Abstract
To understand the hydrochemical characteristics and circulation pattern of groundwater in coastal coal mining areas, we analyzed 81 water samples from different water bodies in the Liangjia coal mine (LCM) area using multivariate statistical analysis and hydrochemical methods. The Quaternary groundwater (QW), accumulated water (AW) in the subsidence area, and mine water (MW) in the LCM all exhibit weakly alkaline to slightly saline water chemistry. The dominant cations and anions in the water are sodium (Na+) and chloride (Cl−), reflecting the influence of seawater intrusion. Some ions in QW, AW, and MW exhibited significant annual variations, but Na+ and Cl− concentrations increased with time. The water samples were divided into four categories through cluster analysis: C1 and C2 (bedrock water samples), C3 (water samples prominently affected by seawater intrusion), and C4 (QW and AW in the surface subsidence area). According to the Piper diagram, QW and AW in the surface subsidence area mainly correspond to the Na−Cl type, whereas the MW mainly consists of Na−Cl and Na−HCO3 types. Factor analysis revealed four main factors: seawater recharge, HCO3-rich bedrock water, alkaline water, and Quaternary groundwater (QW) with eigenvalues of 4.18, 2.44, 1.22, and 1.19 respectively, which explained 81.98% of the original data information. The comprehensive results of hydrochemical analysis and mathematical statistics indicated that the recharge sources of MW in LCM include seawater, QW, AW, HCO3-rich bedrock water, and mixed water. Based on regional hydrogeological conditions, a preliminary groundwater circulation model of the coastal coal mining area was constructed. Groundwater generally flows into the Bohai Sea from southeast to northwest, and coal mining has changed the original local groundwater runoff patterns and intensified seawater intrusion.
Zusammenfassung
Um die hydrochemischen Eigenschaften und den Grundwasserfluss in einem küstennahmen Kohleabbaugebiet zu interpretieren, wurden 81 Wasserproben aus verschiedenen Wasserkörpern im Liangjia-Kohlebergbaugebiet mit Hilfe multivariater statistischer Analysen und hydrochemischer Methoden analysiert. Das quartäre Grundwasser (QW), das sog. akkumulierte Wasser (AW) im Senkungsgebiet des Kohleabbaus sowie das Grubenwasser (mine water, MW) im Liangjia-Kohlebergbaugebiet zeigen schwach alkalische bis leicht salzige Eigenschaften. Hauptkat- und -anionen sind Na + und Cl-, was den Einfluss einer Meerwasserintrusion widerspiegelt. Einige der An- und Kationen in QW, AW und MW wiesen erhebliche jährliche Schwankungsbereiche auf. Die Gehalte an Na + und Cl- nehmen insgesamt mit der Zeit zu. Die Wasserproben wurden durch eine Clusteranalyse in vier Kategorien eingeteilt: C1 und C2 (Wasserproben aus dem anstehenden Gebirge), C3 (Wasserproben, die stark von durch Meerwasser beeinflusst sind) und C4 (QW und AW im Bereich der Oberflächensenkung). Im Piper-Diagramm entsprechen QW und AW im Oberflächensenkungsgebiet hauptsächlich dem Na-Cl-Typ, während das Grubenwasser hauptsächlich aus Na-Cl- und Na-HCO3-Typen besteht. Eine Faktorenanalyse ergab die vier Hauptfaktoren Meerwasseranreicherung, HCO3-reiches Grundwasser, alkalisches Wasser und quartäres Grundwasser (QW), womit 82% der ursprünglichen Daten gedeutet werden konnten. Die Ergebnisse der hydrochemischen und der statistischen Auswertung zeigten, dass das Grubenwasser im Liangjia-Kohlebergbaugebiet sowohl durch Meerwasser, QW, AW, HCO3-reichem Grundwasser sowie durch Mischwasser beeinflusst wird. Auf der Grundlage der regionalen hydrogeologischen Bedingungen wurde ein konzeptionelles Grundwassermodell für das Küstenkohleabbaugebiet erstellt. Das Grundwasser fließt grundsätzlich von Südosten nach Nordwesten in das Bohai-Meer, jedoch hat der Kohleabbau den lokalen Grundwasserfluss verändert und das Eindringen von Meerwasser verstärkt.
