Abstract
Objectives
To investigate a possible association between autonomic dysfunction and fatigue in people with multiple sclerosis.
Methods
In 70 people with multiple sclerosis early in the disease course (51 females, mean age 33.8 ± 9.1), quantitative sudomotor axon reflex tests, cardiovascular reflex tests (heart rate and blood pressure responses to the Valsalva maneuver and heart rate response to deep breathing), and the tilt table test were performed. Participants completed the Composite Autonomic Symptom Score 31, the Modified Fatigue Impact Scale, and the Epworth Sleepiness Scale, as well as the Beck Depression Inventory. Cutoff scores of ≥ 38 or ≥ 45 on the Modified Fatigue Impact Scale were used to stratify patients into a fatigued subgroup (N = 17 or N = 9, respectively).
Results
We found clear associations between fatigue and scores in subjective tests of the autonomic nervous system: fatigued patients scored significantly worse on Composite Autonomic Symptom Score 31, and there was a strong correlation between the Modified Fatigue Impact Scale and the Composite Autonomic Symptom Score 31 (rs = 0.607, p < 0.001). On the other hand, we found only modest associations between fatigue and scores in objective tests of the autonomic nervous system: there was a clear trend for lower sweating outputs at all measured sites, which reached statistical significance for the distal leg and foot. We found weak correlations between the Modified Fatigue Impact Scale and the Valsalva ratio (rs = − 0.306, p = 0.011), as well as between the Modified Fatigue Impact Scale and quantitative sudomotor axon reflex tests of the forearm, proximal, and distal lower leg (rs = − 0.379, p = 0.003; rs = − 0.356, p = 0.005; and rs = − 0.345, p = 0.006, respectively). A multiple regression model showed that the Composite Autonomic Symptom Score 31, Beck Depression Inventory, and Epworth Sleepiness Scale were independent predictors of fatigue (p = 0.005, p = 0.019, and p = 0.010, respectively).
Conclusion
These results suggest that—even early in the course of the disease—people with multiple sclerosis suffer from objective and subjective impairments of the autonomic nervous system. The results also point to an association between autonomic nervous system impairment and multiple sclerosis related fatigue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatigue, defined as extreme and persistent mental and/or physical tiredness, weakness, or exhaustion [1], is one of the most common symptoms of many neurological conditions. It can be primary, when it is considered to be part of the underlying neurological condition, or secondary, when it results from the presence of other concomitant circumstances or diseases [2].
Fatigue is one of the most frequent and burdensome symptoms of multiple sclerosis (MS) [3]. Multiple sclerosis related fatigue (MSRF) is reported in up to 80% of people with MS (pwMS), and over 55% of patients report fatigue as the worst symptom of MS [4]. Several MS-specific processes may be responsible for primary MSFR, such as impaired interactions between functionally related cortical and subcortical areas, activation of proinflammatory cytokines, and dysregulation of the neuroendocrine system [5,6,7]. However, a substantial proportion of pwMS also have concomitant depression and sleep disorders that are strongly related to secondary MSRF [8, 9].
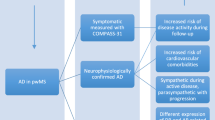
It has recently been suggested that dysregulation of the autonomic nervous system (ANS) can lead to many MS-related clinical symptoms/comorbidities, including fatigue, depression, and sleep disorders [10]. Also, it has been demonstrated that pwMS frequently have ANS dysfunction, and that it is present even in the earliest stages of the disease, with parasympathetic dysfunction present in 5%, sympathetic dysfunction in 42.6%, and sudomotor dysfunction in 32.7% of patients [11].
Hypothesizing that ANS dysfunction is an important contributor to MSRF, we investigated the role of ANS dysfunction in MSRF, taking into consideration the presence of depression and sleep disorders. The results of that investigation are presented in this paper.
Methods
Patients who participated in the BACIS project [12]—in which consecutive patients with CIS were recruited into a two-year prospective clinical and neurophysiological follow-up from October 2014 until April 2016—were asked at their final follow-up visit if they would participate in a MS comorbidities substudy. The study had been approved by the ethical committees of the University Hospital Center Zagreb and the University of Zagreb School of Medicine. Patients who accepted the request signed informed consent forms. ANS testing of the enrolled patients was performed during the same BACIS follow-up visit. This consisted of quantitative sudomotor axon reflex tests (QSARTs) performed with a Q-Sweat system (WR Medical Electronics Co., Maplewood, MN, USA) [13], cardiovascular reflex tests (heart rate and blood pressure responses to the Valsalva maneuver, and the heart rate response to deep breathing), and the tilt table test (Task Force Monitor (TFM), CNSystems Medizintechnik AG, Graz, Austria) [13, 14]. The Composite Autonomic Scoring Scale (CASS) was utilized to quantify autonomic dysfunction [15].
