Abstract
Composite restorations are widely used worldwide, but the polymerization shrinkage is their main disadvantage that may lead to clinical failures and adverse consequences. This review reports, currently available in vitro techniques and methods used for assessing the polymerization shrinkage. The focus lies on recent methods employing three-dimensional micro-CT data for the evaluation of polymerization shrinkage: volumetric measurement and the shrinkage vector evaluation through tracing particles before and after polymerization. Original research articles reporting in vitro shrinkage measurements and shrinkage stresses were included in electronic and hand-search. Earlier methods are easier, faster and less expensive. The procedures of scanning the samples in the micro-CT and performing the shrinkage vector evaluation are time consuming and complicated. Moreover, the respective software is not commercially available and the various methods for shrinkage vector evaluation are based on different mathematical principles. Nevertheless, these methods provide clinically relevant information and give insight into the internal shrinkage behavior of composite applied in cavities and how boundary conditions affect the shrinkage vectors. The traditional methods give comparative information on polymerization shrinkage of resin composites, whereas using three-dimensional micro-CT data for volumetric shrinkage measurement and the shrinkage vector evaluation is a highly accurate method. The methods employing micro-CT data give the researchers knowledge related to the application method and the boundary conditions of restorations for visualizing the shrinkage effects that could not be seen otherwise. Consequently, this knowledge can be transferred to the clinical situation to optimize the material manipulation and application techniques for improved outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More than 260 million direct composite restorations are placed worldwide each year [1]. They became the first choice for direct anterior and posterior restorations owing to their satisfactory esthetic quality, conservation of tooth structure compared to indirect restorations, repairability and their reasonable cost. Their main disadvantage is their contraction upon polymerization [2]. When bonded to cavity walls, they are restricted to contract freely which inevitably leads to shrinkage stresses at the cavity walls with possible detachment and gap formation. The interfacial contraction stresses depend on the total volumetric material shrinkage and the ratio between the free to the bonded surfaces or what has been termed as cavity configuration or C-factor [3]. Interfacial contraction gaps result in microleakage with increased likelihood of post restoration hypersensitivity, adverse pulpal consequences, marginal discoloration, recurrent caries, pulling action on cusps and possible cracking in enamel [4–9]. Current practice of restorative dentistry includes a significant part in replacing failed composite restorations, consistent with the repetitive restorative cycle described by Elderton [2, 10]. Factors related to the patient, operator, tooth, cavity size, and materials have been reported in the literature as potentially relevant for restoration failures, although evidence of this is still limited [2].
Composition of dental resin composites
Typically, a resin composite is composed of three major components: organic matrix of polymeric chains and cross linking agents, inorganic filler particles, coupling agent. Additionally, a number of initiator-activator systems are incorporated for the polymerization reaction to take place.
Composites are classified according to the functional group determining their cure into pure methacrylates, acid modified methacrylates and ring opening epoxides. Pure methacrylates include the classical dental matrix that is composed of Bis-GMA, UDMA and TEGDMA [11]. Classical ormocers differ from the classical monomers in the non-reactive part, the Si–O-network. The acid modified methacrylates consist of compomers which have a more hydrophilic monomer component due to the carboxylic group in addition to the ormocers with carboxylic function. The last group consists of the ring opening epoxides, the siloranes, that are characteristic for their lower shrinkage than the conventional composite matrix [12].
In today’s composites, fillers constitute the major portion of the composite by weight. They are added to the resin matrix to reduce polymerization shrinkage and stress, to reinforce the resin matrix, and to improve the optical properties of the material with a special respect to translucency. The fillers are inorganic particles with variability in size and size distribution such as finely ground quartz or glass, sol–gel derived ceramics microfine silica, or nanoparticles. Most glasses contain heavy-metal oxides such as barium, zinc, yttrium fluoride, or ytterbiumtrifluoride for radiopacity. Fillers are usually silanized to bond to the organic matrix and transfer the stresses to the fillers. However, some researchers kept fillers nonbonded for stress reduction [13]. Fillers upgrade the materials’ physical and mechanical properties through even minute addition of separate components. Therefore, mixtures of large and small fillers are used to incorporate as many fillers as possible [12].
The manufacturers surface-treat the fillers by a silane coupling agent to form a bond between the inorganic and organic phases of the composite. The coupling agent strongly binds the filler to the resin matrix, thereby enhancing the mechanical properties of the composite by transferring the stresses from the mechanically weak matrix to the stronger filler in addition to providing a hydrophobic environment that minimizes water absorption [14].
The function of the initiator-accelerator system is to initialize the network forming polymerization. The polymerization reaction can be started by light-activation, chemical activation, and dual curing which is chemical and light-curing together. Light-activation is usually achieved with blue light at a peak wavelength of 470 nm that is absorbed by a photosensitive agent, most commonly camphorquinone 0.1–1.0 %. The free radical reaction is accelerated in the presence of an organic amine. The disadvantage of camphorquinone is its yellowish tint and the toxicity concerns over the amine co-initiator that is used with camphorquinone [15]. In the silorane composite, camphorquinone, iodonium salts and electron donors generate cations that start the ring opening polymerization process [11].
Alternatives to camphorquinone/amine systems are the phosphine oxide initiators. They initiate through a cleavage mechanism that does not require a co-initiator. They absorb in the visible range, however, exhibit little absorption beyond 420 nm and are, therefore, not ideal for use in dental applications, as dental curing lamps are designed to match the absorbance spectrum of camphorquinone at 470 nm. On the other hand, Lucirin TPO is an acyl phosphine oxide that bleaches out after polymerization, therefore, it can be used for composite bleach shades or colorless protective varnishes. Another initiation system is based on benzoylgermanium derivatives that undergoes photodecomposition to form radicals without the need for a co-initiator. Bulk-fill composites are intended for use in increments up to 4 mm, which is achieved by a combination of camphorquinone, phosphine oxide and a germanium based photoinitiator as Ivocerin. It allows curing in large increments with an absorption maximum in the blue light range around 370-460 nm [16]. It is more reactive than camphorquinone or Lucirin TPO resulting in more rapid polymerization with greater depth of cure [15, 16].
Polymerization kinetics
The free monomer molecules are loosely bonded by weak van der Waals force and upon polymerization, the monomers are tightly linked by covalent bonds in a polymer with a smaller distance between the molecules leading to polymerization shrinkage.
Polymerization contraction strain is time-dependent and proceeds in two stages: pregelation and post-gelation, or rigid contraction [17]. Two macroscopic demarcations occur during polymerization: the gel point conversion at which an incipient gel is formed. The second macroscale demarcation is the vitrification point which represents the conversion at which the polymer becomes glassy, accompanied by an increase in modulus. These polymer networks are extremely heterogenous which grow from microgels arising near sites of initiation [15]. Plastic flow occurs during the initial phase, and internal stresses within the material undergo stress-relaxation, while stress development occurs beyond the gel point and its magnitude depends on the elastic modulus [18].
Hardness is taken as an indirect measure for the degree of cure, and is measured in a longitudinally sectioned specimen from the top of restoration until the bottom, with decreasing hardness values denoting decreasing degree of conversion with increased thickness and distance from the light source [19, 20]. Over 20 years ago, the question about anisotropy of the cure pattern was raised with possible variation of shrinkage patterns within the material, relative to the initiating light source, with respect to material thickness and surface area. In cases where material specimens are imperfectly cured, the measured shrinkage will be correspondingly reduced [20–22].
In recent studies, light beam profiles and their effect on the polymerization of the composites were investigated. Irradiance values calculated by conventional methods assume power uniformity within the light beam but do not describe the distribution of the irradiance delivered. Beam profilers investigated the optically active emitting area, the mean irradiance and the irradiance distribution with differences among various light-curing units. This could propose improper curing of deeper parts of a restoration due to beam inhomogeneity [23, 24]. However, longer curing time could improve the polymerization properties of conventional and bulk-fill composites [25].