为了解近滨海采煤区地下水水化学特征和水循环规律, 采用多元统计和水化学方法分析了梁家煤矿( LCM)不同水体的81个水样。第四系地下水( QW)、塌陷区积水( AW)、梁家煤矿矿井水( MW)均呈弱碱水至微咸水特征。主要阳离子、阴离子是Na+和Cl−, 反映出海水入侵影响。第四系地下水( QW)、塌陷区积水( AW)和梁家煤矿矿井水( MW)的一些离子具有明显年际变化特点, 而Na+和Cl−浓度随时间持续增大。依据聚类分析, 水样被分为四类: C1和C2 (基岩水样)、C3 (受海水入侵影响显著的水样)和C4 (第四系水和塌陷区积水)。根据Piper图解, 第四系水 (QW) 和塌陷区积水 (AW) 主要为Na-Cl型, 梁家煤矿矿井水 (MW) 由Na-Cl型和Na- HCO3型组成。因子分析揭示出4个主要因子: 海水补给、富含HCO3−基岩水、碱性水和第四系地下水, 它们解释了82.0%水源数据信息。综合水化学分析和数理统计的结果表明, 梁家煤矿的矿井水补给来源包括海水、第四系地下水 (QW) 、塌陷区积水(AW)、富含HCO3−基岩水和混合水。基于区域水文地质条件, 建立了滨海采煤区地下水循环的基本模型。地下水总体由东南向西北流向渤海, 煤矿开采改变了局部地下水径流方式, 加剧了海水入侵。
Resumen
Con el objetivo de comprender las características hidroquímicas y el patrón de circulación de las aguas subterráneas en las zonas mineras de carbón de la costa, se analizaron 81 muestras de agua de diferentes masas de agua en la zona de la mina de carbón de Liangjia (LCM) utilizando análisis estadísticos multivariantes y métodos hidroquímicos. El agua subterránea cuaternaria (QW), el agua acumulada (AW) en la zona de hundimiento y el agua de mina (MW) en la LCM presentan una química del agua entre débilmente alcalina y ligeramente salina. Los cationes y aniones dominantes son Na + y Cl-, reflejando la influencia de la intrusión de agua de mar. Algunos iones en QW, AW y MW mostraron variaciones anuales significativas, con las concentraciones de Na + y Cl- aumentando con el tiempo. Las muestras de agua se dividieron en cuatro categorías mediante un análisis de conglomerados: C1 y C2 (muestras de agua del lecho rocoso), C3 (muestras de agua afectadas prominentemente por la intrusión de agua de mar), y C4 (QW y AW en el área de subsidencia de la superficie). Según el diagrama de Piper, el QW y el AW de la zona de subsidencia superficial corresponden principalmente al tipo Na-Cl, mientras que el MW está formado principalmente por los tipos Na-Cl y Na-HCO3. El análisis de factores mostró cuatro factores principales: recarga de agua de mar, agua de lecho de roca rica en HCO3, agua alcalina y agua subterránea cuaternaria (QW), que explicaban el 82,0% de la información de los datos originales. Los resultados exhaustivos del análisis hidroquímico y las estadísticas matemáticas indicaron que las fuentes de recarga de MW en LCM incluyen agua de mar, QW, AW, agua del lecho rocoso rica en HCO3 y agua mixta. Con base en las condiciones hidrogeológicas regionales, se construyó un modelo preliminar de circulación de aguas subterráneas de la zona minera costera de carbón. Las aguas subterráneas desembocan generalmente en el mar de Bohai desde el sureste hasta el noroeste y la minería del carbón ha cambiado los patrones locales de flujo de las aguas subterráneas y ha intensificado la intrusión de agua de mar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal is China’s main source of energy and thus strongly supports the national economy (Tang et al. 2015; Wu et al. 2016). However, the mining of coal resources has negatively affected the hydrogeological environment, causing the deterioration of groundwater resources and many environmental problems (Dong et al. 2020; Li 2018). Coal mining inevitably impacts the flow pattern of aquifers to varying degrees and affects groundwater chemistry, changes the groundwater circulation pattern, and is often accompanied by groundwater contamination issues (Li et al. 2018). In addition, the hydrogeological patterns of China's coal mines are relatively complex and mine water inrushes frequently occur during coal mining operations (Qian et al. 2018).
Groundwater undergoes complex hydrogeochemical reactions with the surrounding environment, such as water–rock interactions, cation exchange, mixing, and evaporation (Bozau et al. 2017; Gu et al. 2018; Liu et al. 2021). The water composition also changes accordingly, leading to unique chemical and physical characteristics. These hydrochemical characteristics can trace back aquifer behaviour, and serve as the foundation of groundwater circulation patterns in mining areas (Liu et al. 2019b; Singh et al. 2016).
Analyzing the hydrochemistry of water bodies in coal mines has important practical significance and can be used to help mitigate mine water hazards (Guan et al. 2019; Zhang et al. 2020). In recent years, research on groundwater chemistry and circulation patterns in coal mining areas has steadily increased, and contributed to a deeper understanding of mine water hydrology. Qian et al. (2016) analyzed the recharge source, geochemical evolution, and hydraulic connectivity of adjacent aquifers in Dingji Coal Mine based on multivariate statistical and hydrochemical methods. Singh et al. (2018) determined the hydrochemical evolution of groundwater, recharge sources, and impacts of mine drainage on water quality in the Korba mining area, India, using major ion and stable isotope data of different water bodies. Liu et al. (2019b) analyzed the hydrochemistry of different aquifers in the Suixiao coal mine in combination with hydrogeological conditions and concluded that the main recharge aquifers were hydraulically connected and that groundwater circulation in the sandstone and limestone aquifers was controlled by faults. However, most such research has focused on coal mining in inland areas, while research studies on hydrochemistry associated with coastal coal mining environments are very limited.
Coastal aquifers form a brackish zone called an estuary. It builds up a dynamic saltwater to freshwater interface. Intrusion of saltwater affects the regional groundwater in these areas and is mainly controlled by the sedimentary environment and ocean dynamics. Coal mining impacts on the evolution of the groundwater cycle more strongly in coastal areas than in inland areas. In coastal areas, coal mining disrupts the flow pattern of aquifer and the hydrodynamic balance between brackish water and freshwater, enlarging and complicating the transition zone of brackish to freshwater compartments.
