Abstract
Objective
The aim of this study was to determine the prevalence of autonomic dysfunction using the composite autonomic scoring scale (CASS) and heart rate variability (HRV) in patients with clinically isolated syndrome (CIS) and to correlate autonomic dysfunction with other measures of MS disease activity.
Methods
CASS, HRV and plasma catecholamines during supine and tilted phase were performed in 104 CIS patients. MRI findings were analyzed for total number of lesions and the presence of brainstem and cervical spinal cord lesions.
Results
Autonomic dysfunction (CASS >1) was present in 59.8 % of patients, parasympathetic dysfunction in 5 %, sympathetic in 42.6 % and sudomotor in 32.7 % of patients. Patients with autonomic dysfunction on CASS had lower level of norepinephrine in the supine position compared to patients without autonomic dysfunction (1.06 ± 0.53 vs. 1.37 ± 0.86, p = 0.048). The CASS score showed positive correlation with s-HF (r = 0.226, p = 0.031), s-SDNN (r = 0.221, p = 0.035), t-HF (r = 0.225, p = 0.032), and t-HFnu (r = 0.216, p = 0.04), and a negative correlation with t-LF/HF (r = −0.218, p = 0.038). More patients with MRI brainstem lesions had a positive adrenergic index (p = 0.038). Patients with MRI brainstem lesions also had a lower t-SDNN (26.2 ± 14.2 vs. 32 ± 13.3, p = 0.036) and a lower t-LF (median 415.0 vs. 575.5, p = 0.018) compared to patients without these lesions. Patients with adrenergic index ≥1 had a significantly higher standing heart rate compared to patients with an adrenergic index of 0 (96 ± 13.5 vs. 90 ± 12, p = 0.032).
Conclusion
Autonomic (primarily sympathetic) dysfunction is present in a large proportion of early MS patients and it seems to be related to brainstem involvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinically isolated syndrome (CIS) is clinically indistinguishable from multiple sclerosis (MS) relapses, except that it is isolated (at least clinically) in time and in most patients in space [1]. More than two-thirds of patients with CIS will have further episodes of neurological dysfunction and convert to MS. Although a number of demographic, genetic, environmental and clinical factors have been identified that confer an increased risk of conversion to MS, the most robust predictor remains conventional MRI abnormalities [1].
Reports about frequency of autonomic dysfunction in MS vary notably, with cardiovascular autonomic dysfunction being present in up to 50 %, bladder dysfunction in up to 97 %, sexual dysfunction in more than 80 %, and bowel dysfunction in more than 50 % of MS patients, while the prevalence of sudomotor dysfunction in MS is still unknown [2]. Identifying autonomic dysfunction in patients with multiple sclerosis is relevant both from clinical (i.e., diagnosis and treatment) and research (i.e., identifying potential treatment targets) perspectives [3]. However, how to define and/or test autonomic dysfunction in multiple sclerosis remains an unanswered question. The composite autonomic scoring scale (CASS) is a 10-point scoring scale of autonomic function, which consists of the quantitative sudomotor axon reflex test (QSART), orthostatic blood pressure and heart rate response to tilt, heart rate response to deep breathing, the Valsalva ratio, and beat-to-beat blood pressure measurements during phases II and IV of the Valsalva maneuver, tilt, and deep breathing [4]. The maximum CASS is 10, with 4 points for adrenergic and 3 points each for sudomotor and cardiovagal failure. CASS has been useful in detecting and monitoring autonomic dysfunction in many conditions; however, it has not been investigated in MS. In a recently published study, we have compared the CASS between 24 CIS patients and 17 healthy controls and found that the proportion of CIS patients with a pathological adrenergic index was statistically significantly higher compared to healthy controls, while there was no difference in cardiovagal index between groups [5]. Furthermore, we found sudomotor dysfunction diagnosed with QSART in 31 % of patients (5 out of 16 patients with QSART available) [5]. With this study, we have shown that the CASS is able to detect autonomic nervous system dysfunction in CIS patients and have confirmed findings from previous studies that ANS dysfunction in active MS (i.e., relapses) is restricted to the sympathetic nervous system (both adrenergic and cholinergic), while the parasympathetic nervous system seems to be preserved [6].
