Abstract
Kawasaki disease (KD), a systemic vasculitis in children, may bring serious complications. However, the etiology of KD remains unclear. AIM2, an intracellular receptor, plays a vital role during the infection caused by a variety of pathogens. However, its role in KD remains unclear. The principal aim of the present research is to concentrate on the relation between AIM2 and KD. We detected the levels of AIM2, IL-18 and IL-1β in all subjects by ELISA. The conventional inflammatory indices were detected in all subjects, such as WBC, HB, CRP and so on. The serum concentrations of AIM2, IL-18 and IL-1β were notably upregulated in the KD group compared to the febrile group and healthy group, respectively. And the three indicators in the KD patients were greatly reduced after interpreted with IVIG. Furthermore, the expressions of IL-18 and IL-1β were positively correlated with AIM2. Meanwhile, the cutoff value of serum AIM2 level for the diagnosis of KD was 541.90 ng/L with the specificity of 60% and sensitivity of 92.5%, compared to the febrile controls. And the area under curve (AUC) of AIM2 was 0.771. And no difference was observed in patients with CALs when compared with patients without CALs. The serum AIM2, IL-18 and IL-1β might play a critical role during the progress of KD. AIM2 can be considered as a candidate indicator for Kawasaki disease diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kawasaki disease (KD), a systemic vasculitis syndrome, occurs preferentially in coronary arteries of infants and children [1]. The etiology of KD has not been clearly clarified, and clinical diagnosis is mainly based on manifestation. At the acute stage of KD, nearly 25% of patients will develop coronary artery dilatation without timely treatment [2]. So how to early pick out KD from febrile disease is very important. Inflammatory signaling has a critical function in occurrences of cardiovascular diseases such as KD, coronary ischemia and cardiomyopathy [3].Up to now, there is no exact laboratory diagnostic indicator for KD except for the inflammatory indices including platelet (PLT), C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). In the study of Song et al., the specificity of CRP for the prediction of KD was 72.7% and sensitivity was 69.0%, and the specificity and sensitivity of PLT were 75 and 70.6% [4]. Many proinflammatory cytokines are significantly upregulated at the early phases of KD. Su et al. identified increased levels of multiple proinflammatory cytokines in KD patients, such as IL-6, IL-1β, TNF-α and IFN-γ [5,6,7,8]. However, these cytokines have a limited role in the diagnosis of Kawasaki disease. A previous study clarified the sensitivity and specificity of IL-6 for predicting incomplete KD were 54.40 and 77.80% [9]. Thus, a better indicator is needed to help identify KD.
As a member of the AIM2-like family, AIM2 (absent in melanoma-2) is a cytoplasmic receptor that triggers the formation and release of inflammasome through the double-stranded DNA (dsDNA) transmitted within the cell, assistant in defending against various pathogens [10, 11]. AIM2 assembles into an inflammasome combined with a key adaptor protein (ASC), which can activate caspase-1 [12]. At the same time, caspase-1 promotes the release of mature IL-1β and IL-18 from cells to the outside and participates in the inflammatory response [12,13,14]. AIM2 plays a crucial role in inflammatory and immune diseases including acute pancreatitis [12, 15,16,17], systemic lupus erythematosus [18] and hepatitis B virus-associated glomerulonephritis [19]. However, it is unclear whether AIM2 participates in the pathogenesis of KD. Therefore, we tested the concentration of AIM2 in KD patients and its correlation with the inflammatory factor IL-18 and IL-1β to determine whether AIM2 is involved in the progress of Kawasaki disease.
Materials and methods
Subjects studied
All enrolled patients were extracted from the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, China, mainly between January 2019 and January 2020. All 40 KD patients met the complete Kawasaki disease diagnostic standard of the American Heart Association. Simultaneously, 20 age and gender-matched patients with the infectious febrile disease were considered as the febrile controls (FC) and 20 healthy children as the healthy controls (NC). Laboratory data including white blood cell (WBC), hemoglobin (HB), platelet count (PLT), platelet hematocrit (PCT), mean platelet volume (MPV), platelet distribution width (PDW) and C-reactive protein (CRP) were obtained. All candidates and their parents received detailed information about the research and signed the consent form. The research was approved by the ethics review boards.
