Abstract
Comparative studies of floral development and morphology have largely contributed to the understanding of taxonomic classification, phylogenetic relationships and evolutionary trends across many angiosperm clades, particularly in the florally diverse family Leguminosae (alternatively Fabaceae). This study aimed to characterize the middle to late stages of floral development and morphological variation of the caesalpinioid genus Tachigali, an evolutionary radiation of predominantly neotropical rainforest trees. Floral buds and flowers of five representative species from Tachigali were analyzed under stereo microscopy, light microscopy and scanning electron microscopy to evaluate informative morphological and developmental characters. Although the genus displays relatively small flowers measuring up to 14 mm long, they are variable in terms of symmetry, structure and size, which have influenced the main taxonomic subdivisions among the species. Here, we show that the floral architecture of Tachigali involves a double whorl of stamens, anthers with dome-shaped connective extension and monosymmetrical hypanthium, owing to the unequal development of its wall at different stages of the floral ontogeny. Such developmental patterns are likely new diagnostic floral characters of Tachigali in the context of the early diverging caesalpinioid clades and reaffirm the circumscription of the genus in order to include the species previously classified within Sclerolobium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Comparative studies of floral development and morphology have informed taxonomic classifications, and enhanced our understanding of phylogenetic relationships and evolutionary trends across many angiosperm clades, particularly in the early diverging clades of the family Leguminosae, where the constituent genera display an extreme diversity of floral architecture (Tucker 1991, 2003; Cardoso et al. 2013a, b; Bruneau et al. 2014; Leite et al. 2014, 2015; Prenner et al. 2015; Prenner and Cardoso 2017). In the recently recircumscribed subfamily Caesalpinioideae, morphological variation in floral organs can be so high that it is difficult to recognize clear synapomorphies that best characterize the close phylogenetic affinity among florally discrepant genera (LPWG 2013, 2017; Prenner and Cardoso 2017).
The focus of this study is the caesalpinioid genus Tachigali Aubl., an evolutionary radiation of ant-housing trees predominantly found in neotropical rainforests (Chomicki et al. 2015). Among the 75 species described in Tachigali, 58 occur in Brazilian territory, and 26 are endemic to the country (Silva and Lima 2007; BFG 2018). Tachigali stands out for its ecological dominance, being one of the most abundant in number of species per sampling area in the Amazon region (ter Steege et al. 2013), as well as for its common mutualistic relationship with ants that inhabit the variously shaped leaf domatia (Fonseca 1999; Fonseca and Benson 2003; Chomicki et al. 2015).
The most recent phylogenetic classification of Tachigali based on molecular data placed the genus in the so-called Tachigali clade together with Arapatiella R.W. Cowan from the Atlantic Forest and Jacqueshuberia Ducke from the Amazon (Haston et al. 2005; Manzanilla and Bruneau 2012; Silva et al. 2016a, b). Traditionally, Tachigali was considered to be closely related to Sclerolobium Vogel because of their shared small flowers in large, dense panicles and cryptosamaroid legumes (Haston et al. 2005; Bruneau et al. 2008). However, these genera have been assembled in a broader circumscription of Tachigali based on similar wood anatomy (Barreta-Kuipers 1981; Macedo et al. 2014), pollen morphology (Graham and Barker 1981), floral and fruit morphological features (Silva and Lima 2007; van der Werff 2008), as well as results from molecular phylogenetic analyses, despite very sparsely sampled (Silva et al. 2016a, b). If Tachigali and Sclerolobium have to be recognized as distinct genera, Van der Werff (2008) pointed out that they would be separated by only one floral character: spathulate vs. linear petals. He also argued that the floral morphological variation of Tachigali was expanded to include both actinomorphic and zygomorphic flowers with spathulate and/or linear petals, as well as the ovary stipe arising in a central portion or laterally displaced in the hypanthium (van der Werff 2008).
In order to contribute to a better characterization of the floral morphological variation in Tachigali, we herein examine the middle to late stages of flower development to reveal the putative homologies between the actinomorphic Sclerolobium-like flowers and the zygomorphic Tachigali-like flowers. We also show how floral developmental morphology can contribute to elucidate diagnostic characters that bring more insight to the current classification of Tachigali.
Materials and methods
For the study of floral morphology, the following species of Tachigali were analyzed: T. beaurepairei (Harms) L.G.Silva & H.C.Lima, T. denudata (Vogel) Oliveira-Filho, T. duckei (Dwyer) Oliveira-Filho, T. paratyensis (Vell.) H.C.Lima & T. spathulipetala L.G.Silva, L.J.T.Cardoso, D.B.O.S.Cardoso & H.C.Lima. The first three species listed were previously circumscribed in Sclerolobium. These species were chosen as they represent the wide variation in floral morphology in Sclerolobium.
The floral buds and flowers of T. paratyensis (RB 435559) were collected at the Arboretum of the Rio de Janeiro Botanical Garden and fixed in glutaraldehyde 2.5% in a 0.1 M sodium phosphate buffer in pH 7.2 (Gabriel 1982). The samples of T. duckei (RB 430982; RB spirit 1617) and T. spathulipetala (RB 460994; RB spirit 1327; RB 459830; RB spirit 1329) were obtained from the Spirit collection of the herbarium of the Rio de Janeiro Botanical Garden and were previously fixed in ethanol 70%. The samples of T. denudata (RB 659134) and T. beaurepairei (RB 38772) were also preserved in ethanol 70% and were taken from the personal collection of Professor Haroldo Lima. The floral buds and flowers of each species were measured using the Leica MZ8 stereo microscope and analyzed and photographed with the Olympus SZ61 stereo microscope with an Olympus SC30 camera to determine the different stages of development.
The flower buds were dehydrated in ethanol series and then embedded in hydroxymethyl methacrylate (Gerrits and Smid 1983) and sectioned with the Leica RM2245 rotary microtome in order to proceed with light microscopy analysis. In this process, glass knives were used to obtain sections 1–3 μm thick, which were stained with 0.05% toluidine blue O (O’Brien et al. 1964) and observed and photographed using the BX-50 light microscope with the Olympus DP73 digital camera.
For scanning electron microscopy (SEM), the materials were dehydrated initially in ethanol series and later in acetone (Bozzola and Russel 1999, modified). The samples were submitted to Leica EM 030 critical point to finish the dehydration and fixed with a carbon tape on stubs and covered with a ca. 20 nm layer of gold (Emitech K550X Sputter Coater). The samples were observed under the Zeiss EVO 40 scanning electron microscope.