Therefore, the aim of the present study was to determine the prevalence and extent of ANS dysfunction using the Composite Autonomic Scoring Scale (CASS) and heart rate variability analysis (HRV) in a large cohort of CIS patients and to correlate autonomic dysfunction with other measures of MS disease activity.
Methods
Design
This was a cross-sectional study which included consecutive patients diagnosed with the first clinical symptom of multiple sclerosis (CIS) from August 1st 2014 until the 1st of December 2015 at the Department of Neurology, University Hospital Center Zagreb—a tertiary medical center and a referral center for MS and ANS disorders. The diagnosis of CIS was made in a patient with acute or subacute development of neurological symptoms and/or signs lasting longer than 48 h in the absence of fever or infection and with at least one demyelinating lesion larger than 3 mm showing on the brain and/or spinal cord MRI. The exception was patients with optic neuritis who were included if the classical clinical triad was present (i.e., rapidly developing impairment of vision, dyschromatopsia and retro-orbital pain), accompanied by prolonged latencies of visual evoked potentials, irrespective of the presence of brain and/or spinal cord MRI lesions.
All patients were recruited within 6 months of the MRI scan and diagnosis of CIS. All autonomic tests were performed on the same day, at least 2 weeks after pulse corticosteroid therapy, if the patient received one.
Ethical committees of the University Hospital Center Zagreb and University of Zagreb, School of Medicine approved the study. All participants signed informed consent.
MRI
All MRIs were performed on a 1.5T MRI scanner. Brain MRI was available for all patients. Cervical spinal cord MRI was analyzed if it was available. MRI sequences included multi-planar dual fast spin-echo PD and T2-WI, FLAIR and T1 postcontrast sequences. A neurologist with at least 5 years of experience with MS reviewed all MRIs for the presence of lesions.
Autonomic nervous system testing
Autonomic nervous system testing was performed in the following order: first, Quantitative Sudomotor Axon Reflex Test (QSART) was performed with the Q-Sweat (WR Medical Electronics Co Maplewood, MN, USA) [7]; second, heart rate and blood pressure responses to Valsalva maneuver; third, heart rate response to deep breathing; and finally, blood pressure response to passive tilt in the duration of 10 min (Task Force Monitor (TFM), CNSystems Medizintechnik AG, Austria) [7, 8]. The results of the autonomic nervous system testing were interpreted in the form of the CASS score [4].
After all the tests were performed, patients were questioned by an experienced neurologist for symptoms of autonomic dysfunction, specifically, symptoms of orthostatic intolerance and symptoms of secretomotor, vasomotor, gastrointestinal, bladder or pupilomotor dysfunction.
Blood samples were collected directly from a peripheral vein catheter, in chilled tubes containing EGTA and reduced glutathione, for determination of plasma catecholamine levels in the 10th minute of the supine phase before the beginning the Valsalva maneuver and in the 10th minute of the tilted phase (Kabevette® N, Kabe Labortechnik GmbH). Plasma levels of catecholamine were measured on high-pressure liquid chromatography (HPLC Prominence; Shimadzu GmbH) with an electrochemical detector CLC 100 (Chromsystems GmbH, Germany) using a commercially available HPLC kit and a reverse-phase analytical column for HPLC analysis of catecholamines in plasma (Chromsystems GmbH, Germany).
Heart rate variability analysis
Power spectral analysis of HRV was performed by Kubios HRV 2.2 software (Department of Applied Physics, University of Eastern Finland, Kuopio, Finland) using time- and frequency-domain methods. In spectral analysis of the frequency domain, the variables autoregressive spectral estimation method was used. Data used for the HRV analysis were recorded with TFM using a sampling frequency of 1000 Hz, and the data were subsequently inspected and edited for any missing data. The medium artefact correction option and Smoothness priors-based detrending approach (Lambda = 500) were used to ensure data quality [9].
