Abstract
Groundwater is the primary source of water for domestic use and agricultural irrigation in Jiaodong Peninsula. This study collected 80 groundwater samples from Jiaodong Peninsula to characterize groundwater hydrogeochemical processes and the suitability of groundwater for domestic use and agricultural irrigation. The groundwater of Jiaodong Peninsula was categorized as slightly alkaline freshwater, with a Piper diagram classifying most samples as SO4·Cl-Ca·Mg and HCO3-Ca·Mg types. Major ions were Ca2+, Na+, SO42−, and HCO3−. The major processes driving the hydrochemistry of groundwater were identified as water-rock interactions as well as evaporation. The dissolution of silicate and cation exchange were the predominant hydrogeochemical processes responsible for groundwater chemistry. Four water samples showed seawater intrusion and some indicated pollution from anthropogenic activities such as industry, agriculture, and domestic sewage discharge. Overall, it was found that groundwater in most areas of Jiaodong Peninsula is suitable for domestic use and agricultural irrigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is a significant water resource and a major source of water for various purposes (Nematollahi et al. 2016; Liu et al. 2018; Liu et al. 2019a). Among the developing countries, China has the greatest area and a large population of 1.4 billion. More than 70% of cities in China use groundwater as a source of domestic water. However, industrialization, urbanization, and economic growth have had a significant impact on the groundwater environment in China (Liu et al. 2018). In addition, water shortages are becoming increasingly severe in China (Yang et al. 2016). The hydrochemical composition of groundwater is controlled by many factors, including hydrogeological conditions, geological structure, climate, topography, elevation, and human activities (Xing et al. 2018). The analysis of the hydrochemical characteristics of groundwater can assist in identifying hydrogeochemical interactions within groundwater and surrounding environments and in revealing the evolution of hydrochemical processes (Marghade et al. 2015; Yang et al. 2016). These analyses can also strengthen the understanding of the hydrogeochemical system (Xiao et al. 2015).
There have been many recent studies on the hydrogeochemistry and quality of groundwater worldwide (Yang et al. 2016; Tiwari et al. 2017; Wu et al. 2018). These studies have found that the weathering of rock and minerals is the major factor affecting the hydrochemical characteristics of groundwater, and that groundwater quality is greatly influenced by human activities (Liu et al. 2017; Singh et al. 2017; Jain and Vaid 2018). The major causes of groundwater pollution include the discharge of industrial pollutants and use of agricultural chemical fertilizers, which result in elevated groundwater concentrations of nitrate (NO3−), nitrite (NO2−), and sulfate (SO42−) (Vikas et al. 2014; Li et al. 2016a). Groundwater quality not only has a direct impact on human health but also has a significant impact on industrial and agricultural development, urbanization, food safety, and environmental sustainability (Adimalla et al. 2018; Li et al. 2018). The sustainable management and safe usage of groundwater resources for domestic, agricultural, and industrial purposes requires a full understanding and consideration of groundwater chemical characteristics and quality.
Jiaodong Peninsula is China’s largest peninsula, located in the northeast coastal area of the North China Plain. The peninsula faces the sea on three sides, and is highly developed. Groundwater for industrial, agricultural, and domestic water use is important for maintaining social and economic development in the region. Yin et al. (2018) investigated the groundwater hydrochemistry of the Dagu River Basin of Qingdao and found that artificial inputs of agricultural nitrogen fertilizer, manure, and domestic sewage were the main factors resulting in elevated groundwater NO3− content. Yu (2018) investigated the quality of groundwater in Yantai City based on a comprehensive index method and found that the main cause of poor groundwater quality was the large discharge of industrial and domestic sewage along with unsustainable exploitation of groundwater. Yi et al. (2015) assessed the environmental quality of groundwater in Huancui District, Weihai City, and concluded that the quality of groundwater in most areas was relatively good. The main water quality variables affecting the quality of groundwater in Jiaodong Peninsula include sulfate, nitrate, and chloride. However, past studies mainly focused on smaller localized impacts within the study area, and there are few studies that have attempted to characterize groundwater quality and hydrochemistry for the entire Jiaodong Peninsula. Therefore, from a groundwater management perspective, there is practical significance in an analysis of groundwater for the entire Jiaodong Peninsula to characterize the quality and hydrochemical characteristics of groundwater and the underlying processes affecting groundwater hydrochemical evolution. In the present study, traditional integrated geochemical methods were used to assess the hydrogeochemistry and quality of groundwater in the Jiaodong Peninsula. The objectives of the present study were (1) the identification of the hydrogeochemical processes controlling groundwater hydrochemistry and (2) the elevation of the groundwater quality of the Jiaodong Peninsula. This paper will be of great significance for the development, utilization, and protection of groundwater in Jiaodong Peninsula.
Study area
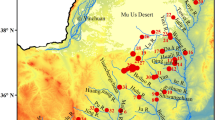
The Jiaodong Peninsula (Fig. 1) has been identified as the area with the greatest potential for economic development in Shandong Province. The peninsula is situated at the junction of the Circum-Bohai marine economic zone and the Asian Yellow Sea Economic Circle in China. The designation “Jiaodong Peninsula” mainly refers to the area east of the Jiaolai River, including Qingdao, Yantai, and Weihai. The peninsula is surrounded by the sea on three sides and lies between lat. 35° 35′ 00″–38° 00′ 00″ N and lon. 119° 30′ 00″–122° 43′ 00″ E, with a total area of ~ 2.98 × 104 km2. Three geomorphological types dominate the study area, namely tectonic erosion landforms, tectonic denudation landforms, and accumulation landforms. Middle-low mountains and low mountains are mainly distributed to the northeast. The terrain is undulating, with an elevation of 500–800 m, with the terrain generally increasing in height with increasing distance inland.