Degree of conversion and depth of cure
The “depth of cure” (DOC), usually referring to the thickness of a resin composite that is “adequately” cured, is limited by light absorption and scatter within the material, which are influenced by numerous factors, including the amount, size and type of fillers [2, 4], RBC shade [5–7], photoinitiator type and concentration [8, 9], refractive index mismatch [2], light irradiation source and irradiation duration. The degree of conversion (DC) refers to the percentage of C=C that have been converted into single bonds to form the polymer chains. The higher the degree of conversion the better the mechanical and physical properties and clinical reliability of the composite material. The light intensity and light polymerization mode can influence the polymerization shrinkage developed by dental composites [26].
Several methods have been used to assess the DOC. A simplistic method has been described in the ISO 4049 standard and is based on measurement with a micrometer of the thickness of the RBC that remains after removal of uncured soft material with a plastic spatula [13]. Other groups have used a penetrometer to measure the DOC based on application of a needle with a constant force to the lower side of the RBC sample [7, 9, 14]. Alternative methods include measurement of the degree of conversion (DC), by Raman or FTIR spectroscopy [15], or of the microhardness (MH) [5, 11] at regular intervals through the depth of the material. Based on these measurements, the DOC is usually described as the depth at which the MH or DC value equals the surface value multiplied by an arbitrary ratio, usually 0.8 [5, 16]. Several factors suggest, however, that these methods might not be appropriate to measure the quality of cure of a RBC at depth. When considering the DC at depth, it is difficult to determine which DC corresponds to “adequate” polymerization. In fact, the DC value on its own is insufficient, as it does not provide any information on the state of network development, i.e., the degree of cross-linking. The highly crosslinked nature of dimethacrylate-based polymers is responsible for a non-linear polymerization process, marked by two macroscopic changes of state: first, gelation, which refers to the change from a viscous liquid to an elastic gel (infinite network) occurring at a relatively low DC [27].
The “total energy concept” states that the process of light-induced polymerization is energy dependent and basically a product of light intensity and time. Peutzfeldt and Asmussen stated that the kinetics of polymerization is complex, and it was hypothesized that degree of cure, flexural strength, and flexural modulus were influenced not only by energy density, but also by power density per se particularly in stress bearing areas [28], adequate polymerization could be considered a crucial factor in obtaining good clinical performance of resin composites [29]. However, higher DC is accompanied with a high degree of polymerization shrinkage and greater risk of interfacial gaps formation [26, 28].
Polymerization shrinkage and shrinkage stress
Since the use of resin composites as restorative materials, a lot of research has been conducted in the field of polymerization and its effects as shrinkage and stress. In vivo tests focus on the indirect evaluation of gap formation by the resin replica technique [30–32]. In vitro test methods investigate the composite in form of a material sample or applied within a cavity prepared in human teeth [33] or other material such as resin composite or ceramic blocks [19, 34].
Composites undergo volumetric shrinkage of 2–6 % upon setting, which creates 5–15 MPa contraction stresses between the composite and the tooth, straining the interfacial bond, leading to debonding, microgaps and cuspal deflection [5]. This stress can even exceed enamel’s tensile strength and result in stress cracking and enamel fractures along the interfaces. Restorative failures as hypersensitivity, pulpitis and secondary caries may occur [6, 7, 35, 36].
The greater the volumetric shrinkage, the greater is the shrinkage stress for a comparable elastic modulus. The development of shrinkage stress depends on the volumetric shrinkage strain and the stiffness of the composite at the time of shrinkage; even low-shrinkage composites might exhibit high stress when having a high elastic modulus. A 2 mm incremental application of composite and polymerizing each increment independently is usually advised to ensure a full depth of cure and to reduce the net effect of polymerization shrinkage. Net shrinkage stress is assumed to be less because a smaller volume of composite is allowed to shrink before successive additions [11]. On the other hand, Versluis et al. showed that the incremental application combined with a well-established bond to the tooth increased the deformation of the restored tooth and in turn the stress level within the tooth-restoration complex [37].
Feilzer et al. showed that the stress development in a bonded composite restoration depends on the restoration geometry, in that the pregelation flow of the material is inhibited when the ratio of the bonded surface exceeds a certain limit; the magnitude of stress can be estimated through the C-factor (configuration factor) which is the ratio of bonded to unbonded areas. The higher the C-factor, the greater the stress level. This observation is related to the description of shrinkage as a vector, having both magnitude and direction [5]. Other studies criticized that the C-factor does not take into account the volume of the applied composite, and Braga et al. related shrinkage stress to microleakage in restorations of larger size [38, 39].
The polymerization shrinkage stress can be measured by various methods. Early shrinkage stress measurements were performed by Bowen [40, 41] and Hegdahl [42] using a Universal Testing (Instron) machine (UTM), while a servo-hydraulic UTM was used by the ACTA group of Davidson, Feilzer, de Gee and Alster who achieved major insights and developments, including the effect of C-factor on the stress magnitudes and eliminating the effect of system compliance. Unfortunately, this approach is expensive and complex, in addition to the basic limitation of eliminating compliance for load measurement systems as they imply finite compliance. [43–49]. An apparatus with a controlled compliance to measure contraction stress was developed by Sakaguchi et al. in which the composite specimen is located between a glass plate, and a steel rod measures the developed force upon curing [50]. Polymerization shrinkage stress is also determined by a tensiometer [5, 48, 51] and strain gauges [52]. A stress–strain analyzer testing machine was developed by Dullin [53] and measures the shrinkage stress with and without compliance [54, 55].
Alternatively, photo-elastic and Moire methods are based on optical fringes [56–61], while the finite element modeling (FEA) consists of a computer based model that determines the type and location of stresses in the model structures [62, 63]. Shrinkage strain-rate and stress is measured during polymerization with a tensiometer which is based on the cantilever beam deflection theory [64–66]. The “ring-slitting method” evaluates residual shrinkage stresses in composite: ring-shaped specimens are cured and slit for the evaluation of the gap distance due to stress release then gap measurements are evaluated with an image analyzer program [67, 68]. The Bioman shrinkage-stress instrument was designed to overcome the difficulties encountered with the servo-hydraulic UTM and its function is based on the fixed beam compliance [39, 49]. Variations in final stress values are found in the literature which can be attributed to the various testing techniques and system compliances. To obtain data that can be related to the clinical situation, the instrument compliance should preferably be similar to that of the prepared tooth [69].
Methods for measuring polymerization shrinkage
Various devices and methods have been used for measuring the polymerization shrinkage in terms of volumetric and linear shrinkage, cuspal displacements, indirect techniques as microleakage, finite element analysis and through the use of three-dimensional micro-CT data.
Volumetric shrinkage measurement
Early attempts to evaluate the polymerization shrinkage of resin composites consisted of measuring the volumetric loss due to the polymerization shrinkage. Volumetric shrinkage can be measured in a mercury dilatometer by determining the linear height changes of a column of fluid connected to a reservoir surrounding the sample. The capillary tube is read like a thermometer [14, 70, 71]. The disadvantages of this method are the potential for errors due to small temperature changes by composite polymerization which can affect the liquid volume; tedious specimen preparation is needed and flowables cannot be measured [72–74]. Access of the light source and opacity of the mercury are difficulties with light-cured dental materials [22]. Due to the potential for environmental mercury contamination and toxic mercury vapors, water-filled dilatometers are preferred. However, the most critical factor for the successful application is maintaining a constant temperature environment for the dilatometer during shrinkage test [75, 76]. The heat from the light source in addition to the heat of the exothermic polymerization reaction contribute to an unavoidable increase in temperature upon curing.
Yamamoto et al. measured the polymerization shrinkage of flowables in a water-filled dilatometer and with speckle contrast measurement. The flowable composite is condensed into a glass tube, irradiated, and the laser-speckle field is recorded in a digital frame [77]. The water-filled dilatometer is also indicated for shrinkage measurement of other types of composites, not only flowables [78].
Measuring the density change of composites before and after polymerization can be done by a gas pycnometer that determines the volume of samples before and after polymerization without contact, and the difference in volume is calculated [79] or by measuring the specific gravity differences between cured and uncured composite test specimens using a modified version of ASTM method D792 “Specific Gravity and Density of Plastics by Displacement” [80]. Buoyancy (density in water) measurements determine the volumetric polymerization shrinkage of composites in real time by measuring the buoyancy change of the specimen in distilled water [81]. The Archimedes method measures the actual shrinkage in volume according to the buoyant force principle [82] and this method was developed into a German Standard (DIN 13907/2005) [83–85]. When testing hydrophilic materials, as compomer or flowables, water sorption is a disadvantage with the Archimedes method that can be overcome by an alternative medium to water, such as oil or mercury.