To address the extent of saltwater intrusion and the impact of mining, we investigated the groundwater hydrochemistry of a coastal coal mining area and constructed a corresponding circulation model. Using the Liangjia coal mine (LCM) in Longkou, Shandong Province, as an example, samples were collected of the main hydrochemical species: Quaternary water (QW), accumulated water (AW) in the surface subsidence area, and mine water (MW) from the mine workings. The hydrochemical characteristics and influencing factors of the major water bodies were analyzed, the recharge source of mine water was identified through multivariate statistical analysis, and a groundwater circulation model was constructed considering regional hydrogeological conditions.
Study Area
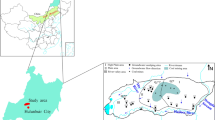
The Liangjia coal mine (LCM) is located in the Longgang Economic Development Zone, Longkou City, Shandong Province (Fig. 1), with the Bohai Sea in the west, Beizao coal mine in the north, Sangyuan coal mine in the northeast, and Wadong coal mine in the east. The mine is ≈ 9–9.5 km long from east to west and 3–6.1 km wide from north to South, covering an area of 47.5 km2. The terrain of the LCM is generally flat, but gradually slopes from the southeast to northwest, and the ground elevation ranges from 2 to 27 m. Surface water systems flowing through the area include the Zhongcun River and Xiaoheng River, which are seasonal. In the rainy season, surface water discharges and flows into the Bohai Sea from southeast to northwest. The Zhongcun River has a total length of 45 km, a catchment area of 120 km2, a flow length of 6 km, and a riverbed width of 100–150 m. In addition, the river is hydraulically connected with the Quaternary alluvial aquifer.
From top to bottom, the coalfield lithology comprises: a Quaternary sand gravel layer, marl, mudstone with marl interbedding, coal seams 1 and 2 and their floor sandstone, and coal seams 3 to 4 along with their floor sandstone, etc. (Fig. 1). Among them, the Quaternary gravel layer comprises grain sizes ranging from fine, medium, coarse sand, to gravel. The sands are mainly quartz and feldspar, whereas the gravel is composed of granite, gneiss, granite, and quartzite. The Quaternary gravel layer has abundant water. The typical coal-bearing sedimentary sequence is composed of sandstone, coal, and carbonaceous mudstone. The upper part of the coal-bearing strata is mainly composed of marl, calcareous mudstone, carbonaceous mudstone, mudstone interbedded with marlstone, mudstone, oil-bearing mudstone, coal and oil shale, and the lower part is mainly composed of claystone, mudstone, sandstone, and oil shale. The roof of the coal seam is soft and prone to deformation, settling, and rock falls. The water content of the marl and sandstone is low to medium, and the hydrochemical water types based on ion equivalents are mainly HCO3·Na, HCO3·Cl−Na, or Cl·HCO3−Na.
The coal seams in the area are soft and brittle, with well-developed endogenetic cleats and locally developed fractures due to the influence of structure, facilitating the storage of fissure water in the coal and rocks. However, the water yield is low in the deep layers because the fracture rate and recharge rate decrease with increasing lithostatic pressure. There were no considerable water-bearing aquifers below the coal-bearing strata. The coal measure aquifers in the area are not directly recharged by precipitation, but mainly by water from fractured bedrock from the southern and eastern mountainous areas. Although faults exist in the coal field, the fault zones are mostly filled with argillaceous rocks, and their water abundance and hydraulic conductivity are low. Under natural conditions, groundwater runoff from the south and east to the northwest is slow, and it normally discharges into the Bohai Sea. The local groundwater flow field has been changed by coal mining activities, but the overall runoff trend has remained constant. Seawater intrusion in the Longkou area began in the mid-1970s. During the 1980s, decreased precipitation and intensive groundwater extraction accelerated the rate of seawater intrusion. After 2000, numerous measures were taken to successfully identify and control seawater intrusion. At present, the intrusion front reaches a steady state.
Materials and Methods
Sampling and Testing
The water chemistry data was made possible by the Shandong Energy Longkou Mining Group, which operates the LCM. A total of 81 samples of AW in the surface subsidence area, QW, and MW were collected from the coal mine area during 2014–2019. Among them, the MW samples were mainly collected from mine workings, including the main tunnel, auxiliary tunnel, transportation tunnel, and material tunnel. The sampling locations are shown in Fig. 1b. QW and AW were continuously sampled, and MW was also continuously sampled at some sampling points. The samples were collected in dry and clean polyethylene plastic bottles (500 ml) the water was sampled after the sampling bottles had been rinsed two to three times with the water being sampled. The water sample was sealed to prevent leakage, and subsequently sent to the water chemistry laboratory of LCM for water quality analysis.
The main cations (Ca2+, Mg2+, Na+ and K+) and main anions (HCO3−, SO42−, Cl−, and NO3−), as well as pH and total dissolved solids (TDS) were determined by water quality analysis. The pH was measured using a pH meter (PHS-3C). The main anions (SO42−, Cl−, and NO3−) were analyzed by ion chromatography, the main cations (Ca2+, Mg2+, Na+, and K+) by atomic absorption spectrometry, and HCO3− was measured by titration, using phenolphthalein and methyl orange as indicators, and the water sample was titrated with an acid standard solution (HCl = 0.05 mol/L).
Analysis Process
Figure 2 presents a flowchart of this study’s methodology. Based on statistical and hydrochemical analysis of the analytical results, a groundwater circulation model for coal mines in coastal areas was constructed.