All images obtained from the different analyses were processed with Adobe Photoshop CS5 Extended software version 12.1, and the schemes were made with Adobe Illustrator CS5 software version 15.1.0.
Results
Morphological characters
In Tachigali, the young floral buds are protected by bracts (Fig. 1), which are deciduous and fall before anthesis. The flowers are small, with the smallest (T. spathulipetala) ca. 4.5 mm long and the biggest (T. paratyensis) ca. 14 mm long. The flowers in the other species analyzed here (T. beaurepairei, T. denudata and T. duckei) are ca. 5.5 mm long. The floral buds have 0.20–0.50 mm long pedicels in T. beaurepairei (Fig. 2d), T. denudata (Fig. 2a) and T. duckei (Fig. 2c) or 1–4 mm long in T. paratyensis (Fig. 2e) and T. spathulipetala (Fig. 2b).
a–e Flower buds, anthetic and postanthetic flowers. Tachigali denudata (a), T. spathulipetala (b), T. duckei (c), T. beaurepairei (d) and T. paratyensis (e). I) Floral buds with sepals and petal whorls removed, showing double whorl of stamens (arrowhead); II) flowers showing the gynoecium, with sepals, petals and stamens removed; III) flowers showing the androecium, with sepals and petals removed; IV) flowers showing petals (arrows), with sepals removed; V) entire flowers, showing sepals (asterisk). Bar: a–e = 1 mm
The flowers and floral buds have monosymmetric hypanthium with simple and glandular trichomes on their outer surface, except for T. paratyensis, which only has simple trichomes. All studied species have five boat-shaped sepals (Fig. 2a–e, V) with simple trichomes on the inner and outer surface, and five petals (Fig. 2a–e, IV) varying between spathulate (T. paratyensis Fig. 2e, IV), linear (T. beaurepairei, Fig. 2d, IV; T. denudata, Fig. 2a, IV; and T. duckei, Fig. 2c; IV) and heteromorphic in T. spathulipetala (Fig. 2b, IV) with spathulate and linear petals in the same flower. All petals have simple trichomes on the inner surface. In the spathulate petals, the trichomes are distributed along the main rib. Tachigali spathulipetala also has simple trichomes on the outer surface of its spathulate petals.
All species have ten stamens (Fig. 2a–e, III; Online Resource 1–5) arranged in two whorls (Fig. 2a–e, I; Online Resource 1–5), which are conspicuous in T. denudata (Fig. 2a, I; Online Resource 1) and T. paratyensis (Fig. 2e, I; Online Resource 2), less so in T. beaurepairei (Fig. 2d, I; Online Resource 3), T. duckei (Fig. 2c, I; Online Resource 4) and T. spathulipetala (Fig. 2b, I; Online Resource 5). After anthesis, the stamens are equal in size and shape in T. beaurepairei (Fig. 2d, III), T. denudata (Fig. 2a, III), T. duckei (Fig. 2c, III) and T. spathulipetala (Fig. 2b, III), but heteromorphic in T. paratyensis (Fig. 2e, III), with three short and thick stamens in the adaxial portion and seven thin stamens from the mid to the abaxial portion of the flower. All stamens have simple trichomes at the base of the filaments. The anthers are dorsifixed (Fig. 2a–e, III; 3a–b) with a dome-shaped connective extension apically (Fig. 3a–d), which can occasionally have simple trichomes (Fig. 3d). This connective extension was more protruded in T. denudata, especially in the anthers of antepetalous stamens.
a–d Anthers of Tachigali.a–bTachigali denudata seen in stereo microscope. cTachigali duckei seen with scanning electron microscopy. dTachigali beaurepairei seen with scanning electron microscopy. a Antesepalous anther/stamen; b antepetalous anther/stamen; a–b arrowhead indicating dome-shaped connective extension; c antepetalous anther/stamen of T. duckei. d antepetalous anther/stamen of T. beaurepairei; c–d anther detail with dome-shaped connective extension (arrowhead). Bar: a–b = 1 mm; c = 100 µm; d = 20 µm
In all species, the superior ovary with simple trichomes is elevated on a stipe (Figs. 2II, a–e, 4d–e). The stipe is centrally attached to the hypanthium (T. beaurepairei, Fig. 4d; T. denudata, Fig. 4a; and T. duckei, Fig. 4c) or laterally displaced (T. paratyensis, Fig. 4e; T. spathulipetala, Fig. 4b). The only style varies in length, being short (up to 0.65 mm long) in T. spathulipetala (Fig. 2b, II); medium-sized (1.00–1.50 mm long) in T. beaurepairei (Fig. 2d, II), T. denudata (Fig. 2a, II) and T. duckei (Fig. 2c, II); and elongated (ca. 2.0 mm long) in T. paratyensis (Fig. 2e, II).
Hypanthium development
In the floral buds, an unequal development of the hypanthium takes place (Fig. 4), giving an asymmetrical aspect to the floral bud from middle to late stages of floral development. This monosymmetry is evidenced by a projection in the adaxial portion of the hypanthium wall (Fig. 4a–e; Online Resource 1–5). In addition, from early stages, the ovary stipe can be found in a central position of the hypanthium in all species, while in later stages decentralization of the stipe appears in T. paratyensis (Fig. 4e) and T. spathulipetala (Fig. 4b). This positional change results from the unequal growth of the hypanthium which becomes more prominent in the adaxial portion.