The Jiaodong Peninsula is located in the warm temperate monsoon semi-humid to continental climate area. The climate of the peninsula is regulated by the ocean, with four distinct seasons and a mild climate. The region has agreeable temperatures with an annual average of 12.3 °C with low variation. The average annual precipitation measured from 1956 to 2009 is 718.8 mm. The rivers in the Jiaodong Peninsula originate in the central mountainous areas, with most flowing into the sea. The rivers are characterized by relatively wide riverbeds and turbulent currents. River runoff is greatly affected by season, with 70–80% of the annual runoff concentrated during the flood season.
Deep metamorphic crystalline rocks such as the Archean gneiss and schist in the Jiaodong area are widely exposed, and extensive volcanic eruptions and acidic magma intrusions occurred at the end of the Cretaceous. The intrusive rocks are widely distributed, accounting for more than half of the bedrock outcrops in Jiaodong. Among them, the Neoproterozoic Sinian and Mesozoic Yanshanian intrusive rocks are the most developed.
Materials and methods
Sampling
Groundwater samples totaling 80 were collected from wells in the Jiaodong Peninsula during May and June, 2017. Sterilized plastic bottles used to collect water samples. The bottles were rinsed 2–3 times with the source water before sampling. All samples were refrigerated and transported to the laboratory as soon as possible for further analysis.
Method of measurement
Electrical conductivity (EC) and pH were determined by a portable multi-meter, whereas total dissolved solids (TDS) was determined by the oven drying method. The major cations K+, Na+, Ca2+, and Mg2+ were determined by the flame atomic absorption spectrophotometry and the major anions (SO42−, NO3− and Cl−) were determined by ion chromatograph (IC). Chemical oxygen demand (COD) and HCO3− were determined by the titrimetric method. The normalized inorganic charge balance (NICB) was obtained using the following formula:
In Eq. (1), TZ+ (cationic charge) = Na+ + K+ + 2Ca2+ + 2Mg2+ and TZ− (anionic charge) = Cl− + HCO3− + 2SO42− + NO3−. The absolute values of NICB of most groundwater samples in Jiaodong Peninsula were less than 5, indicating the accuracy of the measurements.
Analysis of geochemical processes
Piper diagrams have been widely used in the study of groundwater types and in the analysis of the hydrochemical processes controlling groundwater chemical composition (Liu et al. 2018; Zhang et al. 2018). Gibbs plots (Gibbs 1970) can be used to determine which major mechanisms, namely rock-water interaction dominance, precipitation dominance or evaporation dominance, and control water chemistry (Qu et al. 2019). The use of ratio graphs of ions is one of the most effective graphical means determining the origins of solutes and hydrochemical processes (Tiwari et al. 2017; Colado et al. 2018).
The use of the chloro-alkaline index (CAI) is an effective method to investigate the ion exchange process (Schoeller 1965), and CAI can be expressed as follows:
In Eqs. (2) and (3), CAI > 0 indicates that Na+ or K+ from solution is replaced by Ca2+ from sediments, whereas CAI < 0 indicates reverse ion exchange in which Ca2+ from water is replaced by Na+ or K+ from sediments.
The saturation index (SI) indicates the saturation state of water relative to a given mineral and can reflect the tendency of mineral precipitation or dissolution (Li et al. 2016b). SI was calculated using the PHREEQC software and is based on the mathematical relationship between ion activity products (IAP) and a corresponding equilibrium constant (K):
In Eq. (4), SI = 0, SI > 0, and SI < 0 represents equilibrium, oversaturation, and undersaturation, respectively.
Principal component analysis (PCA) is widely used to better understand groundwater geochemical processes. PCA is based on eigenvalues > 1 according to the Kaiser-Meyer-Olkin measure (Nematollahi et al. 2016; Liu et al. 2017).
Groundwater quality assessment
The water quality index (WQI) is a popular method of assessing the quality of groundwater for domestic use (Deepa and Venkateswaran 2018; Rabeiy 2018). The WQI is able to reflect the influences of a number of different water quality variables on groundwater quality. Figure 2 illustrates the process required to calculate the WQI.
The suitability of water for irrigation was examined using several indicators represented as ranges of relative suitability (Jain and Vaid 2018). The current study applied certain methods to assess groundwater suitability for agricultural irrigation, with Table 1 illustrating the equations used.
Results and discussion
Statistical results
As shown in Table 2, the ranges of chemical compositions of the groundwater samples were quite large. The pH level of water indicates acid-base properties, and is a fundamental indicator of the suitability of water for various purposes (Şener et al. 2017). The range of pH among the samples was between 6.6 and 8.4, with an average of 7.6, indicating that groundwater of Jiaodong Peninsula is slightly alkaline. EC varied between 393.73 and 25,032.00 μs cm−1, with the highest EC values and maximum concentrations of major ions observed in samples Q11, Q26, D19, and D32. TDS of samples ranged between 207.30 and 28,063.4 mg L−1 with a median of 602.83 mg L−1. The maximum TDS values were measured at locations Q11, Q26, D19, and D32, which appear to be related to seawater intrusion.