Mathematical Statistics
Cluster analysis (CA) is a multivariate analysis technique. Its basic principle is to quantitatively determine the affinity and sparsity between samples according to their own attributes and some similarity or difference indicators, and cluster the samples accordingly (Liu et al. 2019a; Singh et al. 2016). Factor analysis (FA) is another multivariate statistical analysis method that is widely used in water chemistry analysis (Liu et al. 2018; Martín Del Campo et al. 2014). It uses mathematical methods to reduce the dimensionality of the original water quality data, extract key information from the multivariable water quality data, and create a set of new variables to optimize the original variables. However, there is no correlation between the new variables obtained.
Hydrochemical Methods
The descriptive statistics (maximum, minimum, mean, standard deviation, and coefficient of variation) of the hydrochemical composition were used to determine the general chemical fingerprint of the different water bodies. The graphical hydrochemical toolset used in this study mainly included Piper diagram, Stiff diagram, ion ratios, and saturation index (SI).
Results and Discussion
Hydrochemical Characteristics
The descriptive statistical results of the main hydrochemical parameters of QW, AW, and MW in the LCM area are shown in Table 1. On average, Na+ and Ca2+ were the dominant cations in all water samples, and the following relationship was observed: Na+ > Ca 2+ > Mg2+ > K+. The main anions in QW and AW were Cl− and SO42−, with Cl− > SO42− > HCO3− > NO3−. The dominant anions in the MW were HCO3− and Cl−, and the ions followed the order HCO3− > Cl− > SO42− > NO3−. The concentrations of the major ions significantly varied among the different water bodies, but both Na+ and Cl− remained dominant (Table 1), which indicates that the hydrochemistry in the mining area is affected by seawater intrusion.
The average TDS values in QW (4366 mg/L), AW (4879 mg/L), and MW (5407 mg/L) all exceeded 3000 mg/L (brackish water) (Table 1). The pH values of all sample types were above 7, (7.20, 8.26, and 8.36, respectively). The variation coefficients (CV) of Mg2+, Ca2+, and NO3− in MW were 151.89%, 145.08%, and 166.67%, respectively, indicating wide variations and unstable contents. The Mg2+, Ca2+ and NO3− contents followed the order QW > AW > MW (Table 1); Na+, pH and TDS followed MW > AW > QW; K+ and SO42− followed AW > QW > MW; HCO3− followed MW > QW > AW and; Cl− followed AW > MW > QW.
Temporal Variation Characteristics of the Major Ions
The temporal variation trends of the major ions in the different water bodies of LCM are shown in Fig. 3. The contents of Na+ and Cl− in AW, QW, and MW exhibited a fluctuating upward trend with time (Fig. 3a, b). The contents of Ca2+ and Mg2+ in AW and QW were relatively stable, but their contents in MW first increased and then decreased; the fluctuation of Ca2+ and Mg2+ concentrations in QW was more obvious (Fig. 3c, d). In all water bodies, K+ concentration was relatively stable before 2018, but it significantly fluctuated after 2018 (Fig. 3e). The content of HCO3− in AW and QW did not change prominently with time, but its concentration in MW widely fluctuated and showed an upward trend (Fig. 3f). The content of SO42− in MW showed a decreasing trend with time, while its concentration in AW and QW showed an increasing trend, with a high value in 2017 (Fig. 3g). The content of NO3− in AW and MW showed a fluctuating decrease, while its concentration in QW significantly fluctuated (Fig. 3h), which may be attributable to anthropogenic inputs.
Cluster Analysis (CA)
The hydrochemical data of the different LCM water bodies were analyzed using cluster analysis, and the cluster spectrum is plotted as dendrogram (Fig. 4). The samples were divided into four categories using a distance of 10 between classes: C1 (n = 41), C2 (n = 14), C3 (n = 8), and C4 (n = 18). In addition, the average ion concentrations in mg/L of various water samples were summarized in a Stiff diagram.
The Na+ and Cl− contents in the C1, C3, and C4 water clusters were relatively high (C3 > C1 > C2), which may correspond to different degrees of seawater intrusion. Chemical components corresponding to C1 are characterized by high concentrations of Na+ (1646 mg/L), Cl− (1443 mg/L), and HCO3− (1621 mg/L). In addition, C2 is characterized by high concentrations of Na+ (1225 mg/L) and HCO3− (1802 mg/L). Considering hydrogeological conditions, C1 and C2 represent HCO3−-dominated bedrock groundwater, comprising host rock compositions including marl, mudstone, and interbedded limestone. Hydrochemical components corresponding to C3 are characterized by high concentrations of Na+ (1773 mg/L) and Cl− (2619 mg/L). Based on the dominance of Na+ and Cl− ions, C3 represents water affected by seawater intrusion. In addition to the characteristics of high Na+ (1051 mg/L) and Cl− (1432 mg/L), C4 also contains higher Mg2+ (145 mg/L) and SO42− (990 mg/L) concentrations. The concentrations of Mg2+ and SO42− were comparable to the ionic composition of QW and AW (Table 1). Therefore, considering the major ion concentrations (Table 1) and cluster analysis results (Fig. 4), C1 and C2 can be regarded as HCO3-rich bedrock groundwater, while C3 represents diluted seawater, and C4 represents formation waters of QW and AW.