Furthermore, the establishment of hypanthium monosymmetry is distinct in relation to the maturity of reproductive whorls among the analyzed species (Fig. 5). In T. denudata, the asymmetry begins when the ovule integuments are forming (Fig. 5a), as well as when meiosis takes place in the anthers (Fig. 5b, b’), with callose deposition. In T. spathulipetala, T. beaurepairei and T. duckei, the asymmetry arises later. In sections of T. spathulipetala and T. beaurepairei, functional megaspores are established in the ovule (Fig. 5c), and microgametogenesis with uninuclear pollen grains begins (Fig. 5d, d’). In T. duckei, the megaspores are at the dyad stage (Fig. 5e) and the anthers contain binuclear pollen grains (Fig. 5f, f’). In T. paratyensis, hypanthium asymmetry begins earlier than in the other species analyzed, and the floral buds are asymmetrical when the ovule (Fig. 5g) and the anther walls (Fig. 5h, h’) develop.
a–h’ Development of reproductive organs in floral buds. Tachigali denudata (a–b’), T. spathulipetala (c–d’), T. duckei (e–f’) and T. paratyensis (g–h’). a, c, e, g—ovule details. b–b’ d–d’ f–f’ h–h’—anther and androspore details. a Establishment of integuments (arrowhead); b androspore in meiosis (asterisk); b’ detail of the callose deposition (arrowhead); c functional gynospore established in the ovule (arrowhead); d beginning of androgametogenesis; d’ detail of unicellular pollen grains (arrowhead); e gynospore in meiosis (arrowhead), dyad; f–f’ bicellular pollen grains (arrowhead); g establishment of young ovules (arrowhead); h–h’ establishment of anther wall (arrowhead). Bar a–h = 100 µm; b’–h’ = 25 µm
Vascularization of flower buds
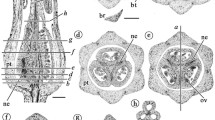
The hypanthium monosymmetry described above is also seen in flower bud vascularization. Among the five species analyzed, there are three distinct vascular patterns (Figs. 6, 7, 8). In all species, the intersection region between the pedicel and the basal portion of the hypanthium is similar, with a continuous cylindrical bicollateral vascular bundle (Figs. 6/1, 7/1, 8/1).
Diagram of Tachigali denudata floral bud vascularization in seven levels (indicated in 8). The vascular bundles are indicated in different colors related to their origin and position. Central cylinder (1–3) and ovary bundles (4–7) in purple; external circle (2–5): dark blue; internal circle (3–5) and stamen bundles (6–7): yellow; light blue (5–6): sepal–petal complex bundles; cyan (6–7): petal bundles. Bar: 7 = 100 µm, 8 = 1 mm
Floral bud vascularization scheme of Tachigali spathulipetala at seven levels (indicated in 8). The vascular bundles are indicated in different colors related to their origin and position. Central cylinder (1–3) and ovary bundles (4–7) in purple; external circle (2–5): dark blue; internal circle (2–5) and stamen bundles (6–7): yellow; light blue (5–6): sepal–petal complex bundles; cyan (6–7): petal bundles. Bar: 7 = 100 µm, 8 = 1 mm
Floral bud vascularization scheme of Tachigali paratyensis in seven levels (indicated in 8). The vascular bundles are indicated in different colors related to their origin and position. Central cylinder (1–3) and ovary bundles (4–7) in purple; external circle (2–4): dark blue; internal circle (3–4) and stamen bundles (5–7): yellow; light blue (5–6): sepal–petal complex bundles; cyan (5–7): petal bundles. Bar: 7 = 100 µm, 8 = 1 mm
In T. beaurepairei, T. denudata and T. duckei, the division of five vascular bundles starts at the hypanthium base from the central cylinder in the abaxial region (Fig. 6/2–4). These vascular bundles will be part of the external circle of hypanthium bundles (Fig. 6/4). Above these bundles, the division of five more vascular bundles begins, also from the central cylinder to the abaxial region of this structure (Fig. 6/3–4). These will be part of the inner circle of hypanthium bundles (Fig. 6/4). Because of the short (cupular) hypanthium, the gap between the divisions of these vascular bundles is reduced. Immediately after the establishment of the vascular bundles in the abaxial region, the separation of bundles from the central cylinder begins (Fig. 6/4). Above the departure of the bundles, a total of twenty vascular bundles are present at the midpoint of the hypanthium composed of two circles of ten bundles (outer and inner) and a vascular cylinder in the middle, which will be part of the carpel stipe (Fig. 6/5). Above the midpoint of the hypanthium, the vascular bundles of the outer circle undergo consecutive divisions. Five of these bundles form the sepal–petal complex, giving new bundles to both sepals and petals; the other five bundles divide, forming new bundles that will only be part of the sepals (Fig. 6/5). At the hypanthium, these bundles are intercalary to the ones that divides above in new bundles in sepals and petals (Fig. 6/5). The petals of T. beaurepairei, T. denudata and T. duckei are linear and have only one vascular bundle from their basal portion to the apex (Fig. 6/6–7). The vascular bundles of the inner circle will be part of the stamens (Fig. 6/3–7). They divide again only when they reach the anthers toward the two thecae (Fig. 6/7). The vascular cylinder of the ovary stipe is transformed into three vascular bundles in the ovary (Fig. 6/7).
In T. spathulipetala (Fig. 7), significant changes were noticed from level 2 (Fig. 7/2). In Fig. 7/2–3, in the abaxial region, the internal circle of vascular bundles has ten vascular bundles arranged in five pairs, originating from the central cylinder (Fig. 7/2–7). The same was observed for the adaxial region (Fig. 7/4–5). The pairs are only separated in the anthers when each bundle becomes part of a theca (Fig. 7/7). In addition, because of petal heteromorphism in this species, all petals have one vascular bundle at the base (Fig. 7/6). However, in spathulate petals, the vascular bundles divide into two or three higher up (Fig. 7/7), increasing in number toward the apical portion.
The third pattern was observed in T. paratyensis (Fig. 8). As a result of the elongated tubular hypanthium, the separation of the bundles of the external and internal circle occurs at some distance from the central cylinder in the abaxial and adaxial regions (Fig. 8/2–4). The petals have one vascular bundle at their base (Fig. 8/5–6); however, they divide into three bundles (Fig. 8/6–7) and, higher up, into several bundles near the middle region.
The supplemental material (sup. material 1–5) also shows the floral development of the species analyzed.
Discussion
Taxonomically informative floral developmental characters
Although the genus Tachigali displays small flowers with the largest up to 14 mm long, they are variable in terms of symmetry, structure and size, resulting in well-delimited species (van der Werff 2008). Within the Tachigali clade (Haston et al. 2005), the genus Tachigali has the smallest flowers compared with Arapatiella and Jacqueshuberia, as one of their distinguishing characters (Silva et al. 2016a, b).