Naoum et al. quantified the polymerization shrinkage with an electromagnetic balance by recording changes in the composite buoyancy during polymerization; it measures real time volumetric shrinkage at small intervals [86]. Volumetric shrinkage is obtained by video-imaging using AcuVol (Bisco Inc., Schaumburg IL, USA) and sample imaging by a digital video-camera [7, 87].
The volumetric shrinkage is approximately equal to three times the linear shrinkage provided the shrinkage occurs identically in all directions [81, 88]. Isotropic shrinkage is shrinkage equal in all directions, which is hardly the case in a restorative material applied into a tooth cavity, as it is confined by the cavity’s boundaries and associated boundary conditions.
Linear shrinkage measurement
Early measurements of linear shrinkage were determined on a cylinder of material utilizing a dilatometer, and the shrinkage percentage equals the difference in length over the original length [89]. The most common measurement for polymerization shrinkage is the bonded-disc method by Watts and Cash, also referred to as the “Watts method” [22]. An earlier precursor was described by Wilson [90] and Bausch et al. [17]. Linear shrinkage measurements are computed with linear displacement transducers such as linear variable differential transformer (LVDT) and the bonded-disc method which has been internationally adopted by a number of academic and industrial research laboratories [22, 84, 91]: a disc-shaped specimen of uncured composite is positioned at the center of a ring attached to a glass slide and covered by a flexible microscope coverslip on which the LVDT probe is placed for measuring the plate deflection; the material is light cured and data are recorded over time by a computer. The bonded-disc method results in lower shrinkage values because only the linear shrinkage of a bonded composite disc is measured and then converted into volume percent. However, the Archimedes method measures the actual volumetric shrinkage according to the buoyant force principle, and both methods are showing a high correlation [82]. Advantages of the bonded-disc method include the adjustment of a defined C-factor, the shrinkage direction is governed by the configuration rather than the direction of light application, axial shrinkage strain corresponds to volumetric shrinkage, specimen diameter matches that of the light guide tip, complete cure of the specimen due to low thickness, and the ease and convenience of use [91].
Various techniques for linear polymerization measurements involve strain gauges [52, 92, 93], a microscope [43] and the linometer whose results are in agreement with those obtained with the mercury dilatometer [94]. When a direct contact displacement transducer such as a strain gauge is employed, some mechanical resistance must be overcome to produce deformation of the gauge. Consequently, the polymerization shrinkage before the gel point is compensated by flow of resin and only the post-gel contraction is assessed [92].
The scanning laser beam determines the linear polymerization shrinkage of light-cured composites, in which the sample is unaffected by the measurement technique and small samples could be analyzed with great accuracy [95]. A laser interferometric method consists of a low power Helium–Neon laser, a Michelson interferometer, amplified photodiode detectors, and a computer data acquisition system. The method measures linear polymerization shrinkage in composites which is expressed as the percent linear contraction [96].
Another method is the 2D particle tracking method in which an optical instrument measures the linear shrinkage without directly contacting the specimen and the images are processed by a custom made software for image processing and analysis (IMAQ Vision and Labview 7.0, National Instrument, Austin, TX, USA). An update of that method involves the fluorescent particle tracking method with computer vision during curing and a software for multi-particle tracking. [88, 97]. Others determine the shrinkage by a video-imaging device [98, 99].
Cuspal deflection measurement
The previously mentioned methods measure the gross shrinkage of resin composite samples as such, but they do not represent or consider the boundary conditions that occur in a tooth cavity, thus, do not predict clinical performance. Quantifying cuspal deflection is indicative of the shrinkage measurement of bonded composite inside a tooth cavity, in an attempt to correlate to the clinical situation. Polymerization shrinkage is greater when measured by a dilatometer than cuspal movement where the composite is restricted by being bonded to the cavity walls [100]. Moreover, it was previously confirmed that shrinkage strains change with the boundary conditions [101].
Cuspal deflection has been thoroughly investigated for class II cavities restored with composites using a variety of techniques including photography/microscopy (microscope with a micrometer stage) [102–104], Michelson interferometry [105], strain gauges [106–110], linear variable differential transformers (LVDT) [111–113], contact stylus profilometry together with computer graphics [4, 114], digital-image-correlation (DIC or texture correlation) [115, 116], the direct current differential transducers [117] or electronic speckle pattern interferometry [118, 119]. The DIC method analyzes an object’s displacement based on the comparison of two similar speckled images; one before deformation and one after. Images are recorded using a CCD camera through an optical microscope, and the local displacements between the images are calculated [116, 120–122].
The cuspal deflections average 15 µm up to 50 µm, but vary according to the technique used. Absence of standardization of tooth size, cavity preparation and restoration technique renders comparison of results difficult, since contraction of cusps depends on the remaining tooth structure. Some studies combine cuspal deflection with microleakage analysis [123] or shrinkage stress determination [124].
Comparisons of results should be done with caution as the methods are not standardized among the various testing labs. One group of researchers have performed various investigations with standardized cavity configurations that allow the comparison of the results among their studies [123, 125–130]. Another difficulty of the interpretation of the results is when partial debonding of the restoration happens, because it would decrease the cuspal movement, while the bond has failed, thus, giving misleading results assuming less shrinkage.
Measurement of adverse effects of polymerization shrinkage
A traditional method for determining the adverse effects of polymerization shrinkage includes in vitro assessments of interfacial adaptation based on dye penetration, or quantitative marginal gap analysis by the replica technique and scanning electron microscopy (SEM) [31, 131–133]. Recent studies applied clinical evaluation criteria such as the use of the World Dental Federation criteria (FDI-criteria) [134] to in vitro investigations for predicting the clinical performance of restorations, thus correlating in vitro results to the in vivo situation [135, 136]. Bond strength tests evaluate the composite-dentin-bonding-agent-complex such as shear, tensile, micro-shear and microtensile bond strength tests [137, 138]. Data from labs worldwide using various tests on the same material achieve different results, even microtests could not eliminate the high degree of variations among results [139]. Others attempt to combine more than one investigation method, for example, a bond strength test with the interfacial gap distance evaluation [9] or with a finite element analysis [140] for obtaining a more diverse perspective on the shrinkage effects. Other in vitro phenomena help to rate the shrinkage effects such as microleakage, nanoleakage, structure and mechanical properties of the bonding interface. These can be related to bond strength values, but they do not necessarily correlate with results of microleakage tests or gap formation at the cavity margin, whereas nanoleakage tests and morphological and chemical characteristics of the bonding interface could be indicative of future dentin bond durability [141].
However, the clinical reliability of these tests are considerably questioned. It is suggested to develop more appropriate bond test and analysis of in vitro phenomena [141], while others suggest to use an interfacial fracture mechanics approach for the analysis of the dentin-adhesive bond for better agreement of test results [139, 140]. Thus, when hydrophilic dentin bonding agents are investigated the dye penetration method bears some difficulties in differentiating between the stained bonding agent and true gaps. Furthermore, the accuracy of the replica technique are found to depend largely on the quality of both the impression and replica for the margin analysis. In addition, it gives information on the length but not the depth of defect which also applies to SEM [142].
Finite element analysis
The finite element analysis is a numerical technique in which the model of an object is divided into many small simple-shaped elements. Thus a model of the tooth and the restoration can be generated from a micro-CT scan. The deformation of the small elements of one structure can be easily calculated and the deformation of the whole structure can be evaluated [143]. In a finite element analysis Versluis et al. questioned whether composites shrink toward the light. A cylindrical cavity with different boundary conditions is simulated by stress analysis and revealed that the shrinkage direction of light-cured composites depends on the boundary conditions, especially on the bonding between the restoration and the tooth rather than the light source [144].
Methods employing three-dimensional micro-CT data for the evaluation of polymerization shrinkage
The micro-computed tomography is a cone beam tomography producing three-dimensional images of high resolution up to few micrometer. The micro-CT scans are extensively used for bone density investigation [145, 146], mineral content assessment in caries research [147–151] and tooth modeling in finite element analysis [143]. Data acquisition for the shrinkage evaluation could be divided into two distinct parts: on one hand the assessment of volumetric changes and resulting gap analysis or detachment of the restorative material from the cavity interface, and on the other hand the shrinkage vector calculations through tracing particles before and after polymerization.