HRV was analyzed in 5-min intervals of beat-to-beat data recorded during the testing [10]. HRV analysis done on the supine phase dataset was performed on the most stable 5-min interval for every patient (‘s’ variables) and most stable 5-min interval between the 1st and 9th minute was used for the analysis of HRV during the tilted phase (‘t’ variables). High-frequency (0.15–0.4 Hz) and low-frequency (0.04–0.15 Hz) power of RR interval expressed in absolute units (HF and LF) were used as an index of cardiovagal activity and combined sympathetic and parasympathetic cardiac activity, respectively [11]. HF expressed in normalized units (HFnu) was used an index of modulation of the parasympathetic branch of the ANS [10]. Low to high frequency ratio (LF/HF) was used as a marker of the sympathovagal balance [7]. The time domain analysis parameter SDNN was used as a marker of overall HRV [10].
Outcomes
The primary outcome was to determine the prevalence and the extent of the autonomic dysfunction in the studied cohort. This was assessed by: (1) the proportion of patients with pathological CASS; (2) the proportion of patients with pathological adrenergic, cardiovagal and sudomotor index of the CASS; and (3) the correlation between autonomic dysfunction measured with the CASS and with the plasma catecholamine levels.
Secondary outcomes were to investigate the possible correlates of autonomic dysfunction in the studied cohort. Widely used measures of disease activity [expanded disability status scale (EDSS) and MRI] were tested with this aim. Furthermore, we wanted to see whether pathological CASS and its indices have clinical consequences. This was assessed by comparing differences in supine heart rate, standing heart rate, systolic blood pressure and diastolic blood pressure in patients with different autonomic dysfunctions. Taking into account that cardioregulatory centers are located in the brainstem, we wanted to see whether lesion location could have influenced heart rate variability. Furthermore, we wanted to determine if HRV parameters could be related to the ANS testing results interpreted in the form of CASS.
Statistical analysis
Statistical analysis was performed using the IBM SPSS software, version 20. The Kolmogorov–Smirnov test was applied to test whether the data have a normal distribution. Differences in the distribution of qualitative variables were determined with the χ 2 test, while the differences in quantitative variables were determined with the use of a parametric t test and nonparametric Mann–Whitney and Kruskal–Wallis test. To determine the correlation between the variables, the Spearman correlation method was used. P values less than 0.05 were considered as significant.
Results
Patients
During the study period, 104 consecutive patients were included (31 males) with a mean age of 32.3 ± 8.9 years. There were 32 cases of optic neuritis, 32 of incomplete transverse myelitis, 27 brainstem/cerebellar cases, 10 cases of hemispheral and 3 cases of multifocal type CIS. Median EDSS was 1.0 (range 0–3.5).
Brain MRI was available for all patients and cervical spinal cord MRI was available for 86 (82.7 %) patients. Ninety-nine patients (95.2 %) had lesions present on brain MRI, 42 (40.4 %) patients had brainstem lesions (15 in the midbrain, 32 in the pons and 15 in the medulla oblongata) and 41 (47.7 %) patients had lesions in the cervical spinal cord. Median number of lesions was 11 (0–84). None of the patients received any MS-specific medications or any other medications which could influence the autonomic nervous system at the time of testing. Table 1 shows the frequency of symptoms related to specific autonomic nervous system function.
Primary outcomes
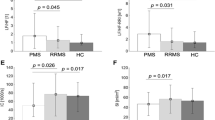
Total CASS was available for 92 participants; three patients could not perform the Valsalva maneuver so adrenergic and cardiovagal indices could not be calculated; and, for 9 patients the sudomotor index could not be calculated due to technical difficulties during the testing. The prevalence of sympathetic, parasympathetic and sudomotor dysfunction in the form of CASS and its indices is presented in Fig. 1. In total, 59.8 % of patients had some form of autonomic dysfunction (CASS >1); parasympathetic dysfunction was present in 5 %, sympathetic in 42.6 % and sudomotor in 32.7 % patients. All parameters used to calculate adrenergic and cardiovagal indices are presented in Tables 2 and 3. The prevalence of abnormal QSART responses is presented in the Table 4 and QSART variables (sweat output and latency) on each tested site (forearm, proximal and distal leg, and foot) are presented in Table 5. Hear rate, blood pressure, catecholamine values and HRV analysis data in the supine and upright positions are presented in Table 6.