The overall flower morphology of Tachigali has already been described in taxonomic studies (Silva 2007; van der Werff 2008; Silva et al. 2016a, b). Within Leguminosae, two stamen whorls, five sepals, five petals and a single carpel are well known (Tucker 2003). However, we herein describe new floral features that have, so far, remained unnoticed in morphological assessments of the genus. Among the floral characters observed in the later flower development of all studied species, two stamens whorls and anthers with dome-shaped connective extension are newly reported for the genus, as well as the glandular trichomes on the outer surface of the hypanthium in T. denudata, T. duckei and T. spathulipetala.
Anthers with connective extensions are common among several angiosperm families, as in Crassulaceae, Proteaceae, Sapindaceae and Rutaceae for example (Endress and Stumpf, 1991). Within Leguminosae, such extensions are often present in the mimosoid clade of Caesalpinioideae, in which they differentiate into secretory structures (Luckow and Grimes 1997; Barros and Teixeira 2016). This feature was also observed in the Detarioideae legume Goniorrhachis marginata Taub. (Prenner and Cardoso 2017). Although the connective extensions observed here are not secretory, they can still be taxonomically useful as a possible diagnostic character for the genus.
Glandular trichomes are also common in Leguminosae, both in vegetative and reproductive organs (Lavin et al. 2001; Horner et al. 2003; Meira et al. 2014; Gagnon et al. 2015; Marinho et al. 2016; Silva et al. 2018; Vargas et al. 2018). Moreover, they show considerable variation in form and therefore often used as a diagnostic character at genus and species levels (Meira et al. 2014; Gagnon et al. 2015; Silva et al. 2018; Vargas et al. 2018). In Tachigali, glandular trichomes have already been studied in the stipules (Pipoly 1995) and hypanthium (Dwyer 1954).
The floral hypanthium is a common feature of Leguminosae (LPWG 2017). It typically appears as an elongated receptacle that elevates perianth and androecium but does not merge with the gynoecium (Weberling 1989). When present, the hypanthium in Caesalpinioideae genera varies between cupular and tubular (LPWG 2017). After its new circumscription, the genus Tachigali began to encompass all species with tubular (cylindrical) hypanthium, as well as the species with cupular hypanthium that were formerly classified in Sclerolobium (Watson and Dallwitz 1983; Silva and Lima 2007).
Van der Werff (2008) has classified the hypanthium shape in Tachigali by its symmetry, i.e., symmetrical when the stipe is in central position in the hypanthium and asymmetrical when the stipe is laterally displaced. This character was used to separate Sclerolobium from Tachigali. Sclerolobium was described as having symmetrical hypanthium and Tachigali as having asymmetric hypanthium (Van der Werff 2008), which was then used in infrageneric classification. However, all species analyzed here have asymmetrical hypanthium, even those with a central stipe, meaning that stipe position is irrelevant in relation to the asymmetry of the hypanthium. Therefore, hypanthium asymmetry as described by Van der Werff (2008), was not confirmed in this study. Rather, it is suggested here that the asymmetry of the hypanthium is a consequence of unequal development of the hypanthium walls which protrude into the adaxial portion of the floral bud in middle to late stages of development. In addition, the hypanthium asymmetry in all species analyzed, including those previously classified as Sclerolobium, corroborates the results of the preliminary molecular phylogeny of Tachigali and Sclerolobium, which places both genera in the same monophyletic group (Silva et al. 2016a, b).
According to Tucker’s (1997) hierarchical theory, floral development corresponds to a succession of cascading events in which the first changes will dictate the final changes. Even according Tucker (1997), characters that develop in middle stages, such as the relative size of the floral organs, the loss or suppression of organs, as well as the fusion of organs, enable the characterization of taxa at generic level. Characteristics that develop late in the formation of the floral bud, such as the formation of papillae and trichomes, promote few changes in flower architecture; therefore, these characteristics are better suited for differentiation of infrageneric taxa. Our results for the hypanthium developmental pattern presented here agree with Tucker’s (1997) hierarchical theory since the monosymmetry in this structure becomes more evident in the middle to late stages of flower development and might supply additional support for the circumscription of Tachigali and Sclerolobium. However, some studies have shown that this hypothesis cannot be applied in all cases of legume classification (Bello et al. 2012; Cardoso et al. 2013a, b; Bruneau et al. 2014). Based on combined data of morphological floral traits and molecular analyses, Bello et al. (2012) demonstrated that the ontogenetic characters supporting the clade comprised of Leguminosae, Quillajaceae and Surianaceae in Fabales originate in middle to late stages of floral development, such as the five/six antesepalous stamen primordia and the disposition of the androecial whorl. Also, Bruneau et al. (2014) show that species differentiation within genera in Detarioidae can be caused by changes that occur at all stages of flower ontogeny.
Conspicuous changes of floral symmetry within Tachigali
Leguminosae are well known for the strongly zygomorphic flowers, as more emblematically exemplified by the papilionate flower of the Papilionoideae, which often involves a highly modified architecture with standard, wing and keel petals, stamens enveloping the ovary, fusion of floral organs and limited access to pollen and nectaries (Cardoso et al. 2013a, b; LPWG 2017). The Leguminosae also embraces actinomorphic, as well as monosymmetrical and zygomorphic yet non-papilionate flowers within the six subfamilies (Cardoso et al. 2013a, b; LPWG 2017).
In the Tachigali clade (Haston et al. 2005), Jacqueshuberia has flowers with slightly zygomorphic corolla. The Tachigali flowers were described as zygomorphic owing to the presence of heteromorphic stamens and an unequal tubular hypanthium (Watson and Dallwitz 1983; van der Werff 2008), as observed here in T. paratyensis, whereas the flowers of the species previously classified as Sclerolobium were described as actinomorphic (Watson and Dallwitz 1983; van der Werff 2008).
In the species analyzed in the present study (Table 1), the floral buds have a bilateral symmetry (zygomorphy) owing to hypanthium monosymmetry. However, in T. beaurepairei, T. denudata and T. duckei (previously Sclerolobium), after anthesis, flowers assume an actinomorphic aspect from the isomorphism of the sepals, petals and stamens. Finally, in addition to the monosymmetric hypanthium described above in the flower buds of T. spathulipetala, Silva et al. (2016a, b) point out the presence of heteromorphic petals: an adaxial, standard-like petal, two lateral, linear petals and two abaxial, spathulate petals. These characteristics confer a slight zygomorphic floral architecture in that species, being considered transitional among the floral morphological variation found in the genus (Silva et al. 2016a, b).