Blood samples were respectively drawn in KD patients before (pre-IVIG) and after IVIG therapy (post-IVIG).
In addition, KD patients were divided into two groups according to the presence of coronary artery lesions (CALs): KD with CALs (n = 18) and KD without CALs (n = 22). According to Japanese guidelines, CALs are defined as a Z-score ≥ 2 [2].
Measurement of serum levels of AIM2, IL‑1β and IL‑18
Peripheral blood (2 ml) was centrifuged at 3000 rpm for fifteen minutes to make the serum, which was stored at − 80 °C until analysis. And the levels of IL-18, IL-1β and AIM2 were determined by enzyme-linked immunosorbent (Boyun Biotech, China) according to the specification.
Statistical analysis
The results were described as mean ± standard error or number and percent (n, %). Quantitative data were analyzed by Student's t test or the one-way ANOVA. Differences in data when accepted IVIG therapy were analyzed by the paired sample t test. Comparisons of qualitative data between groups were tested using Chi-square test. Correlations between AIM2 and other parameters were assessed by Pearson’s correlation analysis. And the sensitivity and specificity of AIM2, IL-18 and IL-1β were calculated by receiver operating characteristic (ROC) curve. The cutoff value of the curve was calculated by the Youden index (sensitivity + specificity-1). If p values were < 0.05, the results were considered significant. SPSS version 22.0 was adopted for statistical data analysis.
Results
Clinical and laboratory results and levels of IL-18, IL-1β and AIM2
The clinical and laboratory data of the three groups of patients are set out in Table 1. There was no significant difference in sex among the NC group, the FC group and the KD group (p > 0.05). Meanwhile, laboratory indicators including WBC and PDW were substantially increased in the KD group compared with the NC group (p < 0.05), but similar to the FC group (p > 0.05). And the concentration of PCT of KD patients was higher than the febrile controls (p < 0.05). The inflammatory mediators CRP and PLT increased in children with KD compared to the NC group or FC group (p < 0.05), while HB and RBC in the KD group decreased significantly (p < 0.05). It showed that the MPV of the KD patients was considerably lower than the NC group.
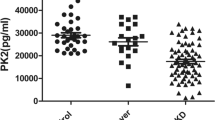
Also, we measured the IL-1β, IL-18 and AIM2 content in the KD group and the controls by ELSA kits. As displayed in Fig. 1, the serum concentration of AIM2 in FC group (602.60 ± 159.67 ng/L) was markedly higher (p < 0.001) than the NC group (356.24 ± 62.94 ng/L), but still not as high as the KD group (858.91 ± 292.77 ng/L). Notably, the concentrations of IL-1β and IL-18 in different groups were approximately similar to the AIM2. The levels of IL-18 were 92.95 ± 26.42 ng/L in KD children, 73.20 ± 16.81 ng/L in febrile controls and 46.92 ± 10.15 ng/L in healthy controls. And the respective serum levels of IL-1β were 30.47 ± 12.64 ng/L, 22.93 ± 7.64 ng/L and 11.31 ± 2.63 ng/L. Compared with the febrile patients, IL-1β was elevated in KD patients (p < 0.05). It was worth mentioning that the expressions of IL-18 (r = 0.357, p < 0.05) and IL-1β (r = 0.612, p < 0.05) were positively associated with AIM2 in KD patients (p < 0.05) (Table 2).