Following ANS testing, 70 participants filled in validated Croatian versions of the Composite Autonomic Symptom Score 31 (COMPASS-31) [16], the Modified Fatigue Impact Scale (MFIS), the Beck Depression Inventory (BDI-2), and the Epworth Sleepiness Scale (ESS) [17]. The BDI-2 result was considered to be clinically relevant if the score was > 18 [18].
Previous studies have used cutoff MFIS scores of ≥ 38 or ≥ 45 to define fatigue, so we used both cutoff points in order to stratify patients into a fatigued subgroup (N = 17 or N = 9, respectively) and a non-fatigued subgroup (N = 53 or N = 61, respectively) [19, 20]. For each participant, the Expanded Disability Status Scale (EDSS) score and the current use of a disease-modifying therapy (DMT) were noted.
The primary objective was to evaluate differences in ANS test results and COMPASS-31, BDI-2, and ESS scores between the fatigued and non-fatigued subgroups. Secondary objectives were to correlate the MFIS with objective tests of ANS function and subjective measures of dysautonomia (COMPASS-31), depression (BDI-2), and daytime sleepiness (ESS).
Finally, in order to examine the influences of age, sex, depression, daytime sleepiness, autonomic symptom burden, and current use of DMT on fatigue (as measured with the MFIS), a multiple linear regression model was used.
Statistical analysis
Statistical analysis was performed using the IBM SPSS software, version 20. The Kolmogorov–Smirnov test was applied to test whether the data had a normal distribution. Differences in the distributions of qualitative variables were determined with the χ2 test (sex), while differences in quantitative variables were determined using the parametric t test (age, respiratory sinus arrhythmia (RSA), Valsalva ratio (VR), QSART results) or the nonparametric Mann–Whitney U test (EDSS, CASS, ESS, BDI-2, COMPASS-31). To determine the correlations between the MFIS and other variables (age, RSA, VR, QSART, COMPASS-31, CASS, ESS, BDI-2, EDSS), the Spearman correlation method was used. A multiple linear regression model based on six predictors (age, sex, depression defined as BDI-2 score > 18, ESS, total COMPASS-31 score, and current use of DMT) was used to determine significant predictors for the presence of fatigue measured with the MFIS and its subtypes (physical fatigue, cognitive fatigue, and psychosocial fatigue). Also, additional regression analysis was performed for every domain of COMPASS-31 using the previously mentioned predictors in the model. For the predictors in the multiple regression models, p values of less than 0.05 were considered significant. In analyses that included multiple comparisons of the same set of data, Bonferroni-corrected p values were considered.
Results
The patients’ baseline characteristics are presented in Table 1. Thirty-five (50%) patients were on DMTs (7 on teriflunomide, 8 on dimethyl fumarate, 6 on interferon beta, 13 on glatiramer acetate, and 1 on alemtuzumab).
Descriptive measures for patients with and without fatigue
Differences in all studied parameters between fatigued and non-fatigued MS patients, defined as either MFIS ≥ 45 or ≥ 38, are presented in Table 2. Regardless of the cutoff value used, fatigued patients were older and scored significantly worse on BDI-2 and COMPASS-31. There was a clear trend of lower sweating outputs at all measured sites, which reached statistical significance for the distal leg (MFIS ≥ 45) and foot (MFIS ≥ 38).
Correlations of subjective and objective ANS tests
Results for the correlations between objective autonomic nervous system test parameters and different domains of COMPASS-31 are presented in Table S1 of the Electronic supplementary material (ESM). There were statistically significant correlations between the RSA and the bladder domain of COMPASS-31 (rs = − 0.358, p = 0.002), between QSART of the proximal leg and the gastrointestinal domain of COMPASS-31 (rs = − 0.383, p = 0.002), between QSART of the distal leg and the secretomotor domain of COMPASS-31 (rs = − 0.394, p = 0.002), and between QSART of the distal leg and the gastrointestinal domain of COMPASS-31 (rs = − 0.381, p = 0.002).