Studies of vascularization patterns have helped in the understanding of floral morphology among angiosperms (Puri 1951; Souza et al. 2005; Novikoff and Jabbour 2014; Silva et al. 2016a, b; De Paula et al. 2018; Leme et al. 2018). Within Leguminosae, floral vascularization analyses are used to recognize floral nectaries (Honner et al. 2003; Paiva and Machado 2008; Kochanovski et al. 2018). Here, vascularization of the floral buds was also helpful to reveal the structure of the monosymmetric hypanthium. The vascular bundles that are part of the abaxial region of the hypanthium originate from the central cylinder before the vascular bundles of the adaxial region. These are only present after the establishment of bundles of the abaxial region.
Overall, three patterns were observed with respect to petal vascularization: (i) linear petals with only one vascular bundle along their entire extension (as in T. beaurepairei, T. denudata and T. duckei); (ii) spathulate petals, with one bundle in their basal portion, which divides into three bundles and later into several, as it approaches the apical portion (as in T. paratyensis); and (iii) spathulate petals in which the initial bundle divides into two bundles and later into several bundles (as in T. spathulipetala). This vascularization pattern agrees with the external morphology of the petals analyzed here. The vascular bundle of the stamen divides only at the thecae (as in T. beaurepairei, T. denudata and T. duckei and T. paratyensis). These petal and stamen vascularization patterns are reported here for the first time in the genus.
Here, we provide a detailed assessment of the apparently simple and small, but structurally complex flowers of Tachigali, a large genus of neotropical trees that has early diversified in the Caesalpinioideae phylogeny. Early-branching genera across all legume subfamilies have been largely marked by conspicuous changes in floral morphology involving symmetry, reduction or proliferation of stamens and petals, multicarpellate gynoecium and petals that can be free and equal or highly differentiated and connate (e.g., Pennington et al. 2000; Prenner and Klitgaard 2008; Cardoso et al. 2012a, b, 2013a, b; Zimmerman et al. 2013; Bruneau et al. 2014; Paulino et al. 2014; Leite et al. 2015; Prenner et al. 2015; Prenner and Cardoso 2017). In contrast to such floral evolutionary lability among closely related genera of early-branching legume clades, major changes in floral architecture at genus level seem to be rare. For example, the speciose genera Inga (300 spp.; Caesalpinioideae-Mimosoid), Dalbergia (250 spp.), Lupinus (230 spp.), Indigofera (700 spp.) and Astragalus (2.300 spp.) (Papilionoideae) (Lewis et al. 2005) all have relatively conserved floral morphology. On the other hand, floral symmetry seems to mark a major subdivision within Tachigali, which indeed has been used to define two different genera (van der Werff 2007). Understanding why the small Tachigali flowers have undergone so many changes during its relatively fast diversification history (Baker et al. 2014) seems to be a promising topic to explore in the future under a robust phylogenetic framework, with new data on floral development across its entire morphological diversity, as well as insights from the developmental genetic mechanisms of MADS-box genes in regulating floral symmetry (e.g., Theissen 2001; Citerne et al. 2000, 2003, 2006; Feng et al. 2006; Wang et al. 2008; Zhang et al. 2010).
References
Baker TR, Pennington RT, Gloor SME, Laurance WF, Alvarez MAE, Araujo A, Arets EJMM, Aymard G, Oliveira AA, Amaral I, Arroyo L, Bonal D, Brienen RJW, Chave J, Dexter KG, Di Fiore A, Eler E, Feldpausch TR, Ferreira L, Lopez-Gonzalez G, van der Heijden G, Higuchi N, Honorio E, Huamantupa I, Killeen TJ, Laurance S, Leaño C, Lewis SL, Malhi Y, Marimon BS, Junior BHM, Mendoza AM, Neill D, Peñuela-Mora MC, Pitman N, Prieto A, Quesada CA, Ramírez F, Angulo HR, Rudas A, Ruschel AR, Salomão RP, Andrade AS, Silva JNM, Silveira M, Simon MF, Spironello W, ter Steege H, Terborgh J, Toledo M, Torres-Lezama A, Vasquez R, Vieira ICG, Vilanova E, Vos VA, Phillips OL (2014) Fast demographic traits promote high diversification rates of Amazonian tress. Eco Lett 17:527–536. https://doi.org/10.1111/ele.12252
Barreta-Kuipers T (1981) Wood anatomy of Leguminosae: its relevance to taxonomy. Part 2. In: Polhill RM, Raven PH (eds) Advances in Legume Systematics. Royal Botanic Gardens, Kew, pp 677–706
Barros TC, Teixeira SP (2016) Revisited anatomy of anther glands in mimosoids (Leguminosae). Int J Pl Sci 177:18–36. https://doi.org/10.1086/683844
Bello MA, Rudall PJ, Hawkins JA (2012) Combined phylogenetic analyses reveal interfamilial relationships and patterns of floral evolution in the eudicot order Fabales. Cladistics 28:393–421. https://doi.org/10.1111/j.1096-0031.2012.00392.x
BFG [Brazil Flora Group] (2018) Brazilian Flora 2020: Innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 69:1513–1527. https://doi.org/10.1590/2175-7860201869402
Bozzola JJ, Russel LD (1999) Electron Microscopy, 2nd edn. Jones and Barlett Publisher Toronto, Canada
Bruneau A, Mercure M, Lewis GP, Herendeen PS (2008) Phylogenetic patterns and diversification in the caesalpinioid legumes. Botany 86:697–718. https://doi.org/10.1139/B08-058
Bruneau A, Klitgaard BB, Prenner G, Fougère-Danezan M, Tucker SC (2014) Floral evolution in the Detarieae (Leguminosae): Phylogenetic evidence for labile floral development in an early-branching legume lineage. Int J Pl Sci 175:393–417. https://doi.org/10.1086/675574
