition. Got Transition Web site. https://www.gottransition.org/six-core-elements/. Published 2014. Updated 2022. Accessed July 1, 2022.
Cooley WC, Sagerman PJ (2011) Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics 128(1):182–200
White PH, Cooley WC, Transitions Clinical Report Authoring et al (2018) (2022) Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics 142(5):e20182587
Rajendran GP, Kessler MS, Manning FA (2009) Congenital central hypoventilation syndrome (Ondine’s curse): prenatal diagnosis and fetal breathing characteristics. J Perinatol 29(10):712–713
Brown NT, Turner JM, Kumar S (2018) The intrapartum and perinatal risks of sleep-disordered breathing in pregnancy: a systematic review and metaanalysis. Am J Obstet Gynecol 219(2):147-161.e141
Maloney MA, Keens TG, Vanderlaan MB, Perez IA (2020) Pregnancy in congenital central hypoventilation syndrome. Am J Obstet Gynecol MFM 2(4):100237
De Montpellier S, Sznajer Y, Amiel J et al (2013) An unusual cause of fetal hypomobility:congenital central hypoventilation syndrome associated with Hirschsprung disease. Eur J Pediatr 173(12):1607–1609
Yousif A, Chandler A, Ghandour M, Akinpeloye A (2021) Congenital central hypoventilation syndrome: what to expect during pregnancy. Curēus 13(9):e17827. https://doi.org/10.7759/cureus.17827
Ballard HA, Leavitt OS, Chin AC et al (2018) Perioperative anesthetic management of children with congenital central hypoventilation syndrome and rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation undergoing thoracoscopic phrenic nerve-diaphragm pacemaker implantation. Paediatr Anaesth 28(11):963–973
Basu SM, Chung FF, AbdelHakim SF, Wong J (2017) Anesthetic considerations for patients with congenital central hypoventilation syndrome: a systematic review of the literature. Anesth Analg 124(1):169–178
Standards for Basic Anesthetic Monitoring. https://www.asahq.org/~/media/Sites/ASAHQ/Files/Public/Resources/standards-guidelines/standards-for-basic-anesthetic-monitoring.pdf. Accessed October 19, 2022.
Boka V, Lefkelidou A, Athanasiadou E (2016) Dental treatment of a child with congenital central hypoventilation syndrome. Eur Arch Paediatr Dent 17(3):211–214
Chen ML, Turkel SB, Jacobson JR, Keens TG (2006) Alcohol use in congenital central hypoventilation syndrome. Pediatr Pulmonol 41(3):283–285
Macey PM, Woo MA, Macey KE et al (2005) Hypoxia reveals posterior thalamic, cerebellar, midbrain, and limbic deficits in congenital central hypoventilation syndrome. J Appl Physiol 98(3):958–969
Saiyed R, Rand CM, Carroll MS et al (2016) Congenital central hypoventilation syndrome (CCHS): circadian temperature variation. Pediatr Pulmonol 51(3):300–307
Acknowledgements
The authors thank Tracey M. Stewart, RRT, Technical Director of the Center for Autonomic Medicine in Pediatrics, and Alison Osborne, APN on HSCR Surgical Team, at Ann & Robert H. Lurie Children’s Hospital, Chicago, IL, USA, for their clinical contributions to the detailed explanations in this review.
Funding
Chicago Community Trust Foundation PHOX2B Patent Fund (C.M.R.). No financial relationships relevant to this article to disclose.
Author information
Authors and Affiliations
Contributions
Conceptualization: SMS, KY, IAP, IC, MLC, KCK, EAS, CMR, DEW-M. Literature Search: SMS, KY, IAP, IC, MLC, KCK, TGK, IK, EAS, AM, HAB, CMR, DEW-M. Writing, original draft: SMS, KY, IAP, IC, MLC, KCK, TGK, IK, EAS, AM, HAB, DEW-M. Writing, review, editing: SMS, KY, IAP, IC, MLC, KCK, TGK, IK, EAS, AM, HAB, CMR, DEW-M. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Slattery, S.M., Perez, I.A., Ceccherini, I. et al. Transitional care and clinical management of adolescents, young adults, and suspected new adult patients with congenital central hypoventilation syndrome. Clin Auton Res 33, 231–249 (2023). https://doi.org/10.1007/s10286-022-00908-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-022-00908-8