Prevalence of autonomic, parasympathetic, sympathetic and sudomotor dysfunction in the studied cohort measured with CASS, cardiovagal index, adrenergic index and sudomotor index, respectively. The CASS consists of quantitative sudomotor axon reflex test, orthostatic blood pressure and heart rate response to tilt, heart rate response to deep breathing, the Valsalva ratio and beat-to-beat blood pressure measurement during the Valsalva maneuver, tilt and deep breathing. The grading of the autonomic dysfunction is divided into three indexes: the sudomotor index 1, 2 and 3, the adrenergic index 1, 2, 3 and 4 and the cardiovagal index 1, 2 and 3. The minimal CASS is 0 and maximum 10. Results are referred to as normal (CASS total score = 0) or abnormal. The abnormalities range from mild generalized autonomic failure (total CASS score 1–3), to moderate (total CASS score 4–6), or severe (total CASS score 7–10)
Plasma catecholamine concentrations were available for 95 patients in the supine position and for 94 patients in standing position. The distribution of both epinephrine and norepinephrine values in the supine and standing positions was the same across all CIS subtypes (all p > 0.05). Patients with a CASS ≥1 had a lower level of norepinephrine in the supine position compared to patients with a CASS of 0 (1.06 ± 0.53 vs. 1.37 ± 0.86, p = 0.048). When we looked at the CASS subscores, there was no difference in plasma catecholamine concentrations between groups of patients with adrenergic, cardiovagal or sudomotor indices ≥1 vs. 0.
There was no difference in the CASS, adrenergic, cardiovagal or sudomotor indices between different CIS types (p = 0.256, p = 0.745, p = 1.000, p = 0.075, respectively).
Secondary outcomes
With regard to correlation between autonomic dysfunction and widely used measures of MS disease activity, there was no correlation between autonomic dysfunction (CASS, adrenergic, cardiovagal or sudomotor indices) and the EDSS (p = 0.055, p = 0.189, p = 0.182, p = 0.190, respectively). Similarly, there were no differences with regard to the presence of autonomic dysfunction (CASS, cardiovagal or sudomotor indices ≥1) between patients with and without brainstem or cervical spinal cord lesions, except for positive adrenergic index and the presence of brainstem lesions (p = 0.038) (Table 7). There was no correlation between CASS, adrenergic, cardiovagal or sudomotor indices and the total number of T2 lesions (p = 0.525, p = 0.909, p = 0.744, p = 0.403, respectively).
To investigate whether pathological CASS and its indices have clinical consequences, we compared differences in supine and standing heart rate (HR), systolic (sBP) and diastolic blood pressure (dBP) in patients with different autonomic dysfunctions, and found that patients with an adrenergic index of ≥1 had significantly higher standing heart rate compared to patients with an adrenergic index of 0 (96 ± 13.5 and 90 ± 12, respectively, p = 0.032). No differences were found regarding sBP and dBP (p > 0.05). In addition, no differences in HR, sBP or dBP in relation to cardiovagal or sudomotor indices or CASS were found (p > 0.05). We compared HR, sBP and dBP values between patients with and without BS lesions on brain MRI and found that patients with BS lesions had significantly higher supine sBP (116.2 ± 12.5 vs 110.6 ± 10.4 mmHg, p = 0.016) and dBP values (73.4 ± 8.9 vs 69.7 ± 7.9 mmHg, p = 0.032). There were no other significant differences in HR or blood pressure values in the supine or tilted phases of testing.