Volumetric evaluation of polymerization shrinkage
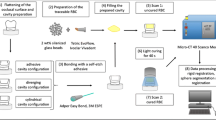
Non-destructive investigations of the marginal adaptation and the adhesive interface are performed [152–154], in addition to the evaluation of the internal adaptation of adhesive restorations [155, 156]. Others investigated the volume loss of resin composites [157–159] in addition to the volume loss in association with a direct non-destructive microleakage investigation [64, 158]. Hirata et al. evaluated the volumetric shrinkage of low shrinkage and bulk-fill composites with and without the application of bonding agents [160] (Fig. 1). The volumetric evaluation measures the composite volumes in the uncured and cured states and can display internal voids as well. It should be noted that the detection of gaps is related to the resolution of scanning which means that gaps are detectable at ×2.5 voxel size which constitutes a limitation to this method. Currently a typical micro-CT resolves 6–8 µm which corresponds to a detectable gap size of 25–30 µm, yet, with a higher resolution, smaller gaps could be visualized.
Methods utilizing the shrinkage vectors methods
The visualization of polymerization shrinkage in form of shrinkage vectors is a technique that has been introduced by Inai et al. and improved by Cho et al. [19, 161]. The basic concept of this technique has been adopted and further developed by Rösch et al., Chiang et al. [33, 142, 162] and other research labs [163, 164].
Medical image registration
The use of radiological images has increased in medical research and healthcare. Generally, image registration can be used for combining images of one subject, thus compensating for motion between scans. This is performed by registration algorithms that automatically register images by a rigid body transformation. On the other hand, non-rigid registration algorithms compensate for tissue deformation or align images from different subjects [165, 166].
Medical image registration methods are important in brain tumor studies for tumor visualization and observation [167]. In dentistry, Kunzelmann, 1996, was one of the first to visualize 3D data for wear analysis and quantification of filling materials in vitro and in vivo [168]. Swennen et al. applied the rigid registration method for detailed visualization of the interocclusal relationship in the course of 3D virtual planning of orthognathic surgery. They designed a 3D splint with the double CT scan procedure to obtain an anatomic 3D virtual augmented model of the skull with detailed dental surface [169]. Sandholzer et al. used quantitative micro-CT data for studying the 3D shrinkage and shape preservation of human teeth upon heating which is relevant information for forensic investigators [170].
Methods for acquiring two-dimensional shrinkage vectors
Shrinkage vectors represent the magnitude and direction in which shrinkage occurs [22]. Inai et al. invented the filler-tracing-method for the visualization of polymerization shrinkage by obtaining real polymerization shrinkage vectors. In their first study, they embedded radiopaque zirconia fillers into a composite, scanned the restoration before and after light-curing to identify the fillers manually in both scans and calculate the movement two-dimensionally [161].
The first automated process was conducted by Chiang et al. in which micro-CT data are combined with the image registration approach to determine and visualize the direction and amount of shrinkage vectors. Radiolucent glass fillers are embedded into a flowable composite scanned with a micro-CT before and after light-curing then the fillers are traced. Using radiolucent glass fillers has the advantage of avoiding reconstruction artifacts. Chiang et al. have studied the two-dimensional displacement vector field in composites which is calculated with an elastic registration algorithm using vector-spline regularization combined with the B-spline based elastic registration [142, 171–174] (Fig. 2).
The basic idea of the elastic registration is the application of a grid to both pre-and-post-polymerization scan images, where the regular grid is applied to the pre-polymerization situation, and the deformed grid to the post-polymerization. The grid is elastic and its deformation costs energy, thus larger deformations require more energy. Then the grid is fit to the new position using the least possible energy, where the points of grid deformation are identical to the markers. Registering two images leads to elastic fields that can be expressed in terms of B-splines. The grid is fit to the new position based on the markers by elastic registration. When markers are not evenly distributed in every 2D section of the 3D scan the missing deformations are assumed. Consequently, the elastic registration based on B-splines regularization is highly dependent on its parameter values and in case of improper assumptions might lead to incorrect results [142, 174].
Methods for acquiring three-dimensional shrinkage vectors
Chiang et al. analyzed the three-dimensional shrinkage vectors in an experimental flowable composite by implementing a method that is based on a different mathematical principal, the block-matching algorithm. The basic idea was to identify each individual embedded glass sphere and follow the position change of the glass spheres due to polymerization shrinkage [33, 142, 162, 174] (Fig. 3).
Data processing is performed in different steps. First, the rigid registration matches the pre- and post-polymerization scans via the outer tooth contours of each sample in which the enamel and the dentino-enamel-junction serve as references for the exact matching of both scans. Afterwards, sphere segmentation and registration is performed for the extraction of glass fillers based on the gray values of the micro-CT scans. Glass beads have a diameter of 40–70 µm and can be identified via the tensor of inertia. Each identified spherical structure (glass filler) is labeled and traced in both scans. The center of each glass filler is determined in the pre-polymerization scan and its corresponding center in the post-polymerization scan. The Euclidian distance of both centers constitutes the shrinkage vector. These calculations are based on the block-matching algorithm.
In another study, Cho et al. imported the micro-CT-scan data of the composite restorations before and after polymerization into a custom made software, the 3D-BON software (Ratoc Systems Engineering, Tokyo, Japan) and used a cluster-labeling algorithm to extract the fillers and perform the pairing procedure. Images are reconstructed from the data by a 3D data visualization tool Avizo 6.2 (Visualization Sciences Group, Burlington, MA). The movement distance of each filler after polymerization is obtained three-dimensionally. However, the analysis of filler movement is limited to the axial direction in relation to the light source by dividing filler movement data into 165 regions within the restoration [19] (Fig. 4).
Takemura et al. have incorporated air bubbles into the composite instead of fillers and traced their movements for visualizing three-dimensional shrinkage vectors [163]. Van Ende et al. have also applied the principal of the B-spline evaluation to their investigation of three-dimensional shrinkage vectors in cavities of different sizes (Fig. 5). They traced inherently present fillers of the flowable composite that were identified instead of adding fillers and studied shrinkage vectors in addition to the accompanying shrinkage stresses in small and large restorations [164]. The various evaluation methods enable the researchers to calculate and visualize the shrinkage vectors, but the methods differ fundamentally as each is based on a specific mathematical principle.
Conclusions
In the light of the currently available methods it can be concluded that the earlier methods for measuring the polymerization shrinkage of composites are easier, faster and less expensive, but they do not give information on the internal shrinkage behavior. The procedures of scanning the samples in the micro-CT and performing the evaluation of the shrinkage vectors are time consuming and complicated. Moreover, the shrinkage vector evaluation can be conducted only with the respective softwares which are not commercially available. Nevertheless, the methods using micro-CT data for measuring the polymerization shrinkage give insight into different resin composite restorations revealing the sites of possible detachment of composite from cavity walls and margins as well as the internal shrinkage behavior and how different boundary conditions affect the shrinkage vectors. This in turn constitutes an advantage that is lacking other methods measuring the polymerization shrinkage. Adopting this method by resin composite materials manufacturers can provide simple, life-image clues of interesting clinical relevance on the materials behavior. We recommend using this method in materials manufacturing for product quality assurance as well as in testing the different manipulation and application techniques. In conclusion, the use of three-dimensional micro-CT data for measuring both the volumetric polymerization shrinkage and calculating the shrinkage vectors is a highly accurate method.
References
Heintze SD, Rousson V. Clinical effectiveness of direct class II restorations—a meta-analysis. J Adhes Dent. 2012;14(5):407–31.
Demarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJM. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater. 2012;28(1):87–101.
Moreira da Silva E, dos Santos GO, Guimaraes JG, Barcellos Ade A, Sampaio EM. The influence of C-factor, flexural modulus and viscous flow on gap formation in resin composite restorations. Oper Dent. 2007;32(4):356–62.