Since the changes in groundwater ion content will correspond to changes in TDS, TDS can to a certain extent act as an indicator of the changing characteristics of the groundwater ion composition. The spatial distribution (Fig. 3) of groundwater TDS (excluding sites Q11, Q26, D19, and D32) indicated that in general groundwater with elevated TDS was mainly distributed in coastal areas, with the highest measurements of TDS exceeding 2000 mg L−1 for sites in the northwest coastal area of Jiaozhou. Since the coastal areas are economically developed and densely populated, human activities have a significant impact on the TDS of groundwater. In addition, groundwater TDS measures of sites located northwest of Jiaodong Peninsula were higher than those of sites in the southeast.
The concentrations of HCO3− and Cl− ranged between 15.03 mg L−1–728.98 mg L−1 and 36.09 mg L−1–14,539.13 mg L−1, respectively. While HCO3− and Cl− are considered the major anions, Ca2+ and Na+ are the predominant cations. The concentrations of Ca2+ and Na+ ranged between 23.92 mg L−1–1063.82 mg L−1 and 19.86 mg L−1–8270.00 mg L−1, respectively. The order of anions and cations according to abundance was HCO3− > Cl− > SO42− > NO3− and Ca2 > Na+ > Mg2+ > K+, respectively. The introduction of NO3− to groundwater mainly originates from agricultural and industrial activities, and this variable is a major contributor to groundwater pollution (Vikas et al. 2014; Li et al. 2016a). The concentrations of NO3− among the samples ranged between 0.80 and 603.39 mg L−1, with the highest concentrations measured at locations D38, Q18, W1, and W11, corresponding to locations hosting intensive industrial activities. Figure 3 shows the spatial distribution of NO3− in groundwater (excluding Q11, Q26, D19, and D32) of Jiaodong Peninsula. The sites with the highest measures of groundwater NO3− were distributed southwest of Rongcheng and northeast of Yantai. Similarly, sites with elevated NO3− were mainly distributed in coastal areas, which indicate that human inputs are the main factor affecting groundwater quality. Groundwater with elevated COD is considered polluted (Amneera et al. 2013). COD measurements of the groundwater samples ranged between 0.51 and 135.54 mg L−1, with most samples within the WHO (2008) standard of 10 mg L−1.
The average concentrations of the main chemical components of groundwater of the Jiaodong Peninsula (excluding water sample points affected by seawater intrusion) were higher compared with those from the adjacent Futuan River Basin, parts of the North China Plain and other coastal areas of China, such as Shenzhen, Tangshan, and Leizhou Peninsula. The average concentrations of TDS, Na+ and Cl− were significantly lower than those of the Tarim River Basin within the Chinese mainland (Xing et al. 2013; Ma et al. 2014; Xiao et al. 2014; Lu et al. 2015; Xiao et al. 2015; Shi et al. 2018; Liu et al. 2019b).
Hydrochemical types
Piper trilinear diagrams have been widely used to increase the understanding of hydrogeochemical regimes and to classify and compare hydrochemical types (Wu et al. 2018). In regards to cation content, most of the groundwater samples were classified as either the calcium type or mixed type (left-hand triangle), collectively accounting for 91.25% of water samples, as shown in Fig. 4. In regards to anion concentrations, the bicarbonate, chloride, and mixed types dominated (right-hand triangle), collectively representing 96.25% of all water samples. Water samples can be further divided into four types (diamond portion). Most of the samples fell within the regions I and IV of the Piper diagram, accounting for 76.25% and 15.00% of the sites, respectively. This result indicates that the primary hydrochemical facies was of the SO4·Cl-Ca·Mg type. The SO4·Cl-Ca·Mg hydrochemical facies also indicated the influences of evaporation and anthropogenic impacts on groundwater chemistry.
Mechanisms controlling groundwater geochemistry
Gibbs diagram
Gibbs diagrams are extensively used to identify the major factors driving the chemical composition of water (Abboud 2018; Qu et al. 2019). The Gibbs diagram indicated that rock weathering, atmospheric precipitation, and evaporation are the three natural processes dominating water hydrochemistry in the Jiaodong Peninsula (Gibbs 1970). In the current study, the Na+:(Na+ + Ca2+) cation weight ratios ranged from 0.16 to 0.95, whereas the Cl−:(Cl− + HCO3−) anion weight ratios ranged from 0.16 to 0.98. Most samples fell within the central part of the Gibbs diagram, suggesting that water-rock interaction is the dominant process driving the chemical composition of groundwater (Fig. 5). On the other hand, the increase in Na+:(Na+ + Ca2+) indicated that cation exchange also has a relevant impact on groundwater hydrochemistry (Li et al. 2016a; Wu et al. 2018). The Gibbs diagram indicated that four water sample points, namely Q11, Q26, D19, and D32, are located in a seawater area, indicating that these four points are affected by seawater intrusion.
Hydrogeochemical processes
End-member diagrams were constructed for further analyses of the types of rock involved within the rock weathering processes driving groundwater hydrochemistry. Gaillardet et al. (1999) proposed end-member diagrams in which the ratios of Ca2+:Na+ were plotted against weight ratios of Mg2+:Na+ and HCO3−:Na+. In the current study, the ratios of Ca2+:Na+, Mg2+:Na+, and HCO3−:Na+ ranged between 0.06–6.18, 0.08–2.50, and 0.01–20.64, respectively. As shown in Fig. 6, silicate was well represented within the groundwater chemical composition, suggesting that the weathering of silicate is a major hydrogeochemical process controlling groundwater hydrochemistry.