Correlations of fatigue with objective and subjective ANS tests, depression, and daytime sleepiness
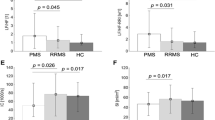
We found significant correlations between MFIS and age (rs = 0.329, p = 0.005), between MFIS and VR (rs = − 0.306, p = 0.011), between MFIS and QSART of the forearm, proximal lower leg, and distal lower leg (rs = − 0.379, p = 0.003; rs = − 0.356, p = 0.005; and rs = − 0.345, p = 0.006, respectively) (Fig. 1), between MFIS and BDI-2 (rs = 0.756, p < 0.001), and between MFIS and COMPASS-31 (rs = 0.607, p < 0.001) (Fig. 2). Also, there were statistically significant correlations between MFIS and every domain of COMPASS-31 (orthostatic intolerance, rs = 0.426, p < 0.001; vasomotor, rs = 0.387, p = 0.001; secretomotor, rs = 0.409, p < 0.001; gastrointestinal, rs = 0.503, p < 0.001; bladder, rs = 0.433, p < 0.001; pupilomotor, rs = 0.720, p < 0.001).
Correlations between Modified Fatigue Impact Scale (MFIS) and quantitative sudomotor axon reflex tests (QSARTs) of the forearm, proximal lower leg, distal lower leg, and foot (rs = − 0.379, p = 0.003; rs = − 0.356, p = 0.005; rs = − 0.345, p = 0.006; and rs = − 0.249, p = 0.051, respectively; Bonferroni-corrected p value = 0.0125)
Regression analysis
A multiple regression model was used to predict the presence of fatigue (measured with the MFIS) based on age, sex, depression (defined as BDI-2 score > 18), daytime sleepiness (measured with ESS), autonomic dysfunction (measured with COMPASS-31), and current use of DMT. The multiple regression model statistically significantly predicted the MFIS score (F = 11.366, p < 0.001), with R2 = 0.536. The total COMPASS-31 score, BDI-2, and ESS were independent predictors of the presence of fatigue measured with the MFIS (B = 0.464, p = 0.005; B = 17.344, p = 0.019; and B = 1.111, p = 0.01, respectively). As the MFIS can distinguish different types of fatigue (physical, cognitive, and psychosocial), we further analyzed the influences of the studied parameters on these three types of MSRF. There was found to be a statistically significant correlation between age and cognitive fatigue (rs = 0.295, p = 0.013). The results of the regression analysis with different types of fatigue (physical, cognitive, and psychosocial) as outcome variables are presented in Table 3. The only statistically significant predictor for cognitive fatigue was COMPASS-31, while the statistically significant predictors for physical fatigue were ESS, BDI-2, and COMPASS-31.
Finally, in order to examine the possible influences of age, sex, depression (defined as BDI-2 score > 18), ESS, different domains of COMPASS-31, and current use of DMT on fatigue (measured with the MFIS), regression analysis was performed (Table S2 in the ESM). The regression analysis showed that orthostatic intolerance and bladder domains of COMPASS-31 were not statistically significant independent predictors of MFIS score, but other domains (vasomotor, secretomotor, gastrointestinal, and pupilomotor) were.
Discussion
We found that pwMS who suffer from fatigue scored significantly worse on COMPASS-31, a patient-related outcome evaluating autonomic symptoms. Furthermore, COMPASS-31 independently predicted fatigue, regardless of depression and/or daytime sleepiness.
Several studies have used different objective tests of ANS in order to investigate the possible role of ANS dysfunction in the development of MSRF. Initial studies focused on correlations of different tests of cardiovascular autonomic function (heart rate and blood pressure variability, responses to postural changes, pressure tests, the Valsalva maneuver, deep breathing, and hyperventilation) and were largely negative; if they were positive, they were confounded by age effects on autonomic tests [21, 22]. In one study, a consistent decrease in blood pressure in response to a sustained grip was observed in combination with increased fatigue [23]. Our study confirmed these observations, as we were unable to show differences in any of the studied cardiovascular reflexes between fatigued and non-fatigued patients.