Tantbirojn D, Versluis A, Pintado MR, DeLong R, Douglas WH. Tooth deformation patterns in molars after composite restoration. Dent Mater. 2004;20(6):535–42.
Feilzer AJ, De Gee AJ, Davidson CL. Setting stress in composite resin in relation to configuration of the restoration. J Dent Res. 1987;66(11):1636–9.
Kleverlaan CJ, Feilzer AJ. Polymerization shrinkage and contraction stress of dental resin composites. Dent Mater. 2005;21(12):1150–7.
Labella R, Lambrechts P, Van Meerbeek B, Vanherle G. Polymerization shrinkage and elasticity of flowable composites and filled adhesives. Dent Mater. 1999;15(2):128–37.
Al-Harbi F, Kaisarly D, Bader D, El Gezawi M. Marginal integrity of bulk versus incremental fill class II composite restorations. Oper Dent. 2016;41(2):146–56.
Al-Harbi F, Kaisarly D, Michna A, ArRejaie A, Bader D, El Gezawi M. Cervical interfacial bonding effectiveness of class II bulk versus incremental fill resin composite restorations. Oper Dent. 2015;40(6):622–35.
Elderton RJ. Restorations without conventional cavity preparations. Int Dent J. 1988;38:112–8.
Sakaguchi RL, Powers JM. Craig’s restorative dental materials. 13th ed. Philadelphia: Elsevier/Mosby; 2012. p. 161–98.
Kunzelmann KH. Aufbau der Kompositfüllungswerkstoffe. In: Heinrich Friedrich Kappert KE, editor. Zahnärztliche Werkstoffe und ihre Verarbeitung, Bd. 2: Werkstoffe unter klinischen Aspekten. Stuttgart, Germany: Georg Thieme Verlag KG; 2008. p. 204–41.
Condon JR, Ferracane JL. Reduction of composite contraction stress through non-bonded microfiller particles. Dent Mater. 1998;14(4):256–60.
Bekkedahl N. Volume dilatometry. J Res Natl Bur Stand. 1949;43(2):145–56.
Cramer NB, Stansbury JW, Bowman CN. Recent advances and developments in composite dental restorative materials. J Dent Res. 2011;90(4):402–16.
Scientific Documentation Tetric EvoCeram® Bulk Fill. In: AG IV, editor. Schaan, Liechtenstein 2013. p. 39.
Bausch JR, de Lange K, Davidson CL, Peters A, de Gee AJ. Clinical significance of polymerization shrinkage of composite resins. J Prosthet Dent. 1982;48(1):59–67.
Braem M, Lambrechts P, Vanherle G, Davidson CL. Stiffness increase during the setting of dental composite resins. J Dent Res. 1987;66(12):1713–6.
Cho E, Sadr A, Inai N, Tagami J. Evaluation of resin composite polymerization by three dimensional micro-CT imaging and nanoindentation. Dent Mater. 2011;27(11):1070–8.
Onose H, Sano H, Kanto H, Ando S, Hasuike T. Selected curing characteristics of light-activated composite resins. Dent Mater. 1985;1(2):48–54.
Watts DC, Amer O, Combe EC. Characteristics of visible-light-activated composite systems. Br Dent J. 1984;156(6):209–15.
Watts DC, Cash AJ. Determination of polymerization shrinkage kinetics in visible-light-cured materials: methods development. Dent Mater. 1991;7(4):281–7.
Price RB, Rueggeberg FA, Labrie D, Felix CM. Irradiance uniformity and distribution from dental light curing units. J Esthet Restor Dent. 2010;22(2):86–101.
Price RB, Labrie D, Whalen JM, Felix CM. Effect of distance on irradiance and beam homogeneity from 4 light-emitting diode curing units. J Can Dent Assoc. 2011;77:b9.
Zorzin J, Maier E, Harre S, Fey T, Belli R, Lohbauer U, et al. Bulk-fill resin composites: polymerization properties and extended light curing. Dent Mater. 2015;31(3):293–301.
da Silva EM, Poskus LT, Guimaraes JG, de Araujo Lima Barcellos A, Fellows CE. Influence of light polymerization modes on degree of conversion and crosslink density of dental composites. J Mater Sci Mater Med. 2008;19(3):1027–32.
Leprince JG, Leveque P, Nysten B, Gallez B, Devaux J, Leloup G. New insight into the “depth of cure” of dimethacrylate-based dental composites. Dent Mater. 2012;28(5):512–20.
Peutzfeldt A, Asmussen E. Resin composite properties and energy density of light cure. J Dent Res. 2005;84(7):659–62.
da Silva EM, Poskus LT, Guimaraes JG. Influence of light-polymerization modes on the degree of conversion and mechanical properties of resin composites: a comparative analysis between a hybrid and a nanofilled composite. Oper Dent. 2008;33(3):287–93.
Opdam NJ, Feilzer AJ, Roeters JJ, Smale I. Class I occlusal composite resin restorations: in vivo post-operative sensitivity, wall adaptation, and microleakage. Am J Dent. 1998;11(5):229–34.
Roulet JF, Salchow B, Wald M. Margin analysis of posterior composites in vivo. Dent Mater. 1991;7(1):44–9.
Qualtrough AJ, Cramer A, Wilson NH, Roulet JF, Noack M. An in vitro evaluation of the marginal integrity of a porcelain inlay system. Int J Prosthodont. 1991;4(6):517–23.
Chiang YC, Rösch P, Dabanoglu A, Lin CP, Hickel R, Kunzelmann KH. Polymerization composite shrinkage evaluation with 3D deformation analysis from microCT images. Dent Mater. 2010;26(3):223–31.
Li J, Li H, Liu X, Fok A, editors. A glass model cavity system for shrinkage stress assessment. IADR/AADR/CADR 89th General Session; 2011; San Diego, CA;1578.
Ferracane JL. Developing a more complete understanding of stresses produced in dental composites during polymerization. Dent Mater. 2005;21(1):36–42.
Ferracane JL. Buonocore Lecture. Placing dental composites—a stressful experience. Oper Dent. 2008;33(3):247–57.
Versluis A, Douglas WH, Cross M, Sakaguchi RL. Does an incremental filling technique reduce polymerization shrinkage stresses? J Dent Res. 1996;75(3):871–8.
Braga RR, Boaro LCC, Kuroe T, Azevedo CLN, Singer JM. Influence of cavity dimensions and their derivatives (volume and ‘C’ factor) on shrinkage stress development and microleakage of composite restorations. Dent Mater. 2006;22(9):818–23.
Watts DC, Satterthwaite JD. Axial shrinkage-stress depends upon both C-factor and composite mass. Dent Mater. 2008;24(1):1–8.
Bowen RL. Adhesive bonding of various materials to hard tooth tissues. VI. Forces developing in direct-filling materials during hardening. J Am Dent Assoc (1939). 1967;74(3):439–45.
Bowen RL, Nemoto K, Rapson JE. Adhesive bonding of various materials to hard tooth tissues: forces developing in composite materials during hardening. J Am Dent Assoc (1939). 1983;106(4):475–7.
Hegdahl T, Gjerdet NR. Contraction stresses of composite resin filling materials. Acta Odontol Scand. 1977;35(4):191–5.
Davidson CL, Feilzer AJ. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J Dent. 1997;25(6):435–40.
Feilzer AJ, De Gee AJ, Davidson CL. Curing contraction of composites and glass-ionomer cements. J Prosthet Dent. 1988;59(3):297–300.
Feilzer AJ, De Gee AJ, Davidson CL. Quantitative determination of stress reduction by flow in composite restorations. Dent Mater. 1990;6(3):167–71.
Feilzer AJ, De Gee AJ, Davidson CL. Increased wall-to-wall curing contraction in thin bonded resin layers. J Dent Res. 1989;68(1):48–50.
Alster D, Feilzer AJ, de Gee AJ, Davidson CL. Polymerization contraction stress in thin resin composite layers as a function of layer thickness. Dent Mater. 1997;13(3):146–50.
Davidson CL, de Gee AJ. Relaxation of polymerization contraction stresses by flow in dental composites. J Dent Res. 1984;63(2):146–8.
Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater. 2003;19(1):1–11.
Sakaguchi RL, Wiltbank BD, Murchison CF. Contraction force rate of polymer composites is linearly correlated with irradiance. Dent Mater. 2004;20(4):402–7.