Water-rock interactions play an important role in groundwater hydrochemistry. Ratio graphs of ions are a popular method for studying the concentrations of different major ions and for determining the major hydrogeochemical processes occurring in groundwater (Yang et al. 2016; Liu et al. 2018). The ratio of Na+:Cl− is close to 1 if Na+ originates mainly from halite. Figure 7 a shows that most of the samples (72.5%) were located below the 1:1 relationship line (Na+:Cl− ratios varied from 0.9 to 1.1), indicating that the dissolution of halite is not the dominant process affecting groundwater hydrochemistry of the region and that cation exchange may be a significant process influencing water chemistry (Li et al. 2016a). However, approximately 21.25% of the samples were located close to the 1:1 line, which may be due to the influence of seawater aerosols.
Relationships between ion concentrations of groundwater samples collected from the Jiaodong Peninsula. a Na+(meq/L), Cl− (meq/L); b Mg2+ + Ca2+ (meq/L), SO42− + HCO3− (meq/L); c Ca2+(meq/L), HCO3− (meq/L); d Mg2+ (meq/L), HCO3− (meq/L); e Mg2+ + Ca2+(meq/L), HCO3− (meq/L); f Mg2+ (meq/L), SO42− (meq/L); g Ca2+(meq/L), SO42− (meq/L); h Ca2+(meq/L), Mg2+ (meq/L)
A ratio of (Ca2+ + Mg2+):(HCO3− + SO42−) of ~ 1 indicates that dissolution of carbonate and sulfate are the predominant reactions affecting hydrochemistry of groundwater (Marghade et al. 2015). Samples located above the 1:1 relationship line are influenced by silicate and sulfate, and those below the line are affected by carbonate and sulfate. As shown in Fig. 7b, approximately 86.25% of the samples were located above the 1:1 line, indicating that the dissolution of silicate as well as cation exchange are the predominant processes driving the hydrochemistry of groundwater in the region.
A ratio of Ca2+: HCO3− close to 1:1 indicates the dissolution of calcite, whereas a ratio of 1:2 indicates dolomite dissolution (Li et al. 2016. As shown in Fig. 7c, most samples were located above the 1:1 line, indicating that calcite and dolomite weathering are not the dominant process. However, samples did fall in the zone between the 1:1 and 1:2 relationship line, indicating that carbonates contribute to the evolution of groundwater hydrochemistry. The bivariate diagram of Mg2+ and HCO3− (Fig. 7d) shows that most samples are located below the 1:1 line, which means that the excess in HCO3− was balanced out by other ions. The plotting of sample points in the area between the 1:1 and 1:2 lines (Ca2+ + Mg2+): HCO3− indicates that the dissolution of calcite or dolomite are the dominant reactions. However, more than 90% of the samples fell above the 1:1 relationship line (Fig. 7e), indicating that the reactions involving dissolution of carbonates are not dominant.
The Ca2+:SO42− ratios in groundwater deviated from the 1:1 line (Fig. 7 g), indicating that gypsum dissolution was not the primary source of SO42− in groundwater. In addition to rock mineral weathering, industrial and agricultural activities can also lead to elevated levels of Ca2+ and SO42− in groundwater. The ratio of Ca2+: Mg2+ has been widely used to study the dissolution of major minerals (Askri 2015; Chesnaux 2015). The sample points falling above the 1:2 relationship line indicate that the dissolution of silicate drives hydrochemistry, whereas those that lie between the 1:2 and 1:1 lines are influenced by calcite (Chesnaux 2015). Figure 7 h shows that the Ca2+: Mg2+ ratios of most of the samples were located above the 1:2 relationship line, indicating that the dissolution of silicate is the primary process affecting groundwater hydrochemistry. However, approximately 25% of the samples were situated between the 1:1 and the 1:2 line, indicating that the dissolution of calcite also occurred.
Saturation index
The mineral balance of water samples can reflect the thermodynamic processes driving hydrochemistry of a natural water system (Liu et al. 2017). The computed SI values of the water samples for dolomite, calcite, and gypsum varied from − 1.60 to 3.00, − 0.92 to 1.38, and − 3.22 to − 0.15, respectively (Table 3). As shown in Fig. 8, most of the SI values of dolomite and calcite were positive, suggesting the mineral saturation of those samples. In contrast, the SI values of anhydrite, gypsum, and halite of all water samples were negative, indicating that anhydrite, gypsum, and halite have a tendency to dissolve. The saturation index of the values of halite was relatively negative (− 2.72 to − 7.66), suggesting that the dissolution of halite was weak. In addition, the SI values were positively correlated with TDS, indicating that the dissolution and precipitation of minerals from sediments are the major processes driving the hydrochemical characteristics of the groundwater in Jiaodong Peninsula (Zhang et al. 2018).