Hydrochemical Types
The Piper diagram is a classical graphical representation of the six common major ion in water samples plotted in terms of meq/L and their relative percentages to total cation and anion percentages. It can be used to analyze the evolutionary trend of the chemical composition of groundwater and infer hydrochemical facies; moreover, it has the advantage of being insensitive to human factors (Piper 1944; Liu et al. 2021). Water samples from QW, AW, MW, and one seawater sample (from a previous study; Wang 2020) were projected on the Piper diagram, as shown in Fig. 5. In the triangle at the bottom left of the Piper diagram, cations basically fall in the area of Na+ dominated water, and C2 in the anion triangle at the right is mainly in the area of HCO3—type water, whereas C1, C3, and C4 mainly correspond to Cl−—type water. After projecting the data from the triangular fields into the central diamond field, the water samples of C1, C3, and C4 are mainly distributed in area ⑦, which corresponds to Na-Cl type water. The sample point of C2 mainly falls in area ⑧, corresponding to Na-HCO3 type water. Therefore, C2 possibly originates from HCO3-rich bedrock water in aquifers such as marls or limestones. In addition, combined with sampling type, QW belong to C4, AW is composed of a mixture of C4 and C3, and MW includes C1, C2, and C3.
The seawater sampling point is located at the junction of MW and AW, that is, in the mixed area of C3 and C4 water, implying that C3 and C4 water may be affected by seawater intrusion. In addition, QW, which is classified as C4 water, also has a high Na+ and Cl− content, which indicates the potential effect of seawater intrusion. Combined with CA and water sample types, AW appears to contain both C4 water of QW and C3 water through seawater intrusion. Therefore, it can be concluded that the water in the surface subsidence area is recharged by QW and seawater to a certain extent.
Analysis of Factors Influencing Water Chemistry
In general, rock weathering, precipitation, and evaporation are the three main factors affecting hydrochemical characteristics (Gibbs 1970), and in coastal areas, hydrochemistry of groundwater and surface water are often influenced by sea water infiltration (Ahmed and Askri 2016; Zghibi et al. 2019). Ion ratio analysis can be performed to further determine the main factors affecting hydrochemical characteristics (Liu et al. 2019a). The molar ratio of Na+ to Cl− concentration is often used to analyze the source of Na+ in water. If the Na+/Cl− ratio is 1, the dissolution of halite may be the main source of Na+ (Eqs. 1), because halite dissolution will release the same amount of Na+ and Cl−. As shown in Fig. 6a, most of the water samples are below the 1:1 line, indicating that the dissolution of halite is not the main source of Na+ in water. If the content of Na+ in water is high, Na+ may originate from other sources, such as silicate rock weathering (Liu et al. 2020) (Eqs. 2) or cation exchange (Gao et al. 2019) (Eqs. 3). A ratio of Na+ to Cl− equal to 1 is clearly associated with halite dissolution, and seawater has an original Na+ to Cl− ratio of 0.86. Therefore, the contents of Na+ and Cl− were very high in different water bodies, and their ratio was close to 0.86 (1.16: 1 line), indicating the influence of seawater. In addition, maybe the Na+ to Cl− concentration data can be associated with sea spray which can affect Quaternary coastal alluvial sediments. The CA and Piper diagram showed that C3 represents the influence of seawater intrusion. C3 and C4 water are distributed close to the 1.16:1 line, and the distribution characteristics are relatively consistent, which also indicate that C4 water is affected by seawater intrusion. Furthermore, in Gibbs diagram (Gibbs 1970) (Fig. 6b), C3 and C4 water are distributed near the upper right corner, indicating that these water samples are affected by seawater to a certain extent.
Similarly, if gypsum dissolution is the only source of Ca2+ and SO42−, the value of Ca2+/SO42− is equal to 1 (Gao et al. 2019) (Eqs. 4). As shown in Fig. 6c, the water sample points are mainly distributed in the upper part of the 1:1 line, indicating that the dissolution of gypsum is not the main source of Ca2+ and SO42−, while the water sample near the 1:1 line is affected by the dissolution of gypsum. The sulfide contained in the coal-bearing formation can oxidize FeS2 to form H2SO4, and react with Fe2+ in the water to form Fe(OH)3, and at the same time release H+ into the groundwater, thereby reducing the pH of the water. As shown in Fig. 6d, SO42− and pH show a certain negative correlation, indicating that the higher SO42− concentration of C4 water may be due to the oxidation of pyrite, as shown in Eqs. 5, 6, 7
Relationship between (Na+ + K+−Cl−) and (HCO3− + SO42−−Mg2+−Ca2+) can be used to identify the cation exchange process. If the chemical composition of water is significantly affected by cation exchange, the water sample point should be distributed along the 1: 1 line (Liu et al. 2019a; Gao et al. 2019). As shown in Fig. 6e, some of the water sample points are distributed close to the 1:1 line and the rest are distributed on either side, indicating the effect of cation exchange to a certain extent. However, it is not the main factor controlling the chemical characteristics. The relationship between HCO3−, Mg2+, and Ca2+ can be used to determine the influence of carbonate weathering on water chemical composition (Li et al. 2019; Liu et al. 2019c). As shown in Fig. 6f, ng, HCO3− has a negative correlation with Ca2+ and Mg2+. The content of HCO3− decreases with increasing Ca2+ and Mg2+ content, indicating that carbonate weathering has a weak influence on hydrochemical characteristics.