On the other hand, we found clear associations between MSRF and subjective measures of ANS dysfunction. Since the introduction of COMPASS-31, it has become possible to systematically assess quantitative autonomic symptoms. One of the first studies to investigate the relationships of autonomic symptom burden to quality of life, fatigue, and other established disease parameters was performed by Cortez and colleagues [24]. That study concluded that autonomic symptom burden is correlated with decreased quality of life and increased fatigue, despite the fact that out of 100 enrolled patients, only 41 completed the COMPASS-31 questionnaire in full. Furthermore, that study did not address possible covariates of fatigue such as depression or daytime sleepiness; nor did the authors test whether the results obtained were still significant after using multivariate regression analysis. Another study showed that COMPASS-31 predicts the level of cognitive fatigue independent of age, disease duration, EDSS, and mental impairment [25]. We confirmed this finding in our study, which showed that COMPASS-31 is the only independent predictor of cognitive fatigue. Furthermore, we replicated the finding that the pupilomotor domain of COMPASS-31 is an important contributor to the MSRF. A recent study investigating cognition and fatigue using pupillary responses to light found a tendency for a smaller pupillary response in pwMS with fatigue, which possibly explains the observation of this association [26].
The association between MSRF and subjective dysautonomia and the lack of association between MSRF and objective dysautonomia could potentially be explained by a discrepancy between patient-reported symptoms and laboratory findings for structural disorders of the ANS. In line with this, one study has shown that even patients with severe orthostatic hypotension can be completely asymptomatic [27]. Similarly, when we correlated domains of the COMPASS-31 with results for the cardiovascular reflexes and QSART, we only observed significant correlations between the QSART results and gastrointestinal and secretomotor domains of the COMPASS-31, suggesting that this discrepancy between subjective and objective dysautonomia may be pertinent to MS too.
The pathophysiology of MSRF is very complex, and the role of structural or functional brain abnormalities as well as the contributions of neurochemical imbalance, neuroendocrine dysfunction, neuroimmune dysregulation, and the peripheral nervous system have all been explored in relation to the generation of this symptom [28].
Several of these hypotheses regarding the possible role of autonomic nervous system dysfunction in MSRF are interesting. The presence of immunological dysregulation is the basis of MS, and inflammatory and neuroendocrine factors may differentially mediate fatigue [29]. It was recently suggested that pathologic interactions between the immune and autonomic systems may fail to trigger anti-inflammatory mechanisms that are crucial to preventing repeated inflammatory attacks, a key pathogenic feature of MS [30]. The parasympathetic part of the ANS plays a major role in alerting the central nervous system to the presence of inflammation via inflammatory cytokines [31]. These afferent signals are transmitted by the vagal nerve and trigger an anti-inflammatory response, termed the “cholinergic anti-inflammatory pathway” [30].
The contribution of the peripheral nervous system to MSRF is a controversial topic, but it has been suggested that sensory perception resulting from a complex integration of physiological, biochemical, and other sensory feedback from the periphery may contribute to MSRF [32]. Interestingly, we found a significant negative correlation between MFIS and sweat volume at different sites measured with QSART. QSART has traditionally been used as a marker of peripheral cholinergic postganglionic nerve affection, so it has mainly been used in the diagnosis of small fiber neuropathy. As MS is a central nervous system disorder, the results of our study can be interpreted in two ways: either QSART can detect central disorders of thermoregulation or the peripheral nervous system is also affected in MS. An argument for the former is a study which showed that QSART values can be abnormal even with preganglionic lesions [33]. An argument for the latter comes from several studies questioning whether MS is a pure central nervous system disorder. Jende and colleagues have demonstrated peripheral nerve lesions in pwMS in vivo by high-resolution MRI [34]. These lesions are defined by an increase in proton spin density and a decrease in T2 relaxation time, indicating changes in the microstructural organization of the extracellular matrix in peripheral nerve tissue in MS. Furthermore, it has also been suggested that pwMS may exhibit significant small fiber damage, which is associated with neurological disability from MS [35, 36]. One of the main consequences of sudomotor dysfunction in MS is heat sensitivity, and it has been suggested that heat sensitivity in MS might contribute to premature fatigue either through passively induced rises in body temperature or through exercise [37]. The results of our study provide a possible pathophysiological explanation for this association, although we did not assess changes in body temperature in our patient group. Despite this theoretical background, the relationship between fatigue symptoms and dysautonomia in MS has received only minor interest.