Cardoso D, Queiroz LP, Pennington T, Lima HC, Fonty E, Wojciechowski MF, Lavin M (2012a) Revisiting the phylogeny of papilionoid legumes: new insights from comprehensively sampled early-branching lineages. Amer J Bot 99:1991–2013. https://doi.org/10.3732/ajb.1200380
Cardoso D, Lima HC, Rodrigues RS, Queiroz LP, Pennington RT, Lavin M (2012b) The realignment of Acosmium sensu stricto with the dalbergioid clade (Leguminosae, Papilionoideae) reveals a proneness for independent evolution of radial floral symmetry among early branching papilionoid legumes. Taxon 61:1057–1073. https://doi.org/10.1002/tax.615011
Cardoso D, Pennington RT, Queiroz LP, Boatwright JS, Van Wyk E, Wojciechowski MF, Lavin M (2013a) Reconstructing the deep branching relationships of the papilionoid legumes. S African J Bot 89:58–75
Cardoso D, Queiroz LP, Lima HC, Suganuma E, van den Berg C, Lavin M (2013b) A molecular phylogeny of the vataireoid legumes underscores floral evolvability that is general to many early-branching papilionoid lineages. Amer J Bot 100:403–421. https://doi.org/10.1016/j.sajb.2013.05.001
Chomicki G, Ward PS, Renner SS (2015) Macroevolutionary assembly of ant/plant symbioses: Pseudomyrmex ants and their ant-housing plant in Neotropics. Proc Roy Soc B Bio Sci 282:1–9. https://doi.org/10.1098/rspb.2015.2200
Citerne HL, Möller M, Cronk QC (2000) Diversity of cycloidea-like genes in Gesneriaceae in relation to floral symmetry. Ann Bot (Oxford) 86:167–176. https://doi.org/10.1006/anbo.2000.1178
Citerne HL, Luo D, Pennington RT, Coen E, Cronk QC (2003) A phylogenomic investigation of CYCLOIDEA-like TCP genes in the Leguminosae. Pl Physiol 131:1042–1053. https://doi.org/10.1104/pp.102.016311
Citerne HL, Pennington RT, Cronk QC (2006) An apparent reversal in floral symmetry in the legume Cadia is a homeotic transformation. Proc Natl Acad Sci USA 103:12017–12020. https://doi.org/10.1073/pnas.0600986103
De Paula OC, Assis LCS, De Craene LPR (2018) Unbuttoning the ancestral flower of angiosperms. Trends Pl Sci 23:551–554
Dwyer JD (1954) The tropical American genus Tachigalia Aubl. (Caesalpinioideae). Ann Missouri Bot Gard 41:223–260. https://doi.org/10.2307/2394605
Endress PK, Stumpf S (1991) The diversity of stamen structure in ‘Lower’ Rosidae (Rosales, Fabales, Proteales, Sapindales). Bot J Linn Soc 107:217–293. https://doi.org/10.1111/j.1095-8339.1991.tb00225a.x
Feng X, Zhao Z, Tian Z, Xu S, Luo Y, Cai Z, Wang Y, Yang J, Wang Z, Weng L, Chen J, Zheng L, Guo X, Luo J, Sato S, Tabata S, Ma W, Cao X, Hu X, Sun C, Luo D (2006) Control of petal shape and floral zygomorphy in Lotus japonicus. Proc Natl Acad Sci USA 103:4970–4975. https://doi.org/10.1073/pnas.0600681103
Fonseca CR (1999) Amazonian ant-plant interactions and the nesting space limitation hypothesis. J Trop Ecol 15:807–825
Fonseca CR, Benson WW (2003) Ontogenetic succession in Amazonian ant trees. Oikos 102:407–412
Gabriel BL (1982) Biological electron microscopy, 1st edn. Van Nostrand Reinhold Company, New York
Gagnon E, Hughes CE, Lewis GP, Bruneau A (2015) A new cryptic species in a new cryptic genus in the Caesalpinia group (Leguminosae) from the seasonally dry inter-Andean valleys of South America. Taxon 64:468–490. https://doi.org/10.12705/643.6
Gerrits PO, Smid L (1983) A new, less toxic polymerization system for the embedding of soft tissues in glycol methacrylate and subsequent preparing of serial sections. J Microscopy 132:81–85. https://doi.org/10.1111/j.1365-2818.1983.tb04711.x
Graham A, Barker G (1981) Palynology and tribal classification in the Caesalpinioideae. part 2. In: Polhill RM, Raven PH (eds) Advances in Legume systematics. Royal Botanic Gardens, Kew, pp 801–834
Haston EM, Lewis GP, Hawkins JA (2005) A phylogenetic reappraisal of the Peltophorum group (Caesalpinieae: Leguminosae) based on the chloroplast trnL-F, rbcL and rps16 sequence data. Amer J Bot 92:1359–1371
Horner HT, Healy RA, Cervantes-Martinez T, Palmert RG (2003) Floral nectary fine structure and development in Glycine max L. (Fabaceae). Int J Pl Sci 164:675–6901. https://doi.org/10.3732/ajb.92.8.1359
Kochanovski FJ, Paulino JV, Teixeira SP, Azevedo AMG, Mansano VF (2018) Floral development of Hymenaea verrucosa: an ontogenetic approach to the unusual flower of Fabaceae subfamily Detarioideae. Bot J Linn Soc 20:1–13. https://doi.org/10.1093/botlinnean/boy006
Lavin M, Pennington TR, Klitgaard BB, Sprent JI, Lima HC, Gasson PE (2001) The dalbergioid legumes (Fabaceae): delimitation of a Pantropical monophyletic clade. Amer J Bot 88:503–533. https://doi.org/10.2307/2657116
Leite VG, Mansano VF, Teixeira SP (2014) Floral ontogeny in Dipterygeae (Fabaceae) reveals new insights into one of the earliest branching tribes in papilionoid legumes. Bot J Linn Soc 174:529–550. https://doi.org/10.1111/boj.12158
Leite VG, Teixeira SP, Mansano VF, Prenner G (2015) Floral development of the early-branching papilionoid legume Amburana cearensis (Leguminosae) reveals rare and novel characters. Int J Pl Sci 176:94–106. https://doi.org/10.1086/678468
Leme FM, Staedler YM, Schönenberger J, Teixeira SP (2018) Ontogeny and vascularization elucidate the atypical floral structure of Ampelocera glabra, a tropical species of Ulmaceae. Int J Pl Sci 179:461–476. https://doi.org/10.1086/697899
Lewis G, Schrire B, Mackinder B, Lock M (2005) Legumes of the world, 1st edn. Royal Botanic Gardens, Richmond
LPWG [Legume Phylogeny Working Group] (2017) A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66:44–77. https://doi.org/10.12705/661.3
LPWG, Legume Phylogeny Working Group (2013) Legume phylogeny and classification in the 21st century: progress, prospects and lessons for other species-rich clades. Taxon 62:217–248. https://doi.org/10.5167/uzh-78167