The SIs of halite and gypsum were both less than 0 (Fig. 6h, i), with average values of − 4.49 and − 2.31, respectively, indicating that halite and gypsum in the water sample are not saturated and can continue to dissolve along the groundwater flow path. The SIs of calcite and dolomite were both greater than 0 (Fig. 6j, k), with average values of 0.91 and 1.69, respectively, indicating that both calcite and dolomite are saturated and have a tendency to precipitate. However, no obvious correlation was observed between the SI of these minerals and TDS content. Thus, the dissolution of this mineral has a relatively weak effect on TDS. In summary, combined with the lithology of the aquifer in the mining area, the hydrochemical characteristics of the LCM area are mainly affected by the weathering of silicate rocks and seawater intrusion.
Quantity and Type of Mine Water Source
Correlation Analysis
Correlation analysis can be performed to determine similarities and dissimilarities between water chemistry parameters as well as consistencies and differences in their sources (Liu et al. 2019a; Chen et al. 2020). In this study, correlation analysis of the main chemical components of MW samples was performed using SPSS 24.0. The correlation analysis matrix scatter plot is presented in Fig. 7. An obvious correlation was observed between Na+ and Cl−, indicating that the two ions may have the same source. In addition, TDS showed a significant positive correlation with Na+ and Cl−, indicating that Na+ and Cl− may largely contribute to TDS content in the MW, which reflects the recharge of seawater to a certain extent. Considering the high concentrations of Ca2+, Mg2+, and SO42− ions in AW and QW (Table 1) and the significant correlation between Ca2+, Mg2+, and SO42− ions in the MW (Fig. 7), the three ions may have a common source, which may be AW and QW. A significant negative correlation was observed between Ca2+ and Mg2+ and HCO3− (Fig. 7), indicating that carbonate weathering was not the source of these components.
Factor Analysis (FA)
In this study, the main chemical components of MW samples were selected for FA to further determine the recharge source of the MW. The Kaiser–Meyer–Olkin (KMO) value was 0.68 and the Bartlett spherical test presented significant values, indicating that the selected hydrochemical data were suitable for FA. Based on the principle of eigenvalues greater than 1, four main factors were determined. The eigenvalues of F1 to F4 were 4.18, 2.44, 1.22, and 1.19, explains 81.98% of the original data information; thus, these four factors reflect most of the MW chemistry. The rotating factor loading matrix and the variance contribution rate are shown in Table 2.
The eigenvalue of F1 was 4.18, explaining 37.99% of the original data information (Table 2); F1 mainly corresponds to Na+, Cl−, and TDS. The Na+ and Cl− content in the MW was high, and exhibited a significant correlation. In addition, LCM is a coastal coal mine, and the coastal area is obviously affected by seawater intrusion. Therefore, F1 can be interpreted as seawater recharge; that is, the MW is recharged by seawater during mining. F2, accounting for 22.15% of the total variance, exhibited a strong negative correlation with HCO3− and a positive correlation with Ca2+ and Mg2+. Aquifers, such as marl, mudstone with interbedded marlstone, and sandstone, in the LCM area are relatively low in water richness, and the main hydrochemical type is HCO3. QW and AW contain relatively high concentrations of Ca2+ and Mg2+, and the HCO3− content is relatively low. Therefore, F2 reflects the recharge of HCO3-rich bedrock water. F3 showed a total variance of 11.06%, with a high CO32− loading. The CO32− content was high and the water was alkaline. Therefore, F3 represents a recharge source of alkaline water, and the recharge of HCO3-rich bedrock water to MW from the side. F4 contributed 10.78% to the total variance with high K+ and NO3− loading. NO3− can reflect the influence of anthropogenic inputs on groundwater to a certain extent, such as agricultural activities. Under natural conditions, groundwater generally has a low K+ content, but the use of potassium-containing fertilizers increases K+ concentrations. QW was obtained at relatively shallow depths, and it is more susceptible to human activities. Thus, F4 mainly reflects the recharge of QW.
Combined with the results of the abovementioned hydrochemical analysis, including cluster analysis, Piper diagram, and ion ratios, the recharge sources of MW in LCM include seawater, QA, AW, HCO3-rich bedrock water, and mixed water.
Analysis of the Groundwater Circulation Model
Considering the region’s hydrogeological conditions, the hydrochemical analysis of the different types of water bodies, and the discrimination of MW sources, a preliminary groundwater circulation model of the coastal coal mining area was constructed (Fig. 8). On the whole, the groundwater in the region mainly originates from atmospheric precipitation, and the direction of runoff flows from the southeast to the northwest into the Bohai Sea. The topography is a major factor controlling groundwater flow.
Mining-induced surface subsidence with associated waterlogging (AW), which was mainly influenced by atmospheric precipitation and QW. The hydrochemical components were obviously affected by seawater intrusion and also pyrite oxidation. Coal mining altered the original groundwater runoff pattern to a certain extent in some areas, forming a new center of confluence in the goaf. In addition, coal mining has promoted seawater intrusion, leading to high Na+ and Cl− contents, which gradually increased with time (Fig. 3). In addition to bedrock water, QW, and AW in the subsidence area, the MW is also recharged by seawater. The concentrations of Na+ and Cl− were particularly high in some mining-related water samples, and the associated hydrochemical type was the Na-Cl type, indicating the influence of seawater intrusion.