According to the ROC curve analysis (Fig. 2), the area under the curve (AUC) of serum AIM2, IL-18 and IL-1β was 0.771, 0.724 and 0.666, respectively (p < 0.05). And the AUC of HB, RBC and PLT was 0.715, 0.762 and 0.697. The cutoff value of serum AIM2 level for the diagnosis of KD was 541.90 ng/L with the specificity of 60% and sensitivity of 92.5%, compared to the patients with other febrile diseases. The sensitivity of serum IL-18 was 62.5%, and specificity was 80% with a cutoff of 81.61 ng/L. And the cutoff of IL-1β was 19.77 ng/L with sensitivity of 90% and the specificity of 55%. What’s more, the sensitivity of PLT was 87.5% and specificity was 55% with a cutoff of 271 × 109/L. The specificity and sensitivity were 65% and 75% with a cutoff of RBC < 4.39 × 1012/L. And the specificity and sensitivity were 75% and 72.5% with a cutoff of HB < 117.50 g/L.
Changes of IL-18, IL-1β, AIM2 and laboratory findings in KD patients after IVIG therapy
In this study, all KD patients received a single dose of IVIG (2.0 g/kg) IV after five days of persistent fever. Blood samples were collected separately from KD patients before (pre-IVIG) and after IVIG therapy (post-IVIG) (Fig. 3). Obviously, we found that IL-18, IL-1β and AIM2 had a visible decrease after IVIG treatment (p < 0.001). Moreover, five inflammatory mediators WBC, HB, RBC, MPV and CRP decreased in KD children after IVIG treatment, especially WBC and CRP (p < 0.05) (Table 3). Regardless of IVIG treatment or not, there was no marked difference in PDW. Interestingly, the expressions of PLT and PCT were markedly upregulated in patients treated with IVIG (p < 0.001) (Table 3).
Differences in serum IL-18, IL-1β, AIM2 and general laboratory findings between the KD children with CALs and without CALs
As shown in Table 4, no noted differences showed in gender, age, WBC, HB, RBC, PLT, PCT, MPV, PDW, CRP, IL-18, IL-1β and AIM2 between KD patients with CALs and without CALs (p > 0.05).
Discussion
To our best knowledge, our study takes the lead in performing an investigation of inflammation-related indicators IL-18, IL-1β and AIM2 between patients and controls as well as subgroups of patients with KD. KD is a systemic vasculitis that primarily affects patients aged < 5 years old. However, the underlying molecular mechanism of KD has not been clarified yet. Our study demonstrated (1) that levels of IL-18, IL-1β and AIM2 in KD patients were higher than the control groups. And (2) after IVIG treatment, AIM2, IL-18 and IL-1β decreased rapidly in KD patients. Meanwhile, (3) AIM2 were positively associated with IL-18 and IL-1β in KD patients. Furthermore, the area under the curve of serum IL-18, IL-1β and AIM2 was 0.724, 0.666 and 0.771, respectively.
Although conventional inflammatory mediators could not be employed as an accurate diagnostic factor of KD, they are still beneficial to identify KD. Clinically, several inflammatory mediators, such as CRP, PLT, ESR and WBC, are often used to diagnose KD combined with clinical symptoms [20]. We found that inflammatory mediators CRP and PLT increased in KD kids compared to febrile controls and the healthy controls, which is in agreement with previous studies [20, 21]. Additionally, WBC in the KD kids was significantly higher than their counterparts in the healthy controls, but similar to the febrile controls. As stated in the 2017 AHA guidelines, these inflammatory mediators including WBC and CRP typically reached a peak during the acute period, while PLT reached the highest point later [2]. This is the reason for the heightened PLT level in patients after treated with IVIG than before, whereas CRP and WBC decreased dramatically in our study. Notably, WBC, CRP and ESR are considered as predictors of the occurrence of coronary damage in KD [22]. However, in this present study, there are no significant differences in these inflammatory indices regardless of coronary artery lesions or not.