Ion exchange
The process of ion exchange has a significant impact on the geochemistry of groundwater (Li et al. 2016a). The present study used CAI (Schoeller 1965) to analyze cation exchange in the study area, with the definitions of the indices given in Eqs. (1) and (2). CAI-I and CAI-II are negative when Ca2+ or Mg2+ in the groundwater is exchanged by Na+ or K+. However, CAI are positive if reverse ion exchange occurs (Li et al. 2018). Most of the water samples showed positive values for CAI (Fig. 9), indicating reverse reactions in groundwater of the Jiaodong Peninsula.
In addition, the ratio of (Na+ + K+ − Cl−): (Ca2+ + Mg2+ − HCO3− − SO42−) has been widely used to analyze the possible role of ion exchange in groundwater. The ratios calculated for the water samples should fall in a line with a slope of − 1 if cation exchange is the dominant process (Li et al. 2018). Figure 9 shows that water samples followed the line with a high correlation (slope = − 0.78, R2 = 0.81), indicating that cation exchange has an important impact on groundwater hydrochemistry in the Jiaodong Peninsula.
Anthropogenic activity
Shandong is one of the most developed provinces in China. The development of the economy has seen a concurrent increase in the population and an acceleration of urbanization. These developments have resulted in increasing exploitation of groundwater, resulting in changes to the regional groundwater dynamics and to groundwater quality (Li et al. 2016a; Li et al. 2018). Coastal areas tend to exhibit more developed economies and therefore higher demands for groundwater resources. Over-exploitation of groundwater in coastal areas results in a significant drop in groundwater levels, resulting in a disruption of the hydrodynamic balance between seawater and freshwater and leading to seawater intrusion. Seawater intrusion seriously affects the quality of groundwater, destroys the soil ecosystem in coastal areas, and impacts industrial and agricultural production and population health. Therefore, sustainable management of groundwater use is needed in coastal areas to prevent seawater intrusion, and strong measures should be taken to reduce groundwater extraction in areas where seawater intrusion occurs. In the present study, seawater intrusion was identified at locations Q11, Q26, D19, and D32.
The NO3−:Ca2+ and SO42−:Ca2+ ratios can act as indicators of the impacts of human activities on groundwater (Zuo et al. 2017; Zhang et al. 2018). As evident in Fig. 10a, the values of these ratios for groundwater samples allowed for the identification of two distinct impacts. The ratio of SO42−:Ca2+ indicated the impact of industrial activities on groundwater, whereas the NO3−:Ca2+ ratio indicated the influence of agricultural activities or municipal wastewater. The ratio of NO3−:Ca2+ showed a much stronger signal than that of SO42−:Ca2+ (Fig. 10a), indicating that the influence of agricultural activities or municipal wastewater on groundwater chemical composition is much more prevalent than that of industrial and mining activities.
Principal component analysis
The variables for PCA were the major cations and anions of groundwater, and a Kaiser-Meyer-Olkin value of 0.711 was obtained, indicating that the chemical variables were suitable for performing PCA. As shown in Table 4, the three most significant factors were obtained which accounted for approximately 91.43% of the total variance in the dataset. Figure 10 b shows the relationship between PCA loadings on groundwater variables.
As shown in Table 4, the first, second, and third eigenvalues had values of 5.213, 1.064, and 1.038, respectively explaining 65.12%, 13.29%, and 12.98% of the total variance, respectively, with the third eigenvalue representing the third component (Table 4). Component 1 explained 65.16% of the total variance, primarily comprising Cl−, Na+, Mg2+, SO42−, K+, and Ca2+. Component 1 likely represents the influence of common natural processes on groundwater hydrochemistry, including weathering and the dissolution of rocks and minerals. Component 2 shows positive loading for NO3− and can be attributed to anthropogenic inputs, such as agricultural and industrial inputs. Component 3 shows positive loadings for HCO3−, and likely reflects the dissolution of carbonate.
Groundwater quality evaluation
Water quality for domestic use
WQI values in the current study were calculated using Eqs. (4)–(7). Water quality indicators (TDS, Ca2+, Mg2+, HCO3−, Cl−, Na+, K+, SO42−, NO3−, pH, and COD) were assessed against the WHO limits (WHO 2008) (Table 5) to evaluate the suitability of groundwater for domestic use. To calculate WQI values, the weightings to be assigned to the indicators were determined according to the relative importance of the indicators for domestic use objectives (Table 5). Nitrates (NO3−) are an indicator of the possible presence of contaminants that can be extremely harmful to human health. NO3− is extremely soluble and can move easily through soil into groundwater (Şener et al. 2017). Therefore, the highest weighting of 5 was assigned to NO3−. The lowest weighting of 1 was assigned to HCO3− because it has the least significance for groundwater quality. The calculated weights (Wx) are shown in Table 5.
The spatial distribution of the calculated WQI values across the study area is shown in Fig. 11. The values of WQI ranged between 24.78 and1736.09, with a median of 67.82. The groundwater quality of the Jiaodong Peninsula ranged from excellent to unsuitable for domestic use. The quality of most groundwater samples was classified as excellent or good, comprising 30% and 40% of the total samples, respectively. Water samples from locations Q11, Q26, D19, and D32 were classified as unsuitable due to the impact of seawater intrusion. The quality of only 18.75% of water samples was classified as poor, with the quality of samples of W11 and D33 classified as very poor due to impacts from agricultural, industrial, or municipal wastewater discharge. In general, it was found that groundwater quality of most sites in the study area was suitable for domestic use. Increased monitoring of groundwater quality and regulation of possible pollution sources is needed to protect groundwater quality and to facilitate sustainable exploitation of groundwater resources. In addition, coastal areas should pay special attention to preventing seawater intrusion into the groundwater table.