A limitation of this study is a possible selection bias, as not all participants of the BACIS project participated in the substudy. It should be emphasized that we enrolled a specific group of pwMS very early in the disease course, and that the percentage of patients with fatigue was comparatively low. Furthermore, not all participants had QSART performed due to financial limitations. Finally, ESS measures daytime sleepiness, a similar feeling to fatigue, but a recent study found that ESS was significantly associated with sleepiness, tiredness, and a lack of energy but not fatigue [38].
Despite this, the results of this study suggest an association between autonomic dysfunction (especially sudomotor dysfunction) and fatigue in MS patients. Further studies with long-term follow-up are warranted.
References
Dittner AJ, Wessely SC, Brown RG (2004) The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res 56:157–170
Penner IK, Paul F (2017) Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol 13:662–675
Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ (1994) The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci 21:9–14
Miller P, Soundy A (2017) The pharmacological and non-pharmacological interventions for the management of fatigue related multiple sclerosis. J Neurol Sci 381:41–54
Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G, Comi G (2002) Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage 15:559–567
Heesen C, Nawrath L, Reich C, Bauer N, Schulz KH, Gold SM (2006) Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry 77:34–39
Gottschalk M, Kümpfel T, Flachenecker P, Uhr M, Trenkwalder C, Holsboer F, Weber F (2005) Fatigue and regulation of the hypothalamo-pituitary-adrenal axis in multiple sclerosis. Arch Neurol 62:277–280
Solaro C, Gamberini G, Masuccio FG (2018) Depression in multiple sclerosis: epidemiology, aetiology, diagnosis and treatment. CNS Drugs 32:117–133
Barun B (2013) Pathophysiological background and clinical characteristics of sleep disorders in multiple sclerosis. Clin Neurol Neurosurg 115(Suppl 1):S82–S85
Sternberg Z (2017) Impaired neurovisceral integration of cardiovascular modulation contributes to multiple sclerosis morbidities. Mol Neurobiol 54:362–374
Habek M, Crnošija L, Lovrić M, Junaković A, Krbot Skorić M, Adamec I (2016) Sympathetic cardiovascular and sudomotor functions are frequently affected in early multiple sclerosis. Clin Auton Res 26:385–393
http://bacis.eu. Accessed 16 Mar 2018
Novak P (2011) Quantitative autonomic testing. J Vis Exp 53:2502. https://doi.org/10.3791/2502
Freeman R (2006) Assessment of cardiovascular autonomic function. Clin Neurophysiol 117:716–730
Low PA (1993) Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 68:748–752
Drulović J, Gavrilović A, Crnošija L, Kisić-Tepavčević D, Krbot Skorić M, Ivanović J, Adamec I, Dujmović I, Junaković A, Marić G, Martinović V, Pekmezović T, Habek M (2017) Validation and cross-cultural adaptation of the COMPASS-31 in Croatian and Serbian patients with multiple sclerosis. Croat Med J 58:342–348
Pecotic R, Dodig IP, Valic M, Ivkovic N, Dogas Z (2012) The evaluation of the Croatian version of the Epworth sleepiness scale and STOP questionnaire as screening tools for obstructive sleep apnea syndrome. Sleep Breath 16:793–802
Sacco R, Santangelo G, Stamenova S, Bisecco A, Bonavita S, Lavorgna L, Trojano L, D’Ambrosio A, Tedeschi G, Gallo A (2016) Psychometric properties and validity of Beck Depression Inventory II in multiple sclerosis. Eur J Neurol 23:744–750
Flachenecker P, Kümpfel T, Kallmann B, Gottschalk M, Grauer O, Rieckmann P, Trenkwalder C, Toyka KV (2002) Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler 8:523–526
Veauthier C, Radbruch H, Gaede G, Pfueller CF, Dörr J, Bellmann-Strobl J, Wernecke KD, Zipp F, Paul F, Sieb JP (2011) Fatigue in multiple sclerosis is closely related to sleep disorders: a polysomnographic cross-sectional study. Mult Scler 17:613–622