Davidson CL, de Gee AJ, Feilzer A. The competition between the composite-dentin bond strength and the polymerization contraction stress. J Dent Res. 1984;63(12):1396–9.
Sakaguchi RL, Peters MC, Nelson SR, Douglas WH, Poort HW. Effects of polymerization contraction in composite restorations. J Dent. 1992;20(3):178–82.
Dullin P. Development of a measuring system for the determination of the polymerization behavior of dental composite materials. “Entwicklung eines Mess-Systems zur Untersuchung des Polymerisationsverhaltens von zahnmedizinischen Kompositfuellungswerkstoffen.” [Thesis in Engineering Technology “Feinwerk und Mikrotechnik”]: University of Munich; 1998.
Chen HY, Manhart J, Hickel R, Kunzelmann KH. Polymerization contraction stress in light-cured packable composite resins. Dent Mater. 2001;17(3):253–9.
Chen HY, Manhart J, Kunzelmann KH, Hickel R. Polymerization contraction stress in light-cured compomer restorative materials. Dent Mater. 2003;19(7):597–602.
Kinomoto Y, Torii M. Photoelastic analysis of polymerization contraction stresses in resin composite restorations. J Dent. 1998;26(2):165–71.
Kinomoto Y, Torii M, Takeshige F, Ebisu S. Comparison of polymerization contraction stresses between self- and light-curing composites. J Dent. 1999;27(5):383–9.
Oliveira KM, Consani S, Goncalves LS, Brandt WC, Ccahuana-Vasquez RA. Photoelastic evaluation of the effect of composite formulation on polymerization shrinkage stress. Braz Oral Res. 2012;26(3):202–8.
Kinomoto Y, Torii M, Takeshige F, Ebisu S. Polymerization contraction stress of resin composite restorations in a model Class I cavity configuration using photoelastic analysis. J Esthet Dent. 2000;12(6):309–19.
Ernst CP, Meyer GR, Klocker K, Willershausen B. Determination of polymerization shrinkage stress by means of a photoelastic investigation. Dent Mater. 2004;20(4):313–21.
Rullmann I, Schattenberg A, Marx M, Willershausen B, Ernst CP. Photoelastic determination of polymerization shrinkage stress in low-shrinkage resin composites. Schweizer Monatsschrift fur Zahnmedizin = Revue mensuelle suisse d’odonto-stomatologie = Rivista mensile svizzera di odontologia e stomatologia/SSO. 2012;122(4):294–9.
Ausiello P, Apicella A, Davidson CL. Effect of adhesive layer properties on stress distribution in composite restorations—a 3D finite element analysis. Dent Mater. 2002;18(4):295–303.
Rodrigues FP, Silikas N, Watts DC, Ballester RY. Finite element analysis of bonded model Class I ‘restorations’ after shrinkage. Dent Mater. 2012;28(2):123–32.
Sun J, Fang R, Lin N, Eidelman N, Lin-Gibson S. Nondestructive quantification of leakage at the tooth-composite interface and its correlation with material performance parameters. Biomaterials. 2009;30(27):4457–62.
Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. Probing the origins and control of shrinkage stress in dental resin-composites: I. Shrinkage stress characterization technique. J Mater Sci Mater Med. 2004;15(10):1097–103.
Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. Probing the origins and control of shrinkage stress in dental resin composites. II. Novel method of simultaneous measurement of polymerization shrinkage stress and conversion. J Biomed Mater Res B Appl Biomater. 2004;71(1):206–13.
Park JW, Ferracane JL. Residual stress in composites with the thin-ring-slitting approach. J Dent Res. 2006;85(10):945–9.
Park JW, Ferracane JL. Measuring the residual stress in dental composites using a ring slitting method. Dent Mater. 2005;21(9):882–9.
Schneider LF, Cavalcante LM, Silikas N. Shrinkage stresses generated during resin-composite applications: a review. J Dent Biomech. 2010;1(1):1–14.
Smith DL, Schoonover IC. Direct filling resins: dimensional changes resulting from polymerization shrinkage and water sorption. J Am Dent Assoc (1939). 1953;46(5):540–4.
Rodriguez VI, Abate PF, Macchi RL. Immediate polymerization shrinkage in light cured restorative resins. Acta Odontol Latinoam: AOL. 2006;19(1):3–7.
Penn RW. A recording dilatometer for measuring polymerization shrinkage. Dent Mater. 1986;2(2):78–9.
de Gee AJ, Davidson CL, Smith A. A modified dilatometer for continuous recording of volumetric polymerization shrinkage of composite restorative materials. J Dent. 1981;9(1):36–42.
Kullmann W. Studies on the course of polymerization shrinkage of self-cured and light-cured composites. Deutsche zahnarztliche Zeitschrift. 1989;44(9):711–3.
Lai JH, Johnson AE. Measuring polymerization shrinkage of photo-activated restorative materials by a water-filled dilatometer. Dent Mater. 1993;9(2):139–43.
Rees JS, Jacobsen PH. The polymerization shrinkage of composite resins. Dent Mater. 1989;5(1):41–4.
Yamamoto A, Miyazaki M, Rikuta A, Kurokawa H, Takamizawa T. Comparison of two methods for measuring the polymerization characteristics of flowable resin composites. Dent Mater. 2007;23(7):792–8.
Oberholzer TG, Grobler SR, Pameijer CH, Rossouw RJ. A modified dilatometer for determining volumetric polymerization shrinkage of dental materials. Meas Sci Technol. 2002;13(1):78.
Cook WD, Forrest M, Goodwin AA. A simple method for the measurement of polymerization shrinkage in dental composites. Dent Mater. 1999;15(6):447–9.
Puckett AD, Smith R. Method to measure the polymerization shrinkage of light-cured composites. J Prosthet Dent. 1992;68(1):56–8.
Lee IB, Cho BH, Son HH, Um CM. A new method to measure the polymerization shrinkage kinetics of light cured composites. J Oral Rehabil. 2005;32(4):304–14.
Weinmann W, Thalacker C, Guggenberger R. Siloranes in dental composites. Dent Mater. 2005;21(1):68–74.
Soltesz U, Bath P, Klaiber B. Dimensional behavior of dental composites due to polymerization shrinkage and water sorption. In: Christel P, Meunier A, Lee A, editors. Biological and biomechanical performance of biomaterials. Amsterdam: Elsevier; 1986. p. 123–8.
Watts DC, Marouf AS. Optimal specimen geometry in bonded-disk shrinkage-strain measurements on light-cured biomaterials. Dent Mater. 2000;16(6):447–51.
Filtek LS, Technical Product Profile. USA: 3M ESPE Dental Products; 2007.
Naoum SJ, Ellakwa A, Morgan L, White K, Martin FE, Lee IB. Polymerization profile analysis of resin composite dental restorative materials in real time. J Dent. 2012;40(1):64–70.
Sharp LJ, Choi IB, Lee TE, Sy A, Suh BI. Volumetric shrinkage of composites using video-imaging. J Dent. 2003;31(2):97–103.
Lee IB, Min SH, Seo DG. A new method to measure the polymerization shrinkage kinetics of composites using a particle tracking method with computer vision. Dent Mater. 2012;28(2):212–8.
Lee HL Jr, Swartz ML, Smith FF. Physical properties of four thermosetting dental restorative resins. J Dent Res. 1969;48(4):526–35.
Wilson HJ. Properties of radiation-cured restorative resins. Proceedings of the International Symposium on Fotofil Dental Restorative. London: Franklin Scientific Projects; 1978.
Watts DC, Hindi AA. Intrinsic ‘soft-start’ polymerisation shrinkage-kinetics in an acrylate-based resin-composite. Dent Mater. 1999;15(1):39–45.
Sakaguchi RL, Sasik CT, Bunczak MA, Douglas WH. Strain gauge method for measuring polymerization contraction of composite restoratives. J Dent. 1991;19(5):312–6.
Sakaguchi RL, Versluis A, Douglas WH. Analysis of strain gage method for measurement of post-gel shrinkage in resin composites. Dent Mater. 1997;13(4):233–9.
de Gee AF, Feilzer AJ, Davidson CL. True linear polymerization shrinkage of unfilled resins and composites determined with a linometer. Dent Mater. 1993;9(1):11–4.