Water quality for irrigation
Groundwater of the Jiaodong Peninsula is also used for agricultural irrigation. Groundwater suitability for irrigation is important because high levels of ions in water can affect plant growth (Nematollahi et al. 2016).
The quality of irrigation water can be categorized into four classes according to Richards (1954) (Table 6, Fig. 12a) based on salinity, namely low, medium, high, and very high salinity categories. The classification of the samples for suitability for irrigation was based on EC values, with 6.25%, 66.25%, and 27.5% of the samples classified as having very high, high, and medium salinities, respectively. The sodium or alkali hazard of groundwater is typically presented as the sodium absorption ratio (SAR). Groundwater can be classified into four categories according to the SAR, namely the low, medium, high, and very high alkali waters. The SAR values for the groundwater samples ranged from 0.48 to 49.92, with an average of 2.90, and with 95.00%, 1.25%, 1.25%, and 2.5% of the samples classified as excellent, good, doubtful, and unsuitable, respectively. As shown in Fig. 12a, 91.25% of the samples fell within the C2S1 and C3S1 zones, suggesting that groundwater has low alkalinity and medium to high salinity. Overall, groundwater of the Jiaodong Peninsula was found to be acceptable for irrigation. However, the samples plotted in C4 (6.25%) contained high salinity and were not suitable for irrigation.
Elevated Na+ content of irrigation water can promote the exchange of soil sodium with calcium and magnesium, thereby reducing soil permeability (Li et al. 2016a; Jain and Vaid 2018). Irrigation water can be classified into five categories according to the percentage sodium (Na%) (Table 6). As shown in Table 6, Na% of groundwater samples ranged from 10.69 to 77.94%, with a mean of 30.32%. A Wilcox diagram (Wilcox 1948) showed that 88.75% of the water samples could be classified as excellent, good, or permissible for irrigation, whereas 6.25% of the samples were unsuitable, 3.75% were classed as doubtful to unsuitable and only 1.25% were classed as permissible to doubtful (Fig. 12b). These results indicate that generally the groundwater of the Jiaodong Peninsula is suitable for irrigation.
The concentrations of CO32− and HCO3− in water also determine the suitability of groundwater for irrigation (Nematollahi et al. 2016). As shown in Table 6, the residual sodium carbonate index (RSC) (Eaton 1950) ranged from − 106.78 to 0.86 with an average value of − 7.70, suggesting that groundwater was suitable for irrigation. The classification of the suitability of the groundwater for irrigation according to the KR value (Kelley et al. 1940) found that groundwater of the Jiaodong Peninsula can be classified into three categories, namely suitable (KR < 1), marginally suitable (1 < KR < 2) and unsuitable (KR > 1) (Li et al. 2018). The KR values ranged from 0.12 to 3.47, indicating that most of the samples (91.25%) can be regarded as excellent for irrigation. An excess of magnesium ions in water can lead to alkaline soil and decrease crop yield (Nematollahi et al. 2016). The MR (Szabolcs and Darab 1964) of the groundwater samples was between 5.06 and 77.21, suggesting that groundwater from most sites (93.75%) was suitable for agricultural irrigation.
Conclusions
Groundwater is the main source of agricultural, industrial, and domestic water in Jiaodong Peninsula. The current study analyzed the major processes driving the hydrochemistry of groundwater of the Jiaodong Peninsula and the suitability of groundwater from this region for domestic and irrigation use. The main conclusions of the current study can be summarized as follows:
-
(1)
The orders of anions and cations in the groundwater samples in terms of content were HCO3− > Cl− > SO42− > NO3− and Ca2+ > Na+ > Mg2+ > K+, respectively. TDS of the samples ranged from 207.3 to 28,063.4 mg L−1, with elevated TDS of four samples due to seawater intrusion. The impacts of anthropogenic activities were responsible for elevated concentrations of NO3− in four samples. The concentrations of COD were in the range of 0.51 mg L−1 to 135.54 mg L−1, and most water samples were within the WHO standards.
-
(2)
Most of the groundwater samples were classified as the SO4·Cl-Ca·Mg or HCO3-Ca·Mg types. The major process influencing groundwater hydrochemistry was found to be water-rock interactions, although evaporation was also found to affect groundwater to a certain extent. The dissolution of silicate and cation exchange were the major contributors to the formation of the chemical components of groundwater. In addition, seawater intrusion was identified in the coastal area, which resulted in deterioration of groundwater quality.
-
(3)
It is clear that groundwater is affected by human activities, and the influence of agricultural activities/municipal wastewater on groundwater chemical composition is much prevalent than that of industrial and mining activities. The results of the WQI, assessment showed that the groundwater of the Jiaodong Peninsula is generally suitable for domestic purposes, with some samples found to be unsuitable for domestic use due to the influences of human activities and seawater intrusion. In addition, the quality of groundwater at most sampling sites was found to suitable for irrigation according to the calculated values of EC, SAR, Na%, RSC, KR, and MR.
References
Abboud, I. A. (2018). Geochemistry and quality of groundwater of the Yarmouk basin aquifer, north Jordan. Environmental Geochemistry and Health, 40, 1405–1435.
Adimalla, N., Li, P., & Venkatayogi, S. (2018). Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environment Process, 5(8), 1–21.