Suggestions for Coastal Coal Mining
Coal development is of great significance in promoting the rapid development of China’s national economy and improving peoples’ living standard. However, in the process of mining coal resources, groundwater resources are inadvertently but inevitably damaged. The regional hydrogeology system in the coal mining influenced strata is deteriorated and the natural groundwater circulation state is altered. In coastal areas, groundwater is affected by the sedimentary environment and marine dynamics. Mining induces changes in the aquifer structure and hydrodynamic balance condition between salt and fresh water, thus affecting groundwater migration and evolution. Therefore, in the process of coastal coal mining, it is necessary to: (1) carry out systematic hydrogeological surveys of mining areas, standardize mining, and protect the regional aquifer structure; (2) avoid the discharge of mine water to prevent pollutants like sulfate and iron from entering the groundwater environment; (3) and strengthen the monitoring of groundwater dynamics (groundwater level, water quality) in mining areas. In addition, special attention needs to be paid to seawater intrusion induced by coal mining within coastal areas. In this manner, the impact of coal mining on groundwater resources can be minimized, and the sustainable development of coal and the management and rational use of water resources can be achieved.
Conclusions
-
1.
The average TDS contents of QW, AW in the surface subsidence area, and MW in the LCM area exceeded 4000 mg/L, and the pH was more than pH = 7, corresponding to weakly alkaline brackish water. The main chemical components showed significantly different contents in different types of water, but Cl− and Na+ were consistently the dominant anions and cations.
-
2.
The chemical components in different water bodies changed significantly with time, but the Na+ and Cl− content showed a fluctuating upward trend. Based on CA, the water samples could be divided into four categories: C1 and C3 represent bedrock water, C2 represents water influenced by seawater intrusion, and C4 represents QW and AW. The Piper diagram showed that QW and AW are mainly Cl-Na type water, while MW is mainly Cl-Na and HCO3-Na type water. Hydrochemical analysis revealed that silicate weathering and seawater intrusion are the main factors affecting the hydrochemical characteristics of water in the LCM.
-
3.
The comprehensive results of hydrochemical analysis and mathematical statistics showed that seawater, QA, AW, HCO3-rich bedrock water, and mixed water are the recharge sources of MW in the LCM. A preliminary groundwater circulation model of the coastal coal mining area was constructed based on the hydrogeological conditions, the hydrochemistry and recharge source identification of MW. In general, groundwater flows into the Bohai Sea from southeast to northwest. Coal mining has changed the original groundwater runoff pattern in some areas and increased the influence of seawater on freshwater resources.
References
Ahmed AT, Askri B (2016) Seawater intrusion impacts on the water quality of the groundwater on the northwest coast of Oman. Water Environ Res 88(8):732–740. https://doi.org/10.2175/106143016X14609975747045
Bozau E, Licha T, Ließmann W (2017) Hydrogeochemical characteristics of mine water in the Harz Mountains, Germany. Geochemistry 77(4):614–624. https://doi.org/10.1016/j.chemer.2017.10.001
Chen J, Qian H, Gao Y, Wang H, Zhang M (2020) Insights into hydrological and hydrochemical processes in response to water replenishment for lakes in arid regions. J Hydrol 581:124386. https://doi.org/10.1016/j.jhydrol.2019.124386
Dong S, Feng H, Xia M, Li Y, Wang C, Wang L (2020) Spatial–temporal evolutions of groundwater environment in prairie opencast coal mine area: a case study of Yimin Coal Mine, China. Environ Geochem Health 42(10):3101–3118. https://doi.org/10.1007/s10653-020-00544-z
Gao Z, Liu J, Feng J, Wang M, Wu G (2019) Hydrogeochemical characteristics and the suitability of groundwater in the alluvial-diluvial plain of southwest Shandong Province, China. Water. https://doi.org/10.3390/w11081577
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088. https://doi.org/10.1126/science.172.3985.870
Gu H, Ma F, Guo J, Zhao H, Lu R, Liu G (2018) A spatial mixing model to assess groundwater dynamics affected by mining in a coastal fractured aquifer, China. Mine Water Environ 37(2):405–420. https://doi.org/10.1007/s10230-017-0505-x
Guan Z, Jia Z, Zhao Z, You Q (2019) Identification of inrush water recharge sources using hydrochemistry and stable isotopes: a case study of Mindong No 1 coal mine in north-east Inner Mongolia, China. J Earth Syst Sci 128(7):200. https://doi.org/10.1007/s12040-019-1232-4
Li P (2018) Mine water problems and solutions in China. Mine Water Environ 37(2):217–221. https://doi.org/10.1007/s10230-018-0543-z
Li P, Wu J, Tian R, He S, He X, Xue C, Zhang K (2018) Geochemistry, hydraulic connectivity and quality appraisal of multilayered groundwater in the Hongdunzi coal mine, northwest China. Mine Water Environ 37(2):222–237. https://doi.org/10.1007/s10230-017-0507-8