Fano V, Ortalli I, Pizzi S, Bonanini M. Polymerization shrinkage of microfilled composites determined by laser beam scanning. Biomaterials. 1997;18(6):467–70.
Fogleman EA, Kelly MT, Grubbs WT. Laser interferometric method for measuring linear polymerization shrinkage in light cured dental restoratives. Dent Mater. 2002;18(4):324–30.
Kweon HJ, Ferracane J, Kang K, Dhont J, Lee IB. Spatio-temporal analysis of shrinkage vectors during photo-polymerization of composite. Dent Mater. 2013;29(12):1236–43.
Yamamoto T, Kubota Y, Momoi Y, Ferracane JL. Polymerization stresses in low-shrinkage dental resin composites measured by crack analysis. Dent Mater. 2012;28(9):e143–9.
Simon Y, Mortier E, Dahoun A, Gerdolle DA. Video-controlled characterization of polymerization shrinkage in light-cured dental composites. Polym Test. 2008;27(6):717–21.
Suliman AH, Boyer DB, Lakes RS. Polymerization shrinkage of composite resins: comparison with tooth deformation. J Prosthet Dent. 1994;71(1):7–12.
Sakaguchi RL, Wiltbank BD, Shah NC. Critical configuration analysis of four methods for measuring polymerization shrinkage strain of composites. Dent Mater. 2004;20(4):388–96.
Suliman AA, Boyer DB, Lakes RS. Cusp movement in premolars resulting from composite polymerization shrinkage. Dent Mater. 1993;9(1):6–10.
Rees JS, Jagger DC, Williams DR, Brown G, Duguid W. A reappraisal of the incremental packing technique for light cured composite resins. J Oral Rehabil. 2004;31(1):81–4.
Gonzalez-Lopez S, Lucena-Martin C, de Haro-Gasquet F, Vilchez-Diaz MA, de Haro-Munoz C. Influence of different composite restoration techniques on cuspal deflection: an in vitro study. Oper Dent. 2004;29(6):656–60.
Suliman AA, Boyer DB, Lakes RS. Interferometric measurements of cusp deformation of teeth restored with composites. J Dent Res. 1993;72(11):1532–6.
Alomari QD, Reinhardt JW, Boyer DB. Effect of liners on cusp deflection and gap formation in composite restorations. Oper Dent. 2001;26(4):406–11.
Taha NA, Palamara JE, Messer HH. Cuspal deflection, strain and microleakage of endodontically treated premolar teeth restored with direct resin composites. J Dent. 2009;37(9):724–30.
Donly KJ, Wild TW, Bowen RL, Jensen ME. An in vitro investigation of the effects of glass inserts on the effective composite resin polymerization shrinkage. J Dent Res. 1989;68(8):1234–7.
Versluis A, Tantbirojn D, Douglas WH. Distribution of transient properties during polymerization of a light-initiated restorative composite. Dent Mater. 2004;20(6):543–53.
Morin DL, Douglas WH, Cross M, DeLong R. Biophysical stress analysis of restored teeth: experimental strain measurement. Dent Mater. 1988;4(1):41–8.
Pearson GJ, Hegarty SM. Cusp movement of molar teeth with composite filling materials in conventional and modified MOD cavities. Br Dent J. 1989;166(5):162–5.
Meredith N, Setchell DJ. In vitro measurement of cuspal strain and displacement in composite restored teeth. J Dent. 1997;25(3–4):331–7.
Lee MR, Cho BH, Son HH, Um CM, Lee IB. Influence of cavity dimension and restoration methods on the cusp deflection of premolars in composite restoration. Dent Mater. 2007;23(3):288–95.
DeLong R, Pintado M, Douglas WH. Measurement of change in surface contour by computer graphics. Dent Mater. 1985;1(1):27–30.
Chuang SF, Chang CH, Chen TY. Contraction behaviors of dental composite restorations—finite element investigation with DIC validation. J Mech Behav Biomed Mater. 2011;4(8):2138–49.
Chuang SF, Chang CH, Chen TY. Spatially resolved assessments of composite shrinkage in MOD restorations using a digital-image-correlation technique. Dent Mater. 2011;27(2):134–43.
Romanita R, Ilici C, Gatin E, Matei E, Didilescu A, Nicola C, et al. Cuspal deflection and adhesive interface integrity of low shrinking posterior composite restorations. Acta Stomatol Croat. 2010;44(3):142–51.
Bouillaguet S, Gamba J, Forchelet J, Krejci I, Wataha JC. Dynamics of composite polymerization mediates the development of cuspal strain. Dent Mater. 2006;22(10):896–902.
Lang H, Rampado M, Mullejans R, Raab WH. Determination of the dynamics of restored teeth by 3D electronic speckle pattern interferometry. Lasers Surg Med. 2004;34(4):300–9.
Huang YH, Quan C, Tay CJ, Chen LJ. Shape measurement by the use of digital image correlation. Opt Eng. 2005;44(8):087011–8.
Chuang SF, Chen TY, Chang CH. Application of digital image correlation method to study dental composite shrinkage. Strain. 2008;44(3):231–8.
Li J, Fok AS, Satterthwaite J, Watts DC. Measurement of the full-field polymerization shrinkage and depth of cure of dental composites using digital image correlation. Dent Mater. 2009;25(5):582–8.
Moorthy A, Hogg CH, Dowling AH, Grufferty BF, Benetti AR, Fleming GJ. Cuspal deflection and microleakage in premolar teeth restored with bulk-fill flowable resin-based composite base materials. J Dent. 2012;40(6):500–5.
Kwon Y, Ferracane J, Lee IB. Effect of layering methods, composite type, and flowable liner on the polymerization shrinkage stress of light cured composites. Dent Mater. 2012;28(7):801–9.
Abbas G, Fleming GJ, Harrington E, Shortall AC, Burke FJ. Cuspal movement and microleakage in premolar teeth restored with a packable composite cured in bulk or in increments. J Dent. 2003;31(6):437–44.
Cara RR, Fleming GJ, Palin WM, Walmsley AD, Burke FJ. Cuspal deflection and microleakage in premolar teeth restored with resin-based composites with and without an intermediary flowable layer. J Dent. 2007;35(6):482–9.
Fleming GJ, Cara RR, Palin WM, Burke FJ. Cuspal movement and microleakage in premolar teeth restored with resin-based filling materials cured using a ‘soft-start’ polymerisation protocol. Dent Mater. 2007;23(5):637–43.
Fleming GJ, Hall DP, Shortall AC, Burke FJ. Cuspal movement and microleakage in premolar teeth restored with posterior filling materials of varying reported volumetric shrinkage values. J Dent. 2005;33(2):139–46.
Fleming GJ, Khan S, Afzal O, Palin WM, Burke FJ. Investigation of polymerisation shrinkage strain, associated cuspal movement and microleakage of MOD cavities restored incrementally with resin-based composite using an LED light curing unit. J Dent. 2007;35(2):97–103.
Palin WM, Fleming GJ, Nathwani H, Burke FJ, Randall RC. In vitro cuspal deflection and microleakage of maxillary premolars restored with novel low-shrink dental composites. Dent Mater. 2005;21(4):324–35.
Lutz F, Luscher B, Ochsenbein H. In vitro evaluation of the adaptation and quality of the margins in various composite systems. Schweizerische Monatsschrift fur Zahnheilkunde = Revue mensuelle suisse d’odonto-stomatologie SSO. 1977;87(8):752–63.
Qvist V, Qvist J. Replica patterns on composite restorations performed in vivo with different acid-etch restorative procedures. Scand J Dent Res. 1985;93(4):360–70.
van Dijken JW, Horstedt P, Meurman JH. SEM study of surface characteristics and marginal adaptation of anterior resin restorations after 3–4 years. Scand J Dent Res. 1985;93(5):453–62.
Hickel R, Peschke A, Tyas M, Mjor I, Bayne S, Peters M, et al. FDI World Dental Federation—clinical criteria for the evaluation of direct and indirect restorations. Update and clinical examples. J Adhes Dent. 2010;12(4):259–72.