We included 103 patients in the HRV analysis; one patient was excluded because of the inadequate length of the recording period due to the development of vasovagal syncope upon head-up tilting. We investigated possible differences in HRV parameters when comparing certain CIS groups with the rest of the sample. We found differences only in the BS CIS group; these patients had a higher t-HFnu (26 ± 15.4 vs. 18.6 ± 10.1, p = 0.026) and a lower t-LF/HF (median 2.77 vs. 4.79, p = 0.021). Disregarding the CIS type, patients with BS lesions evident on brain MRI had lower t-SDNN (26.2 ± 14.2 vs. 32 ± 13.3, p = 0.036) and lower t-LF (median 415 vs. 575.5, p = 0.018) compared to patients without those lesions.
We also wanted to investigate whether HRV results could be related to the findings of ANS testing expressed in the form of the CASS. We found that patients with a positive total CASS score had significantly higher s-HF (median 729 vs. 321.5, p = 0.006), s-HFnu (49.1 ± 18.3 vs. 41.1 ± 12.7, p = 0.017), and s-SDNN (41.4 ± 18.8 ms vs. 33.1 ± 13.7 ms, p = 0.025). These patients also had significantly lower t-LF/HF (median 3.76 vs. 4.82, p = 0.018). Furthermore, total CASS score showed positive correlation with s-HF (r = 0.226, p = 0.031), s-SDNN (r = 0.221, p = 0.035), t-HF (r = 0.225, p = 0.032), and t-HFnu (r = 0.216, p = 0.04), and a negative correlation with t-LF/HF (r = −0.218, p = 0.038). We also found that patients with a positive adrenergic CASS index had significantly higher s-SDNN (42.9 ± 20.3 vs. 35.5 ± 13.8 ms, p = 0.043), s-LF (968.7 ± 938.8 vs. 647.3 ± 514.6 ms2, p = 0.047), and a higher t-HFnu (23 ± 12.1 vs. 18.2 ± 11.5, p = 0.047). The adrenergic CASS score positively correlated with t-HFnu (r = 0.219, p = 0.029) and negatively with t-LF/HF (r = −0.220, p = 0.028). No differences or correlations were found regarding the cardiovagal CASS score.
To investigate the possible influence of supine or standing NA on HRV results, we performed a correlation analysis. This resulted in a negative correlation between supine NA values and s-HF (r = −0.242, p = 0.019) and a positive correlation with s-LF/HF (r = 0.412, p < 0.001). Standing NA values showed negative correlation with s-HF (r = −0.206, p = 0.048) and t-HF (r = −0.256, p = 0.013) and a positive correlation with s-LF/HF (r = 0.338, p = 0.001).
Discussion
The present study has shown that sympathetic cardiovascular function is affected in 42,6 % and sudomotor function in 32,6 % of CIS patients. This is further supported with the finding of lower norepinephrine levels in the supine position in patients with ANS dysfunction. Furthermore, sympathetic cardiovascular dysfunction is probably the consequence of brainstem lesions.
Our study has confirmed previous findings which indicate that the sympathetic nervous system is affected in the acute stages of MS (relapses), with the parasympathetic nervous system being spared in the initial stages of MS [5, 6, 12]. Furthermore, it has been shown that brainstem MS lesions account for most of the cardiovascular abnormalities in MS patients [13]. This is especially interesting since damage to the locus coeruleus, which is located in the brainstem and is the principal site for brain norepinephrine synthesis, could lead to MS sympathetic dysfunction [15].