Keselbrener L, Akselrod S, Ahiron A, Eldar M, Barak Y, Rotstein Z (2000) Is fatigue in patients with multiple sclerosis related to autonomic dysfunction? Clin Auton Res 10:169–175
Merkelbach S, Dillmann U, Kölmel C, Holz I, Muller M (2001) Cardiovascular autonomic dysregulation and fatigue in multiple sclerosis. Mult Scler 7:320–326
Flachenecker P, Rufer A, Bihler I, Hippel C, Reiners K, Toyka KV, Kesselring J (2003) Fatigue in MS is related to sympathetic vasomotor dysfunction. Neurology 61:851–853
Cortez MM, Nagi Reddy SK, Goodman B, Carter JL, Wingerchuk DM (2015) Autonomic symptom burden is associated with MS-related fatigue and quality of life. Mult Scler Relat Disord 4:258–263
Sander C, Hildebrandt H, Schlake HP, Eling P, Hanken K (2017) Subjective cognitive fatigue and autonomic abnormalities in multiple sclerosis patients. Front Neurol 8:475
de Rodez Benavent SA, Nygaard GO, Harbo HF, Tønnesen S, Sowa P, Landrø NI, Wendel-Haga M, Etholm L, Nilsen KB, Drolsum L, Kerty E, Celius EG, Laeng B (2017) Fatigue and cognition: pupillary responses to problem-solving in early multiple sclerosis patients. Brain Behav 7:e00717
Arbogast SD, Alshekhlee A, Hussain Z, McNeeley K, Chelimsky TC (2009) Hypotension unawareness in profound orthostatic hypotension. Am J Med 122:574–580
Ayache SS, Chalah MA (2017) Fatigue in multiple sclerosis—insights into evaluation and management. Neurophysiol Clin 47:139–171
Gold SM, Krüger S, Ziegler KJ, Krieger T, Schulz KH, Otte C, Heesen C (2011) Endocrine and immune substrates of depressive symptoms and fatigue in multiple sclerosis patients with comorbid major depression. J Neurol Neurosurg Psychiatry 82:814–818
Racosta JM, Kimpinski K (2016) Autonomic dysfunction, immune regulation, and multiple sclerosis. Clin Auton Res 26:23–31
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5
Noakes TD, Clair Gibson A, Lambert EV (2005) From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans: summary and conclusions. Br J Sports Med 39:120–124
Davis SL, Wilson TE, Vener JM, Crandall CG, Petajan JH, White AT (1985) Pilocarpine-induced sweat gland function in individuals with multiple sclerosis. J Appl Physiol 2005(98):1740–1744
Jende JME, Hauck GH, Diem R, Weiler M, Heiland S, Wildemann B et al (2017) Peripheral nerve involvement in multiple sclerosis: demonstration by magnetic resonance neurography. Ann Neurol 82:676–685
Khan A, Kamran S, Ponirakis G, Akhtar N, Khan R, George P, Babu BM, Ibrahim FM, Petropoulos IN, Canibano BG, Wilins SS, Deleu D, Shuaib A, Malik RA (2018) Peripheral neuropathy in patients with multiple sclerosis. PLoS One 13:e0193270
Adamec I, Crnošija L, Junaković A, Krbot Skorić M, Habek M (2018) Progressive multiple sclerosis patients have a higher burden of autonomic dysfunction compared to relapsing remitting phenotype. Clin Neurophysiol 129:1588–1594
Marino F (2009) Heat reactions in multiple sclerosis: an overlooked paradigm in the study of comparative fatigue. Int J Hyperthermia 25:34–40
Chervin RD (2000) Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest 118:372–379
Funding
This study was funded by the Installation Research project HRZZ UIP-11-2013-2622 of the Croatian Science Foundation and University of Zagreb research support for the academic years 2017/2018.
Author information
Authors and Affiliations
Contributions
Study concept and design: MH. Acquisition of data: MKS, LC, IA, BB, TG, TS, IS, TP, IP, JD, TP, MH. Analysis and interpretation of data: MKS, LC, IA, BB, TG, TS, IS, TP, IP, JD, TP, MH. Drafting of the manuscript: MH. Critical revision of the manuscript for important intellectual content: MKS, LC, IA, BB, TG, TS, IS, TP, IP, JD, TP, MH. Administrative, technical, and material support: MKS, LC, IA, BB, TG, TS, IS, TP, IP, JD, TP, MH.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krbot Skorić, M., Crnošija, L., Adamec, I. et al. Autonomic symptom burden is an independent contributor to multiple sclerosis related fatigue. Clin Auton Res 29, 321–328 (2019). https://doi.org/10.1007/s10286-018-0563-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-018-0563-6