For a long time, many epidemiological investigations have focused on screening clinical characteristics and laboratory indicators for early diagnosis of KD, which is precisely the main purpose of our research. In this series, we discovered that level of AIM2 was upregulated in the KD patients, compared with the healthy controls and febrile controls, which indicated that AIM2 might promote the development of inflammation at the acute stage of KD. Talita et al. found that NLRP3 or AIM2 inflammasome might be involved in Kawasaki disease vasculitis by mediating the oligomerization of ASC, which was compatible with our results [23]. Meanwhile, AIM2 dropped obviously after receiving IVIG treatment. Base on the above observations, we propose a hypothesis that AIM2 is coordinated with the progress of KD disease.
In our study, it was observed that IL-18 and IL-1β were conspicuously increased in KD children compared with the healthy controls and febrile controls. And we found that the serum levels of IL-18 and IL-1β decreased markedly in KD children who underwent a therapy of IVIG. Asano et al. confirmed elevated serum cytokine IL-18 and IL-1β in KD children, which concords with our results [24]. When the dsDNA is released by the pathogen or host is recognized, AIM2 forms AIM2 inflammasome, thereby releasing IL-18 and IL-1β [25]. A recent research clarified that in glomerular cells attacked with virus, the levels of IL-18 and IL-1β were dramatically subsided after siRNA-mediated silencing of the AIM2 gene [19]. Another novel study had shown that IL-1β in AIM2-/- mice was lower expressed, and the incidence of renal fibrosis as well as inflammation was reduced compared with wild-type mice [26]. Consistent with previous studies, we found that IL-18 and IL-1β were positively correlated with AIM2 in the acute stage of KD, further indicating that AIM2 may participate in the inflammatory process of KD by regulating IL-18 and IL-1β.
Next, we further explored the function of AIM2, IL-18 and IL-1β in coronary damage at the acute stage of KD. Maani et al. observed that AIM2 level was elevated markedly around the necrosis of atherosclerotic vessels and in the neovascularization of aortic aneurysms, simultaneously with the release of IL-18 and IL-1β [27, 28]. It is noteworthy that a few clinical and experimental reports have demonstrated that IL-18 and IL-1β promote the progress of a few cardiovascular diseases including abdominal aortic aneurysms and atherosclerosis [29, 30]. Conversely, our study addressed that no statistical difference in AIM2 was observed regardless the damage of the coronary artery. And we did not find any evidence that IL-1β and IL-18 involved in the process of coronary artery damage in the early stage of KD. However, our study is inconsistent with that of Lee et al., who elucidated that IL-1β could induce coronary aneurysm formation in KD mice, and the use of IL-1 receptor inhibitors can significantly extenuate coronary damage [31, 32]. This result may ascribe to the small sample size. Therefore, the role of IL-1β, IL-18 and AIM2 in the pathogenesis of coronary damage of KD cannot be easily denied, and all of that require further larger-size study.
Although there is little information about AIM2 and KD in the literature, we obtained the finding that AIM2 may participate in the inflammatory process of KD by regulating IL-18 and IL-1β. But whether AIM2 can serve as a new diagnostic indicator of KD remains to be elucidated. Therefore, we utilized the ROC curve to analyze further the role of AIM2 in predicting KD. The AUC value for the diagnosis of KD was 0.771 with the specificity of 60% and sensitivity of 92.5%. And ROC curves showed that 541.90 ng/L of AIM2 could distinguish KD from other high fever diseases. Currently, HB, RBC and PLT have a certain diagnostic function for Kawasaki disease. At the ROC curves, RBC had a sensitivity of 75% and specificity of 65% for the prediction of KD with the AUC value of 0.715. And the AUC value of HB was 0.762 with the sensitivity of 72.5% and specificity of 75%. Meanwhile, the AUC value of PLT was 0.697 with the sensitivity of 87.5% and specificity of 55%. These results suggested AIM2 owned a better predictability than the three laboratory indicators, especially its higher sensitivity. Hence, AIM2 can be used as a novel marker for the prediction of KD.