This notion is further supported by the results of HRV analysis. Results indicate that BS CIS patient groups have lower sympathetic cardiac activity in response to tilt when compared to the rest of CIS patients indicated by lower t-LF/HF and higher t-HFnu. Also, when we grouped patients into those with BS lesions and those without them, disregarding the CIS group affiliation, patients with BS lesions had lower cardiac sympathetic activity as indicated by the lower LF values during tilt. However, this was not reflected in the lower HR values during tilt in these patients. On the contrary, we found that patients with BS lesions had higher supine sBP and dBP, which is somewhat confounding. It has been found that reducing central nervous system sympathetic outflow, using clonidine, causes up-regulation of β-adrenergic receptors on peripheral blood mononuclear cells [14]. Perhaps, a similar adaptation of the blood vessel’s smooth muscle cells to the diminished sympathetic output in patients with BS lesions might explain higher supine blood pressure in these patients compared to those without BS lesion. Interestingly, we also found that the standing heart rate is higher in CIS patients with sympathetic dysfunction. Moreover, studies on MS patients showed reduced brain norepinephrine and reduced intracellular catecholamine concentrations in the peripheral blood lymphocytes, suggesting the dysfunction of both central and peripheral sympathetic functions [15, 16]. Interestingly, we found a positive correlation between the supine and tilted phase NA values and the index of cardiac sympathovagal balance during supine phase of testing (i.e., s-LF/HF). Thus, the results of the present study might support these findings. Patients with a positive adrenergic CASS score also had higher parasympathetic (i.e., lower sympathetic) cardiac activity during tilt, indicated by t-HFnu. Moreover, both total CASS and adrenergic CASS scores correlated negatively with the LF/HF ratio, the index of cardiac sympathovagal balance, during tilt, while both correlated positively with the index of parasympathetic activity (HFnu) during tilt. Taken together, these results suggest an impairment of sympathovagal balance (i.e., diminished central sympathetic output) in response to various stresses demanding a response of sympathetic nervous system, such as orthostasis or blood pressure response to the Valsalva manuever. We also found that patients with a positive total CASS score exhibited higher cardiac parasympathetic (i.e., lower sympathetic) activity when supine, which may indicate that sympathovagal balance is disrupted not only during stress, but also during periods of rest (i.e., supine phase of ANS testing). Patients with BS lesions showed lower overall heart rate variability during tilt, most probably caused by impaired activation of the sympathetic nervous system during tilt, as indicated by lower t-LF. Although reduction of overall HRV during tilt is considered as a normal response [10], previous studies also suggest that in MS patients this reduction is caused by diminished activation of the sympathetic system [17]. Therefore, lower-than-normal sympathetic output seems to be the main cause for sympathovagal imbalance in MS patients and this has already been argued for [17].
Unlike cardiovascular reflexes, research on the sweating response in early MS is scarce. QSART has rarely been studied in MS, mainly because it is considered that an absent response indicates a lesion of the postganglionic axon. However, it can become abnormal even if the preganglionic lesion is present, which has been nicely shown in a study investigating cholinergic sweating responses with pilocarpine iontophoresis. The authors observed diminished peripheral sweating responses as a consequence of impairments in autonomic control of sudomotor function [18]. This has been confirmed both with our previous work and with the present study, which showed a sudomotor index ≥1 in one-third of CIS patients [5].
Results of the present study are important for several reasons. The first one is that dysregulation of the ANS system can lead to arrays of clinical symptoms often observed in MS patients, including cognitive dysfunction, sleep disorders, depression and migraine [17]. Especially interesting are findings of ANS testing in migraine patients, concerning the higher prevalence of migraine in MS patients compared to healthy controls. Several studies have found abnormalities in autonomic nervous system testing, low levels of plasma norepinephrine, and an impaired sweating function in migraine patients during headache free intervals, findings which are very similar to the findings observed in MS patients [19, 20]. Despite all of this, studies of the association of ANS dysfunction with cognitive dysfunction, sleep disorders, depression and migraine in MS are lacking.
The second reason is the finding of a connection between dysfunction of autonomic cardiovascular reflexes and development of cardiac side effects of several drugs that are used in MS treatment. MS relapses are treated with high-dose intravenous methylprednisolone, and cardiac complications including sinus bradycardia, supraventricular tachycardia, atrial fibrillation, ventricular tachycardia and myocardial infarction have all been reported in association with this treatment. These side effects have been additionally associated with autonomic dysfunction in MS patients [21]. Furthermore, fingolimod, an SP1 receptor modulator, which is used in the treatment of highly active MS, may cause prolonged or more prominent HR slowing in some MS patients [22, 23].