Luckow M, Grimes J (1997) A survey of anther glands in the mimosoid legume tribes Parkieae and Mimoseae. Amer J Bot 84:285–297. https://doi.org/10.2307/2446002
Macedo TM, Barros CF, Lima HC, Costa CG (2014) Wood anatomy of seven species of Tachigali (Caesalpinioideae: Leguminosae). IAWA J 35:19–30. https://doi.org/10.1163/22941932-00000044
Manzanilla V, Bruneau A (2012) Phylogeny reconstruction in the Caesalpinieae grade (Leguminosae) based on duplicated copies of the sucrose synthase gene and plastid markers. Molec Phylogenet Evo 65:149–162. https://doi.org/10.1016/j.ympev.2012.05.035
Marinho CR, Oliveira RB, Teixeira SP (2016) The uncommon cavitated secretory trichomes in Bauhinia s.s. (Fabaceae): the same roles in different organs. Bot J Linn Soc 180:104–122. https://doi.org/10.1111/boj.12354
Meira RMS, Francino DMT, Ascensão L (2014) Oleoresin trichomes of Chamaecrista dentata (Leguminosae): structure, function, and secretory products. Int J Pl Sci 175:336–345. https://doi.org/10.1086/673538
Novikoff AV, Jabbour F (2014) Floral anatomy of Delphinieae (Ranunculaceae): comparing flower organization and vascular patterns. Modern Phytomorphol 5:35–44. https://doi.org/10.5281/zenodo.161001
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373. https://doi.org/10.1007/BF01248568
Paiva EAS, Machado SR (2008) The floral nectary of Hymenaea stigonocarpa (Fabaceae: Caesalpinioideae): structural aspects during floral development. Ann Bot (Oxford) 101:125–133. https://doi.org/10.1093/aob/mcm268
Paulino JV, Prenner G, Mansano VF, Teixeira SP (2014) Comparative development of rare cases of a polycarpellate gynoecium in an otherwise monocarpellate family, Leguminosae. Amer J Bot 101:572–586. https://doi.org/10.3732/ajb.1300355
Pennington RT, Klitgaard BB, Ireland H, Lavin M (2000) New insights into floral evolution of basal Papilionoideae from molecular phylogenies. In: Herendeen PS, Bruneau A (eds) Advances in Legume Systematics. Royal Botanic Gardens, Kew, pp 233–248
Pipoly JJ III (1995) A new Tachigali (Fabaceae: Caesalpinioideae) from Western Amazonia. Sida 16:407–411
Prenner G, Cardoso D (2017) Flower development of Goniorrhachis marginata reveals new insights into the evolution of the florally diverse detarioid legumes. Ann Bot (Oxford) 119:417–432. https://doi.org/10.1093/aob/mcw223
Prenner G, Klitgaard BB (2008) Towards unlocking the deep nodes of Leguminosae: floral development and morphology of the enigmatic Duparquetia orchidacea (Leguminosae, Caesalpinioideae). Amer J Bot 95:1349–1365. https://doi.org/10.3732/ajb.0800199
Prenner G, Cardoso D, Zartman CE, Queiroz LP (2015) Flowers of the early-branching papilionoid legume Petaladenium urceoliferum display unique morphological and ontogenetic features. Amer J Bot 102:1780–1793. https://doi.org/10.3732/ajb.1500348
Puri V (1951) The role of floral anatomy in the solution of morphological problems. Bot Rev 17:451–553. https://doi.org/10.1007/BF02882536
Silva LFG (2007) Taxonomia de Tachigali Aublet (Leguminosae, Caesalpinioideae) na Mata Atlântica. Dissertação de Mestrado. Escola Nacional de Botânica Tropical, Rio de Janeiro
Silva LFG, Lima HC (2007) Mudanças nomenclaturais no gênero Tachigali Aubl. (Leguminosae–Caesalpinioideae) no Brasil. Rodriguésia 58:397–401. https://doi.org/10.1590/2175-7860200758214
Silva AL, Trovó M, Coan AI (2016a) Floral development and vascularization help to explain merism evolution in Paepalanthus (Eriocaulaceae, Poales). PeerJ 2811:1–33. https://doi.org/10.7717/peerj.2811
Silva LFG, Cardoso LJT, Cardoso DBOS, Lima HC (2016b) Tachigali spathulipetala, A new threatened caesalpinioid tree species (Leguminosae) from Brazilian Atlantic Forest. Syst Bot 41:971–976. https://doi.org/10.1600/036364416X694080
Silva NF, Arruda RCO, Alves FM, Sartori ALB (2018) Leaflet anatomy of the Dipterygeae clade (Faboideae: Fabaceae): evolutionary implications and systematics. Bot J Linn Soc 187:99–117. https://doi.org/10.1093/botlinnean/boy009
Souza RCOS, De Toni KLG, Andreata RHP, Costa CG (2005) Anatomia e vascularização das flores estaminadas e pistiladas de Smilax fluminensis Steudel (Smilacaceae). Rodriguesia 56:107–121. https://doi.org/10.1590/2175-78602005568708
ter Steege H, Pitman NCA, Sabatier D, Baraloto C, Salomão RP, Guevara JE, Phillips OL, Castilho CV, Magnusson WE, Molino JF, Monteagudo A, Núñez-Vargas P, Montero JC, Feldpausch TR, Coronado ENH, Killeen TJ, Mostacedo B, Vasquez R, Assis RL, Terborgh J, Wittmann F, Andrade A, Laurance WF, Laurance SGW, Marimon BS, Marimon BH Jr, Vieira ICG, Amaral IL, Brienen R, Castellanos H, Cárdenas-López D, Duivenvoorden JF, Mogollón HF, Matos FDA, Dávila N, García-Villacorta R, Diaz PRS, Costa F, Emilio T, Levis C, Schietti J, Souza P, Alonso A, Dallmeier F, Montoya AJD, Piedade MTF, Araujo-Murakami A, Arroyo L, Gribel R, Fine PVA, Peres CA, Toledo M, Aymard CGA, Baker TR, Cerón C, Engel J, Henkel TW, Maas P, Petronelli P, Stropp J, Zartman CE, Daly D, Neill D, Silveira M, Paredes MR, Chave J, Lima-Filho DA, Jørgensen PM, Fuentes A, Schöngart J, Valverde FC, Di Fiore A, Jimenez EM, Peñuela-Mora MC, Phillips JF, Rivas G, van Andel TR, von Hildebrand P, Hoffman B, Zent EL, Malhi Y, Prieto A, Rudas A, Ruschell AR, Silva N, Vos V, Zent S, Oliveira AA, Schutz AC, Gonzales T, Nascimento MT, Ramirez-Angulo H, Sierra R, Tirado M, Medina MNU, van der Heijden G, Vela CIA, Torre EV, Vriesendorp C, Wang O, Young KR, Baider C, Balslev H, Ferreira Mesones CI, Torres-Lezama A, Giraldo LEU, Zagt R, Alexiades MN, Hernandez L, Huamantupa-Chuquimaco I, Milliken W, Cuenca WP, Pauletto D, Sandoval EV, Gamarra LV, Dexter KG, Feeley K, Lopez-Gonzalez G, Silman MR (2013) Hyperdominance in the Amazonian tree flora. Science 342:325–335. https://doi.org/10.1126/science.1243092