Amneera, W. A., Wahidatul, A. Z. N. N., Mohd Yusof, S. R., & Ragunathan, S. (2013). Water quality index of Perlis River, Malaysia. International Journal of Civil & Environmental Engineering, 13, 1–6.
Askri, B. (2015). Hydrochemical processes regulating groundwater quality in the coastal plain of Al Musanaah, Sultanate of Oman. Journal of African Earth Sciences, 106, 87–98.
Chesnaux, R. (2015). Scenarios of groundwater chemical evolution in a region of the Canadian Shield based on multivariate statistical analysis. Journal of Hydrology: Regional Studies, 4, 246–266.
Colado, D. M., Carreño, F., Rasines, R., & Iepure, S. (2018). Hydrogeochemical characterization of a shallow alluvial aquifer: 1 baseline for groundwater quality assessment and resource management. Science of the Total Environment, 639, 1110–1125.
Deepa, S., & Venkateswaran, S. (2018). Appraisal of groundwater quality in upper Manimuktha sub basin, Vellar river, Tamil Nadu, India by using Water Quality Index (WQI) and multivariate statistical techniques. Modeling Earth Systems and Environment, 4, 1165–1180.
Eaton, F. M. (1950). Significance of carbonates in irrigation waters. Soil Science, 69, 123–134.
Gaillardet, J., Dupré, B., Louvat, P., & Allègre, C. J. (1999). Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chemical Geology, 159, 3–30.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 170, 870.
Jain, C. K., & Vaid, U. (2018). Assessment of groundwater quality for drinking and irrigation purposes using hydrochemical studies in Nalbari district of Assam, India. Environment and Earth Science, 77, 254.
Kelley, W. P., Brown, S. M., & Liebig, G. F. J. (1940). Chemical effects of saline irrigation water on soils. Soil Science, 49, 95–108.
Li, P. Y., Wu, J. H., & Qian, H. (2016a). Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua County, China. Arabian Journal of Geosciences, 9, 1–17.
Li, P. Y., Zhang, Y. T., Yang, N., Jing, L., & Yu, P. (2016b). Major ion chemistry and quality assessment of groundwater in and around a mountainous tourist town of China. Exposure Health, 8(2), 239–252.
Li, X., Tang, C. Y., Han, Z. W., & Cao, Y. J. (2016). Hydrochemical characteristic and interaction process of surface and groundwater in mid-lower reach of Hanjiang River, China. Environment and Earth Science, 75, 418.
Li, X. Y., Wu, H., Qian, H., & Gao, Y. Y. (2018). Groundwater chemistry regulated by hydrochemical processes and geological structures: a case study in Tongchuan, China. Water, 10, 338.
Liu, J. T., Gao, Z. J., Wang, M., Li, Y. Z., Ma, Y. Y., Shi, M. J., & Zhang, H. Y. (2018). Study on the dynamic characteristics of groundwater in the valley plain of Lhasa City. Environment and Earth Science, 77, 646.
Liu, J. T., Gao, Z. J., Wang, M., Li, Y. Z., Ma, Y. Y., Shi, M. J., & Zhang, H. Y. (2019a). Hydrochemical characteristics and possible controls in the groundwater of the Yarlung Zangbo River Valley, China. Environment and Earth Science, 78, 76.
Liu, J. T., Feng, J. G., Gao, Z. J., Wang, M., Li, G. H., Shi, M. J., & Zhang, H. Y. (2019b). Hydrochemical characteristics and quality assessment of groundwater for drinking and irrigation purposes in the Futuan River Basin, China. Arabian Journal of Geosciences, 12, 560.
Liu, P., Hoth, N., Drebenstedt, C., Sun, Y., & Xu, Z. (2017). Hydro-geochemical paths of multi-layer groundwater system in coal mining regions - using multivariate statistics and geochemical modeling approaches. Science of the Total Environment, 601-602, 1–14.
Lu, Y. T., Tang, C. Y., Chen, J. Y., & Chen, J. H. (2015). Groundwater recharge and hydrogeochemical evolution in Leizhou Peninsula. Chinese Journal of Chemistry, 2015(2015), 1–12.
Ma, F. S., Wei, A. H., Deng, Q. H., & Zhao, H. J. (2014). Hydrochemical characteristics and the suitability of groundwater in the coastal region of Tangshan. Journal of Earth Sciences, 25(6), 1067–1075.
Marghade, D., Malpe, D. B., & Rao, N. S. (2015). Identification of controlling processes of groundwater quality in a developing urban area using principal component analysis. Environment and Earth Science, 74, 5919–5933.
Nematollahi, M. J., Ebrahimi, P., Razmara, M., & Ghasemi, A. (2016). Hydrogeochemical investigations and groundwater quality assessment of Torbat-Zaveh plain, Khorasan Razavi, Iran. Environmental Monitoring and Assessment, 188, 2.
Qu, B., Zhang, Y. L., Kang, S. C., & Sillanp, M. (2019). Water quality in the Tibetan Plateau: major ions and trace elements in rivers of the “Water Tower of Asia”. Science of the Total Environment, 649, 571–581.
Rabeiy, E. S. (2018). Assessment and modeling of groundwater quality using WQI and GIS in Upper Egypt area. Environmental Science and Pollution Research, 25(31), 30808–30817.