However, there are several limitations that cannot be ignored. Firstly, this was only a single-center survey with an insufficient number of patients. Secondly, we mainly concentrated on the influences of proinflammatory cytokines in KD but did not probe the interrelationship among these cytokines. Thirdly, we only analyzed the serum levels of these inflammatory indicators including AIM2, IL-1β and IL-18 without further mechanism research. Thus, it is powerfully necessary to conduct a large-scale study on the specific mechanism of AIM2 in KD.
In a nutshell, this is the first investigation to illustrate that AIM2 levels rose in KD patients. Along with the judicious application of IVIG, the level of AIM2 dropped significantly. Additionally, our research highly suggested that cytokines IL-1β and IL-18 had positive correlations with AIM2 at the acute stage of KD. And ROC curves showed that AIM2 had a specificity of 60% and sensitivity of 92.5% for predicting KD at a cutoff value of 541.90 ng/L. These findings above indicated that AIM2 might have an indispensable effect during the progression of KD and be prone to be a predictor of KD. Meanwhile, these novel observations provide several new insights into the underlying pathogenesis of KD, which could be quite helpful for developing effective therapies.
Availability of data and materials
The data analyzed are available from the corresponding author on reasonable request.
References
Kawasaki T, Kosaki F, Okawa S, et al. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54(3):271–6.
McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–99.
Li X, Deroide N, Mallat Z. The role of the inflammasome in cardiovascular diseases. J Mol Med. 2014;92(4):307–19.
Xiu-Yu S, Jia-Yu H, Qiang H, et al. Platelet count and erythrocyte sedimentation rate are good predictors of Kawasaki disease: ROC analysis. J Clin Lab Anal. 2010;24(6):385–8.
Su Y, Feng S, Luo L, et al. Association between IL-35 and coronary arterial lesions in children with Kawasaki disease. Clin Exp Med. 2019;19(1):87–92.
Si F, Wu Y, Gao F, et al. Relationship between IL-27 and coronary arterial lesions in children with Kawasaki disease. Clin Exp Med. 2017;17(4):451–7.
Chen SY, Wan L, Huang YC, et al. Interleukin-18 gene 105A/C genetic polymorphism is associated with the susceptibility of Kawasaki disease. J Clin Lab Anal. 2009;23(2):71–6.
Hachiya A, Kobayashi N, Matsuzaki S, et al. Analysis of biomarker serum levels in IVIG and infliximab refractory Kawasaki disease patients. Clin Rheumatol. 2018;37(7):1937–43.
Wu Y, Liu FF, Xu Y, et al. Interleukin-6 is prone to be a candidate biomarker for predicting incomplete and IVIG nonresponsive Kawasaki disease rather than coronary artery aneurysm. Clin Exp Med. 2019;19(2):173–81.
Wang B, Tian Y, Yin Q. AIM2 Inflammasome Assembly and Signaling. Adv Exp Med Biol. 2019;1172:143–55.
Rathinam VAK, Jiang Z, Waggoner SN, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11(5):395–402.
Man SM, Karki R, Kanneganti TD. AIM2 inflammasome in infection, cancer, and autoimmunity: Role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46(2):269–80.
Hou X, Niu X. The NMR solution structure of AIM2 PYD domain from Mus musculus reveals a distinct alpha2-alpha3 helix conformation from its human homologues. Biochem Biophys Res Commun. 2015;461(2):396–400.
Cai X, Chen J, Xu H, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156(6):1207–22.
Lozano-Ruiz B, Bachiller V, Garcia-Martinez I, et al. Absent in melanoma 2 triggers a heightened inflammasome response in ascitic fluid macrophages of patients with cirrhosis. J Hepatol. 2015;62(1):64–71.
Algaba-Chueca F, de Madaria E, Lozano-Ruiz B, et al. The expression and activation of the AIM2 inflammasome correlates with inflammation and disease severity in patients with acute pancreatitis. Pancreatology. 2017;17(3):364–71.