Finally, the third reason is the finding that cardiovascular and thermoregulatory autonomic dysfunctions in MS have considerable potential to adversely affect exercise [24]. Blunted heart rate responses to isometric handgrip exercise, the inability to increase arterial pressure during handgrip exercise, attenuated elevations in heart rate and systolic blood pressure in MS patients during graded arm ergometry, and blunted heart rate and systolic blood pressure responses to graded cycling have all been found in MS patients compared to healthy controls [25–28]. Furthermore, the change in rectal temperature following 60 min of cycling was almost doubled in the MS patients compared to the healthy control group in one study [29]. Collectively, these data argue that cardiovascular and thermoregulatory autonomic dysfunction impacts exercise tolerance and capacity; however, little is known and it remains to be investigated whether ANS dysfunction measured with CASS can predict this exercise intolerance.
In conclusion, the results of this study strongly suggest a positive correlation between brainstem involvement and autonomic (primarily sympathetic) dysfunction in early MS patients. Also, since there is growing evidence indicating the importance of autonomic dysfunction in a number of different aspects of this disease (i.e., pathophysiology, comorbidities, treatment response, quality of life, etc.), ANS function testing may prove to be a valuable tool in the assessment and treatment of MS patients.
Abbreviations
- ANS:
-
Autonomic nervous system
- CIS:
-
Clinically isolated syndrome
- MS:
-
Multiple sclerosis
- MRI:
-
Magnetic resonance imaging
- CASS:
-
Composite autonomic scoring scale
- HRV:
-
Heart rate variability
- QSART:
-
Quantitative sudomotor axon reflex test
- TFM:
-
Task force monitor
- HF:
-
High frequency
- LF:
-
Low frequency
- nu:
-
Normalized units
- HR:
-
Heart rate
- sBP:
-
Systolic blood pressure
- dBP:
-
Diastolic blood pressure
- BS:
-
Brainstem
References
Brownlee WJ, Miller DH (2014) Clinically isolated syndromes and the relationship to multiple sclerosis. J Clin Neurosci 21:2065–2071
Adamec I, Habek M (2013) Autonomic dysfunction in multiple sclerosis. Clin Neurol Neurosurg 115(Suppl 1):S73–S78
Racosta JM, Sposato LA, Morrow SA, Cipriano L, Kimpinski K, Kremenchutzky M (2015) Cardiovascular autonomic dysfunction in multiple sclerosis: a meta-analysis. Mult Scler Relat Disord 4:104–111
Low PA (1993) Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 68:748–752
Crnošija L, Adamec I, Lovrić M et al (2016) Autonomic dysfunction in clinically isolated syndrome suggestive of multiple sclerosis. Clin Neurophysiol 127:864–869
Flachenecker P, Reiners K, Krauser M, Wolf A, Toyka KV (2001) Autonomic dysfunction in multiple sclerosis is related to disease activity and progression of disability. Mult Scler 7:327–334
Novak P (2011) Quantitative autonomic testing. J Vis Exp (53).doi:10.3791/2502
Freeman R (2006) Assessment of cardiovascular autonomic function. Clin Neurophysiol 117:716–730
Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA (2014) Kubios HRV–heart rate variability analysis software. Comput Methods Programs Biomed 113:210–220
Malik M, Bigger JT, Camm AJ et al (1996) Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 17:354–381
Sztajzel J (2004) Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly 134:514–522
Nasseri K, Uitdehaag BM, van Walderveen MA, Ader HJ, Polman CH (1999) Cardiovascular autonomic function in patients with relapsing remitting multiple sclerosis: a new surrogate marker of disease evolution? Eur J Neurol 6:29–33