Li Y, Liu J, Gao Z, Wang M, Yu L (2019) Major ion chemistry and water quality assessment of groundwater in the Shigaze urban area, Qinghai-Tibetan Plateau. China Water Supply 20(1):335–347. https://doi.org/10.2166/ws.2019.167
Liu J, Gao Z, Wang M, Li Y, Ma Y, Shi M, Zhang H (2018) Study on the dynamic characteristics of groundwater in the valley plain of Lhasa City. Environ Earth Sci 77(18):646. https://doi.org/10.1007/s12665-018-7833-4
Liu J, Gao Z, Wang M, Li Y, Shi M, Zhang H, Ma Y (2019a) Hydrochemical characteristics and possible controls in the groundwater of the Yarlung Zangbo River Valley, China. Environ Earth Sci 78(3):76. https://doi.org/10.1007/s12665-019-8101-y
Liu J, Hao Y, Gao Z, Wang M, Liu M, Wang Z, Wang S (2019b) Determining the factors controlling the chemical composition of groundwater using multivariate statistics and geochemical methods in the Xiqu coal mine, North China. Environ Earth Sci 78(12):364. https://doi.org/10.1007/s12665-019-8366-1
Liu Y, Chen L, He Y, Wang L, Zhang J, Chen Y (2019c) Groundwater chemical characteristics and circulation mode in the Suixiao coal-mining district. Q J Eng Geol Hydroge 53(2):227–235. https://doi.org/10.1144/qjegh2018-208
Liu J, Gao Z, Wang Z, Xu X, Sii Q, Wang S, Qu W, Xing T (2020) Hydrogeochemical processes and suitability assessment of groundwater in the Jiaodong Peninsula, China. Environ Monit Assess 192(6):384. https://doi.org/10.1007/s10661-020-08356-5
Liu J, Peng Y, Li C, Gao Z, Chen S (2021) An investigation into the hydrochemistry, quality and risk to human health of groundwater in the central region of Shandong Province, north China. J Cleaner Prod 282:125416. https://doi.org/10.1016/j.jclepro.2020.125416
Martín Del Campo MA, Esteller MV, Expósito JL, Hirata R (2014) Impacts of urbanization on groundwater hydrodynamics and hydrochemistry of the Toluca Valley aquifer (Mexico). Environ Monit Assess 186(5):2979–2999. https://doi.org/10.1007/s10661-013-3595-3
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Eos 25(6):914–928. https://doi.org/10.1029/TR025i006p00914
Qian J, Wang L, Ma L, Lu Y, Zhao W, Zhang Y (2016) Multivariate statistical analysis of water chemistry in evaluating groundwater geochemical evolution and aquifer connectivity near a large coal mine, Anhui, China. Environ Earth Sci 75(9):747. https://doi.org/10.1007/s12665-016-5541-5
Qian J, Tong Y, Ma L, Zhao W, Zhang R, He X (2018) Hydrochemical characteristics and groundwater source identification of a multiple aquifer system in a coal mine. Mine Water Environ 37(3):528–540. https://doi.org/10.1007/s10230-017-0493-x
Singh AK, Varma NP, Mondal GC (2016) Hydrogeochemical investigation and quality assessment of mine water resources in the Korba coalfield, India. Arab J Geosci 9(4):278. https://doi.org/10.1007/s12517-015-2298-1
Singh R, Venkatesh AS, Syed TH, Surinaidu L, Pasupuleti S, Rai SP, Kumar M (2018) Stable isotope systematics and geochemical signatures constraining groundwater hydraulics in the mining environment of the Korba Coalfield, central India. Environ Earth Sci 77(15):548. https://doi.org/10.1007/s12665-018-7725-7
Tang X, Snowden S, McLellan BC, Höök M (2015) Clean coal use in China: challenges and policy implications. Energy Policy 87:517–523. https://doi.org/10.1016/j.enpol.2015.09.041
Wang Y (2020) Hydrogeochemistry in the process of seawater intrusion in Longkou area. China University of Geosciences, Shandong Province ([in Chinese])
Wu Q, Li B, Chen Y (2016) Vulnerability assessment of groundwater inrush from underlying aquifers based on variable weight model and its application. Water Resour Manag 30(10):3331–3345. https://doi.org/10.1007/s11269-016-1352-4
Zghibi A, Mirchi A, Zouhri L, Taupin J, Chekirbane A, Tarhouni J (2019) Implications of groundwater development and seawater intrusion for sustainability of a Mediterranean coastal aquifer in Tunisia. Environ Monit Assess 191(11):696. https://doi.org/10.1007/s10661-019-7866-5
Zhang J, Chen L, Chen Y, Ge R, Ma L, Zhou K, Shi X (2020) Discrimination of water-inrush source and evolution analysis of hydrochemical environment under mining in Renlou coal mine, Anhui Province, China. Environ Earth Sci 79(2):61. https://doi.org/10.1007/s12665-019-8803-1
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (51774194), the “Taishan Scholars Project” (tsqn201812067), the Major Basic Research Funding Project of Shandong Provincial Natural Science Foundation (ZR2018ZC0740), the Natural Science Foundation of Shandong Province (ZR2021QD048), and Liangjia Coal Mine of Shandong Energy Longkou Mining Group Co., Ltd. We thank the anonymous reviewers and editors for their useful comments, which have helped improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., Liu, J., Wang, F. et al. Hydrochemical Analysis of Groundwater in Coastal Coal Mining Areas—A Case Study of the Liangjia Coal Mine, North China. Mine Water Environ 41, 415–427 (2022). https://doi.org/10.1007/s10230-022-00855-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-022-00855-w