Theissen G (2001) Development of floral organ identity: stories from the MADS house. Curr Opin Pl Biol 4:75–85. https://doi.org/10.1016/S1369-5266(00)00139-4
Tucker SC (1991) The role of floral development in studies of legume evolution. Canad J Bot 70:692–700. https://doi.org/10.1139/b92-089
Tucker SC (1997) Floral evolution, development, and convergence: The hierarchical-significance hypothesis. Int J Pl Sci 158:143–146. https://doi.org/10.1086/297514
Tucker SC (2003) Floral development in legumes. Pl Physiol 131:911–926. https://doi.org/10.1104/pp.102.017459
van der Werff H (2008) A synopsis of the genus Tachigali (Leguminosae: Caesalpinioideae) in northern South America. Ann Missouri Bot Gard 95:618–660. https://doi.org/10.3417/2007159
Vargas W, Fortuna-Perez AP, Lewis GP, Piva TC, Vatanparast M, Machado SR (2018) Ultrastructure and secretion of glandular trichomes in species of subtribe Cajaninae Benth. (Leguminosae, Phaseoleae). Protoplasma 255:1–15. https://doi.org/10.1007/s00709-018-1307-0
Wang Z, Luo Y, Li X, Wang L, Xu S, Yang J, Weng L, Sato S, Tabata S, Ambrose M, Rameau C, Feng X, Hu X, Da Luo (2008) Genetic control of floral zygomorphy in pea (Pisum sativum L.). Proc Natl Acad Sci USA 105:10414–10419. https://doi.org/10.1073/pnas.0803291105
Watson L, Dallwitzz MJ (1983) The genera of Leguminosae- Caesalpinioideae: anatomy, morphology, classification and keys, 1st edn. The Australian National University Research School of Biological Sciences, Canberra
Weberling F (1989) Morphology of flowers and inflorescences. Cambridge University Press, Cambridge
Zhang W, Kramer EM, Davis CC (2010) Floral symmetry genes and the origin and maintenance of zygomorphy in a plant-pollinator mutualism. Proc Natl Acad Sci USA 107:6388–6393. https://doi.org/10.1073/pnas.0910155107
Zimmerman E, Prenner G, Bruneau A (2013) Floral ontogeny in Dialiinae (Caesalpinioideae: Cassieae), a study in organ loss and instability. S African J Bot 89:188–209. https://doi.org/10.1016/j.sajb.2013.06.020
Acknowledgements
This paper is part of the first author’s MSc. thesis prepared for the postgraduate program of the Escola Nacional de Botânica Tropical of the Rio de Janeiro Botanical Garden and supported by a grant from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. We thank Dr. Vidal Mansano for the contribution to the author’s MSc. thesis and the manuscript, Dr. David Martin for the review of the English grammar and two anonymous reviewers for the detailed comments and suggestions that greatly improved the manuscript. DC also thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the Research Productivity Fellowship (process 308244/2018-4) and Prêmio CAPES de Teses (process 23038.009148/2013-19) and FAPESB (process APP0037/2016) for financially supporting his research on legume morphology and systematics.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter K. Endress.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Information on Electronic Supplementary Material
Information on Electronic Supplementary Material
The electronic supplementary material attached to this manuscript are high-quality images of the floral development from the five Tachigali species analyzed in this study, whorl by whorl, which allows us to better understand their floral architecture.
Online Resource 1. Floral buds at different developmental stages of Tachigali denudata. a Floral buds showing the sepals; b showing the petals with the sepals removed; c showing the androecium with the sepals and petals removed; d the gynoecium with the sepals, petals and androecium removed.
Online Resource 2. Floral buds at different developmental stages of Tachigali paratyensis. a Floral buds showing the sepals; b showing the petals with the sepals removed; c showing the androecium with the sepals and petals removed; d showing the gynoecium with the sepals, petals and androecium removed; arrowheads indicating the projection of the hypanthium wall.
Online Resource 3. Floral buds at different developmental stages of Tachigali beaurepairei. a Floral buds showing the sepals; b showing the petals with sepals removed; c showing the androecium with sepals and petals removed; d showing the gynoecium with sepals, petals and androecium removed; arrowheads indicating the projection of the hypanthium wall.
Online Resource 4. Floral buds at different developmental stages of Tachigali duckei. a Floral buds showing the sepals; b showing the petals with the sepals removed; c showing the androecium with the sepals and petals removed; d showing the gynoecium with the sepals, petals and androecium removed; arrowheads indicating the projection of the hypanthium wall.
Online Resource 5. Floral buds at different developmental stages of Tachigali spathulipetala. a Floral buds showing the sepals; b showing the petals with the sepals removed; c showing the androecium with the sepals and petals removed; d showing the gynoecium with the sepals, petals and androecium removed; arrowheads indicating the projection of the hypanthium wall.
Rights and permissions
About this article
Cite this article
Casanova, J.M., Cardoso, D., Barros, C.F. et al. Floral morphology and development in Tachigali (Caesalpinioideae, Leguminosae), a predominantly rainforest tree genus with contrasting flower architectures. Plant Syst Evol 306, 17 (2020). https://doi.org/10.1007/s00606-020-01642-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00606-020-01642-2