Lupfer CR, Rodriguez A, Kanneganti TD. Inflammasome activation by nucleic acids and nucleosomes in sterile inflammation... or is it sterile? FEBS J. 2017;284(15):2363–74.
Yang CA, Huang ST, Chiang BL. Sex-dependent differential activation of NLRP3 and AIM2 inflammasomes in SLE macrophages. Rheumatology (Oxford). 2015;54(2):324–31.
Zhen J, Zhang L, Pan J, et al. AIM2 mediates inflammation-associated renal damage in hepatitis B virus-associated glomerulonephritis by regulating caspase-1, IL-1β, and IL-18. Mediators Inflamm. 2014;2014:1–9.
Parthasarathy P, Agarwal A, Chawla K, et al. Upcoming biomarkers for the diagnosis of Kawasaki disease: a review. Clin Biochem. 2015;48(16–17):1188–94.
Eleftheriou D, Levin M, Shingadia D, et al. Management of Kawasaki disease. Arch Dis Child. 2013;99(1):74–83.
Feng S, Su Y, Luo L, et al. Serum levels of C1q/tumor necrosis factor-related protein-1 in children with Kawasaki disease. Pediatr Res. 2018;83(5):999–1003.
Domiciano TP, Wakita D, Jones HD, et al. Quercetin inhibits inflammasome activation by Interfering with ASC Oligomerization and Prevents Interleukin-1 Mediated Mouse Vasculitis. Sci Rep. 2017;7(1):41539.
Asano T, Ogawa S. Expression of monocyte chemoattractant protein-1 in Kawasaki disease: the anti-inflammatory effect of gamma globulin therapy. Scand J Immunol. 2000;51(1):98–103.
Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–20.
Komada T, Chung H, Lau A, et al. Macrophage uptake of necrotic cell DNA activates the AIM2 inflammasome to regulate a proinflammatory phenotype in CKD. J Am Soc Nephrol. 2018;29(4):1165–81.
Yin Y, Yan Y, Jiang X, et al. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22(2):311–22.
Hakimi M, Peters A, Becker A, et al. Inflammation-related induction of absent in melanoma 2 (AIM2) in vascular cells and atherosclerotic lesions suggests a role in vascular pathogenesis. J Vasc Surg. 2014;59(3):794–803.
Ramji DP, Davies TS. Cytokines in atherosclerosis: key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015;26(6):673–85.
Dihlmann S, Erhart P, Mehrabi A, et al. Increased expression and activation of absent in melanoma 2 inflammasome components in lymphocytic infiltrates of abdominal aortic aneurysms. Mol Med. 2014;20:230–7.
Lee Y, Wakita D, Dagvadorj J, et al. IL-1 Signaling Is critically required in stromal cells in Kawasaki disease vasculitis mouse model: role of both IL-1alpha and IL-1beta. Arterioscler Thromb Vasc Biol. 2015;35(12):2605–16.
Lee Y, Schulte DJ, Shimada K, et al. Interleukin-1β Is crucial for the induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation. 2012;125(12):1542–50.
Acknowledgements
We are grateful for their help in the sample collection for this study.
Funding
This study was funded by the Science and Technology Projects of Wenzhou (Grant nos. Y20180007 and Y20170134).
Author information
Authors and Affiliations
Contributions
Zhenquan Wang and Qiaoyu Wang contributed equally to this work. All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interests about the publication of this paper.
Ethics approval
All candidates and their parents received detailed information about the research and signed the consent form. The research was approved by the Ethics Committee of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University.
Informed consent
Informed consent was obtained from legal guardians.
Consent for publication
This study only collected the patient's gender, age, and laboratory data and did not involve the patient's personal privacy.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Wang, Q., Jin, J. et al. The diagnostic role of AIM2 in Kawasaki disease. Clin Exp Med 21, 41–47 (2021). https://doi.org/10.1007/s10238-020-00669-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-020-00669-6