Saari A, Tolonen U, Pääkkö E, Suominen K, Pyhtinen J, Sotaniemi K, Myllylä V (2004) Cardiovascular autonomic dysfunction correlates with brain MRI lesion load in MS. Clin Neurophysiol 115:1473–1478
Zoukos Y, Thomaides T, Pavitt DV, Leonard JP, Cuzner ML, Mathias CJ (1992) Up-regulation of beta-adrenoceptors on circulating mononuclear cells after reduction of central sympathetic outflow by clonidine in normal subjects. Clin Auton Res 2:165–170
Polak PE, Kalinin S, Feinstein DL (2011) Locus coeruleus damage and noradrenaline reductions in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain 134:665–677
Rajda C, Bencsik K, Fuvesi J, Seres E, Vecsei L, Bergquist J (2006) The norepinephrine level is decreased in the lymphocytes of long-term interferon-beta-treated multiple sclerosis patients. Mult Scler 12:265–270
Sternberg Z (2016) Impaired neurovisceral integration of cardiovascular modulation contributes to multiple sclerosis morbidities. Mol Neurobiol. doi:10.1007/s12035-015-9599-y
Davis SL, Wilson TE, Vener JM, Crandall CG, Petajan JH, White AT (1985) Pilocarpine-induced sweat gland function in individuals with multiple sclerosis. J Appl Physiol 2005(98):1740–1744
Gotoh F, Komatsumoto S, Araki N, Gomi S (1984) Noradrenergic nervous activity in migraine. Arch Neurol 41:951–955
Gomi S, Gotoh F, Komatsumoto S, Ishikawa Y, Araki N, Hamada J (1989) Sweating function and retinal vasomotor reactivity in migraine. Cephalalgia 9:179–185
Vasheghani-Farahani A, Sahraian MA, Darabi L, Aghsaie A, Minagar A (2011) Incidence of various cardiac arrhythmias and conduction disturbances due to high dose intravenous methylprednisolone in patients with multiple sclerosis. J Neurol Sci 309:75–78
Rossi S, Rocchi C, Studer V, Motta C et al (2015) The autonomic balance predicts cardiac responses after the first dose of fingolimod. Mult Scler 21:206–216
Hilz MJ, Intravooth T, Moeller S, Wang R, Lee DH, Koehn J, Linker RA (2015) Central autonomic dysfunction delays recovery of fingolimod induced heart rate slowing. PLoS One 10:e0132139
Huang M, Jay O, Davis SL (2015) Autonomic dysfunction in multiple sclerosis: implications for exercise. Auton Neurosci 188:82–85
Thomaides TN, Zoukos Y, Chaudhuri KR, Mathias CJ (1993) Physiological assessment of aspects of autonomic function in patients with secondary progressive multiple sclerosis. J Neurol 240:139–143
Pepin EB, Hicks RW, Spencer MK, Tran ZV, Jackson CG (1996) Pressor response to isometric exercise in patients with multiple sclerosis. Med Sci Sports Exerc 28:656–660
Senaratne MP, Carroll D, Warren KG, Kappagoda T (1984) Evidence for cardiovascular autonomic nerve dysfunction in multiple sclerosis. J Neurol Neurosurg Psychiatry 47:947–952
Cohen JA, Hossack KF, Franklin GM (1989) Multiple sclerosis patients with fatigue: relationship among temperature regulation, autonomic dysfunction, and exercise capacity. Neurorehabil Neural Repair 3:193–198
Huang M, Morris NB, Jay O, Davis SL. Thermoregulatory dysfunction in multiple sclerosis patients during moderate exercise in a thermoneutral environment. FASEB J 2014;28 (Supplement 1),1104.17
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial and competing interest disclosure
None of the authors have relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Funding
This study was funded by the Installation Research project HRZZ UIP-11-2013–2622 of the Croatian Science Foundation.
Rights and permissions
About this article
Cite this article
Habek, M., Crnošija, L., Lovrić, M. et al. Sympathetic cardiovascular and sudomotor functions are frequently affected in early multiple sclerosis. Clin Auton Res 26, 385–393 (2016). https://doi.org/10.1007/s10286-016-0370-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-016-0370-x