Richards, L. A. (1954). Diagnosis and improvement of saline and alkali soils. Agricultural Handbook No. 60. Washington DC: United States Department of Agriculture.
Schoeller, H. (1965). Qualitative evaluation of groundwater resources. In Methods and techniques of groundwater investigation and development. Water Research Series (Vol. 33, pp. 54–83). UNESCO: Paris, France.
Şener, Ş., Şener, E., & Davraz, A. (2017). Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Science of the Total Environment, 584-585, 131–144.
Shi, X. Y., Wang, Y., Jiao, J. J., Zhong, J. L., Wen, H. G., & Dong, R. (2018). Assessing major factors affecting shallow groundwater geochemical evolution in a highly urbanized coastal area of Shenzhen City, China. Journal of Geochemical Exploration, 184, 17–27.
Singh, C. K., Kumar, A., Shashtri, S., Kumar, A., Kumar, P., & Mallick, J. (2017). Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. Journal of Geochemical Exploration, 175, 59–71.
Szabolcs, I., & Darab, C. (1964). The influence of irrigation water of high sodium carbonate content of soils. In Proceedings of 8th international congress of ISSS, transmission (Vol. 2, pp. 803–812).
Tiwari, A. K., Ghione, R., Maio, M. D., & Lavy, M. (2017). Evaluation of hydrogeochemical processes and groundwater quality for suitability of drinking and irrigation purposes: a case study in the Aosta Valley region, Italy. Arabian Journal of Geosciences, 10, 264.
Vikas, C., Kushwaha, R., Ahmad, W., Prasannakumar, V., Dhanya, P. V., & Reghunath, R. (2014). Hydrochemical appraisal and geochemical evolution of groundwater with special reference to nitrate contamination in aquifers of a semi-arid terrain of NW India. Water Quality Exposure and Health, 7, 1–15.
WHO. (2008). Guidelines for drinking-water quality. Geneva: World Health Organization.
Wilcox LV (1948) The quality of water for irrigation use. United States Department of Agriculture, Washington DC, Tech Bull 1962
Wu, C., Wu, X., Qian, C., & Zhu, G. (2018). Hydrogeochemistry and groundwater quality assessment of high fluoride levels in the Yanchi endorheic region, northwest China. Applied Geochemistry, 98, 404–417.
Xiao, J., Jin, Z. D., & Wang, J. (2014). Assessment of the hydrogeochemistry and groundwater quality of the Tarim River Basin in an extreme arid region, NW China. Environmental Management, 53(1), 135–146.
Xiao, J., Jin, Z. D., Wang, J., & Zhang, F. (2015). Hydrochemical characteristics, controlling factors and solute sources of groundwater within the Tarim River Basin in the extreme arid region, NW Tibetan Plateau. Quaternary International, 380-381, 237–246.
Xing, L. N., Guo, H. M., & Zhan, Y. H. (2013). Groundwater hydrochemical characteristics and processes along flow paths in the North China Plain. Journal of Asian Earth Sciences, 70-71, 250–264.
Xing, L. T., Huang, L. X., Hou, X. Y., Yang, L. Z., Chi, G. Y., Xu, J. X., & Zhu, H. H. (2018). Groundwater hydrochemical zoning in inland plains and its genetic mechanisms. Water, 10, 752.
Yang, Q. C., Li, Z. J., Ma, H. Y., Wang, L. C., & Martín, J. D. (2016). Identification of the hydrogeochemical processes and assessment of groundwater quality using classic integrated geochemical methods in the Southeastern part of Ordos basin, China. Environmental Pollution, 218, 879–888.
Yi, W. H., Wang, S. T., Wu, Z., & Song, W. (2015). Assessment of groundwater environmental quality in Huancui District of Weihai city. Shangdong Land and Resources, 31, 43–45.
Yin, Z. Y., Lin, Q., & Xu, S. H. (2018). Spatial-temporal variations and controlling factors of groundwater hydrochemical characteristics in the Dagu River basin. Geological Review, 4, 1030–1043.
Yu, L. H. (2018). Comprehensive assessment of groundwater quality in Yantai City in Shandong Peninsula Blue Economic Zone. Shangdong Land and Resources, 35, 71–77.
Zhang, Y. H., Xu, M., Li, X., Qi, J. H., Zhang, Q., Guo, J., Yu, L. L., & Zhao, R. (2018). Hydrochemical characteristics and multivariate statistical analysis of natural water system: a case study in Kangding County, Southwestern China. Water, 10, 80.
Zuo, Y. Z., An, Y. L., Wu, Q. X., Qu, K. J., Fan, G. H., Ye, Z. X., Qin, L., Qian, J. T., & Tu, C. L. (2017). Study on the hydrochemical characteristics of Duliu River basin in Guizhou Province. China Environmental Science, 37, 2684–2690.
Acknowledgments
The authors sincerely thank the researchers and staff members for their help and thank editors and reviewers for reviewing the manuscript.
Funding
This research was supported by the Shandong Geological Environmental Monitoring Station, Qingdao Geologic-engineering Exploration Institute, the First Institute of Oceanography, Ministry of Natural Resources, and the National Natural Science Foundation of China (U1806212; 41406072).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Gao, Z., Wang, Z. et al. Hydrogeochemical processes and suitability assessment of groundwater in the Jiaodong Peninsula, China. Environ Monit Assess 192, 384 (2020). https://doi.org/10.1007/s10661-020-08356-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-08356-5