Al-Harbi F, Kaisarly D, Bader D, El Gezawi M. Marginal integrity of bulk versus incremental fill class II composite restorations. Oper Dent. 2016;41(2):146–56.
Heintze SD, Monreal D, Peschke A. Marginal quality of class II composite restorations placed in bulk compared to an incremental technique: evaluation with SEM and stereomicroscope. J Adhes Dent. 2015;17(2):147–54.
Braga RR, Meira JB, Boaro LC, Xavier TA. Adhesion to tooth structure: a critical review of “macro” test methods. Dent Mater. 2010;26(2):e38–49.
Armstrong S, Geraldeli S, Maia R, Raposo LH, Soares CJ, Yamagawa J. Adhesion to tooth structure: a critical review of “micro” bond strength test methods. Dent Mater. 2010;26(2):e50–62.
Scherrer SS, Cesar PF, Swain MV. Direct comparison of the bond strength results of the different test methods: a critical literature review. Dent Mater. 2010;26(2):e78–93.
Pongprueksa P, De Munck J, Karunratanakul K, Barreto BC, Van Ende A, Senawongse P, et al. Dentin bonding testing using a mini-interfacial fracture toughness approach. J Dent Res. 2016;95(3):327–33.
Tagami J, Nikaido T, Nakajima M, Shimada Y. Relationship between bond strength tests and other in vitro phenomena. Dent Mater. 2010;26(2):e94–9.
Chiang YC, Rösch P, Kunzelmann KH. Polymerization shrinkage with light-initiated dental composites. Germany: Ludwig-Maximilians-Universitaet Muenchen; 2009.
Magne P. Efficient 3D finite element analysis of dental restorative procedures using micro-CT data. Dent Mater. 2007;23(5):539–48.
Versluis A, Tantbirojn D, Douglas WH. Do dental composites always shrink toward the light? J Dent Res. 1998;77(6):1435–45.
Wagner DW, Lindsey DP, Beaupre GS. Deriving tissue density and elastic modulus from microCT bone scans. Bone. 2011;49(5):931–8.
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res: Off J Am Soc Bone Miner Res. 2010;25(7):1468–86.
Clementino-Luedemann TNR, Dabanoglu A, Ilie N, Hickel R, Kunzelmann K. Micro-computed tomographic evaluation of a new enzyme solution for caries removal in deciduous teeth. Dent Mater J. 2006;25(4):675–83.
Clementino-Luedemann TNR, Kunzelmann K. Mineral concentration of natural human teeth by a commercial micro-CT. Dent Mater J. 2006;25(1):113–9.
Huang TTY, Jones AS, He LH, Darendeliler MA, Swain MV. Characterisation of enamel white spot lesions using X-ray micro-tomography. J Dent. 2007;35(9):737–43.
Schwass DR, Swain MV, Purton DG, Leichter JW. A system of calibrating microtomography for use in caries research. Caries Res. 2009;43(4):314–21.
Zou W, Gao J, Jones AS, Hunter N, Swain MV. Characterization of a novel calibration method for mineral density determination of dentine by X-ray micro-tomography. Analyst. 2009;134(1):72–9.
De Santis R, Mollica F, Prisco D, Rengo S, Ambrosio L, Nicolais L. A 3D analysis of mechanically stressed dentin-adhesive-composite interfaces using X-ray micro-CT. Biomaterials. 2005;26(3):257–70.
Kakaboura A, Rahiotis C, Watts D, Silikas N, Eliades G. 3D-marginal adaptation versus setting shrinkage in light-cured microhybrid resin composites. Dent Mater. 2007;23(3):272–8.
Meleo D, Manzon L, Pecci R, Zuppante F, Bedini R. A proposal of microtomography evaluation for restoration interface gaps. Annali dell’Istituto superiore di sanita. 2012;48(1):83–8.
Kwon O-H, Park S-H. Evaluation of internal adaptation of dental adhesive restorations using micro-CT. Restor Dent Endod. 2012;37(1):41–9.
Kim HJ, Park SH. Measurement of the internal adaptation of resin composites using micro-CT and its correlation with polymerization shrinkage. Oper Dent. 2014;39(2):E57–70.
Sun J, Lin-Gibson S. X-ray microcomputed tomography for measuring polymerization shrinkage of polymeric dental composites. Dent Mater. 2008;24(2):228–34.
Sun J, Eidelman N, Lin-Gibson S. 3D mapping of polymerization shrinkage using X-ray micro-computed tomography to predict microleakage. Dent Mater. 2009;25(3):314–20.
Zeiger DN, Sun J, Schumacher GE, Lin-Gibson S. Evaluation of dental composite shrinkage and leakage in extracted teeth using X-ray microcomputed tomography. Dent Mater. 2009;25(10):1213–20.
Hirata R, Clozza E, Giannini M, Farrokhmanesh E, Janal M, Tovar N, et al. Shrinkage assessment of low shrinkage composites using micro-computed tomography. J Biomed Mater Res B Appl Biomater. 2015;103(4):798–806.
Inai N, Katahira N, Hashimoto K, Tagami J, Hirakimoto A, Marshall SJ, et al., editors. Microfocus X-ray CT analysis of shrinking direction in resin composite 2002. California: San Diego; 2014.
Rösch P, Chiang YC, Kunzelmann K. Quantification of local polymerisation shrinkage from 3D micro CT images of dental composites. Int J Comput Assist Radiol Surg. 2009;4(Suppl. 1):200–1.
Takemura Y, Hanaoka K, Kawamata R, Sakurai T, Teranaka T. Three-dimensional X-ray micro-computed tomography analysis of polymerization shrinkage vectors in flowable composite. Dent Mater J. 2014;33(4):476–83.
Van Ende A, Van de Casteele E, Depypere M, De Munck J, Li X, Maes F, et al. 3D volumetric displacement and strain analysis of composite polymerization. Dent Mater. 2015;31(4):453–61.
Hill DL, Batchelor PG, Holden M, Hawkes DJ. Medical image registration. Phys Med Biol. 2001;46(3):R1–45.
Fischer B, Modersitzki J. Ill-posed medicine-an introduction to image registration. Inverse Problems. 2008;24(3):034008(p 16).
Bauer S, Wiest R, Nolte LP, Reyes M. A survey of MRI-based medical image analysis for brain tumor studies. Phys Med Biol. 2013;58(13):R97–129.
Kunzelmann K. Analysis and quantification of wear of filling materials in vivo and in vitro. Verschleißanalyse und -quantifizierung von Füllungsmaterialien in vivo und in vitro. “Habilitationsschrift”: University of Munich; 1996.
Swennen GRJ, Barth EL, Eulzer C, Schutyser F. The use of a new 3D splint and double CT scan procedure to obtain an accurate anatomic virtual augmented model of the skull. Int J Oral Maxillofac Surg. 2007;36(2):146–52.
Sandholzer MA, Walmsley AD, Lumley PJ, Landini G. Radiologic evaluation of heat-induced shrinkage and shape preservation of human teeth using micro-CT. J Forensic Radiol Imaging. 2013;1(3):107–11.
Arganda-Carreras I, Sorzano CS, Marabini R, Carazo J, Ortiz-de-Solorzano C, Kybic J. Consistent and elastic registration of histological sections using vector-spline regularization. In: Beichel R, Sonka M, editors. Computer vision approaches to medical image analysis. Lecture Notes in Computer Science. Berlin: Springer; 2006. p. 85–95.
Sorzano COS, Thevenaz P, Unser M. Elastic registration of biological images using vector-spline regularization. IEEE Trans Biomed Eng. 2005;52(4):652–63.
Kybic J, Unser M. Fast parametric elastic image registration. IEEE Trans Image Process. 2003;12(11):1427–42.
Chiang YC, Rösch P, Lin CL, Hickel R, Kunzelmann K. Deformation analysis of composite polymerization shrinkage from μCT Images. Annual Meeting of the Academy of Dental Materials. 2008.
Acknowledgments
The authors would like to thank Prof. Dr. Karl-Heinz Kunzelmann for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kaisarly, D., Gezawi, M.E. Polymerization shrinkage assessment of dental resin composites: a literature review. Odontology 104, 257–270 (2016). https://doi.org/10.1007/s10266-016-0264-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-016-0264-3