Abstract
In mining areas, fresh groundwater plays an essential role in meeting the water needs for coal production, agriculture, animal husbandry, and human consumption. The objective of this study is to obtain a deeper understanding of the groundwater evolution processes, and to assess the groundwater quality and its suitability for domestic and irrigation purposes in Chenqi coalfield, located in the Hulunbuir grassland of Inner Mongolia, China. 21 groundwater samples were collected from three different landforms (high plain, plain, and river valley) for major ions and trace metals analyses. General hydrochemistry, correlation, and hierarchical cluster analysis methods were employed. The results show that the groundwater is predominantly of the HCO3–Na·Ca type and HCO3–Ca·Na in the plain and river valley areas, and of the Cl·(HCO3·SO4)–Na·Ca in the high plain area. The groundwater circulation is regulated by natural processes: in the plain and river valley areas, rock weathering, dissolution of silicates (especially plagioclase), and reverse cation exchange are the major hydrogeochemical processes occurring, whereas, in the high plain area, evaporation and dissolution of halite, silicates, and gypsum are the main hydrogeochemical processes. Besides, mining activities have a certain impact on groundwater quality due to the change of groundwater circulation conditions. Groundwater in the plain and river valley areas is suitable for drinking and irrigation purposes, with low concentrations of all parameters. However, in the high plain area, total hardness (TH), high total dissolved solids (TDS), and Cl− and SO42− concentrations limit the suitability of the groundwater for drinking purposes, while high EC and Na concentration makes it unsuitable for irrigation. These results aid in sustainable management of water resources in the study area, and provide a reference for the sustainable utilization of water resources and the rational exploitation of natural resources in other coal mining areas of the world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global demand for freshwater has raised significantly in recent times owing to population explosion, industrial development, and intensive irrigation activities (Rajesh et al. 2015). The availability of freshwater is particularly crucial in mining areas (Li et al. 2013; Roisenberg et al. 2016); however, mining activities threaten the quality and quantity of groundwater resources in many parts of the word. For example, Choubey (1991), Gupta (1999), Singh (1988), and Tiwary (2001) studied the impacts of coal mining on the water quality and water regimes in certain locations in India. Similar investigations of the effects of mining on groundwater quality have been conducted in Turkey (Agartan and Yazicigil 2012; Arkoc et al. 2016), China (Wu et al. 2017a, b) and in some other countries (Nair et al. 2017; Utom et al. 2013). These studies have shown that groundwater quality has been significantly influenced by the effects of coal mining.
In addition, the quality of groundwater can also be affected by a range of climatic and geological factors and by anthropogenic pollution. The hydrochemical characteristics of groundwater are a key indicator for assessing its quality and possible changes therein (Huang et al. 2017). The water chemistry in any given area depends on both the geological and hydrogeological conditions and the intensity of human activities (Towfiqul Islam et al. 2017; Li et al. 2017a, b; Wu et al. 2017b), which vary significantly in different environments. Therefore, it is essential that a full understanding is developed of the hydrogeochemical processes occurring in a groundwater resource to effectively manage the system.
The Piper trilinear diagram (Piper 1944) is a traditional method for classifying groundwater on the basis of its chemical composition and is still widely utilized (Hutchins et al. 1999; DelgadoOuteiriño et al. 2009). The Gibbs diagram (Gibbs 1970) uses the relationships between TDS and Na/(Na + Ca) and Cl/(Cl + HCO3), respectively, to assess the probable sources of chemical constituents in groundwater (Huang et al. 2017). This plot in combination with other mathematical statistical methods can help to identify the processes that have influenced the developed of groundwater quality.
Correlation relationships between chemical elements can also be used to study the effects of water–rock interaction on groundwater chemistry (Redwan et al. 2016). Another commonly used method is hierarchical cluster analysis (HCA), which is a powerful statistical method that has been used to understand the sources of chemical constituents and the factors that have influenced groundwater quality in many parts of the world including Turkey (Kurunc et al. 2016), India (Patel et al. 2016), and Malaysia (Fulazzaky et al. 2010).
In China, which is the focus of the current study, much research has been conducted to assess groundwater quality and its suitability for domestic and irrigation purposes (Li et al. 2012; Chen and Feng 2013). The coal industry is of primary concern to the quality of groundwater here, as there are a large number of coal mines in China. Coal is comfortably China’s primary energy source, contributing up to 70% of the national energy production (Zhang et al. 2009). With the rapid development in the country’s economy, coal mines in the eastern grasslands have gradually been exploited. However, these grasslands are an ecologically vulnerable zone, and it is difficult to guarantee the harmonious development between human beings and the environment (Huang et al. 2017; Li et al. 2017a, b). It is, therefore, of key importance to obtain a full understanding of the evolution processes and the groundwater quality in the mining areas.
The present study relates specifically to the Chenqi coalfield, located in the largest grassland in China—namely, the Hulunbuir grassland in Inner Mongolia. Here, groundwater is the main source of domestic drinking and irrigation water, but the groundwater evolution processes in this region and the water quality are little documented and understood.
Against this backdrop, therefore, the aim of this study is to identify the groundwater evolution processes, and to assess the suitability of water quality for domestic and irrigation purposes in the Chenqi coalfield. The results obtained provide a reference for the better management of groundwater resources and for the rational exploitation of natural resources in the study area.
Materials and methods
Location, climate, and hydrology
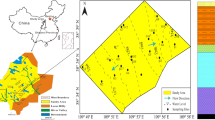
The study area in the Chenqi coalfield is located at a latitude of 49°06′N–49°42′N and longitude of 119°08′E–120°18′E (Fig. 1). Chenqi coalfield is one of the four large coal fields in Hulunbuir city and is characterized by its low-sulfur content and its high calorific value. The study covers an area of 3995 km2, with an east-to-west length of about 80 km and a north-to-south width of about 50 km. The average elevation of the undulating plain ranges from 600 to 750 m above the mean sea level. There are three types of landforms in this area: high plain (I), plain (II), and river valley (III) (Fig. 1). Animal husbandry (such as cattle and sheep rearing), planting (such as wheat and oilseed rape), and coal mining are the three traditional pillar industries in this area.
The study area belongs to a temperate continental semiarid climatic zone characterized by long cold winter and short hot summer. The annual mean temperature is – 1.5 °C, with the lowest temperature occurring in January and the highest temperature occurring in June. Typically, the ground is covered with snow for about 6 months a year from early November to early May. The average annual rainfall is 346.8 mm and the average annual evaporation is 1329.0 mm. The rainy season is from June to September. Rainfall during the rainy season accounts for 90% of the annual precipitation.
In this area, the surface water system is part of the Argun River system. The major rivers are the Hailaer River flowing from east to west and the Mo River, which is a branch of the Hailaer, flowing from north to south. Both the Hailaer and Mo rivers merge into the Argun River. The run-off of the Hailaer River is about 34.76 × 108 m3/a, and that of the Mo River is about 0.994 × 108 m3/a. The surface water from these rivers is one of the major water sources for domestic and irrigation purposes in this area.
Geology and hydrogeology
Quaternary formations, including Holocene alluvium and diluvium (Q al+pl4 ) and an upper Pleistocene ice accumulation layer (Q fgl3 ), cover the whole study area. The Holocene alluvium and diluvium are widely distributed with a thickness of 3–20 m in the Hailar and Mo river valley areas and in the plain area, and the strata are chiefly composed of white sandy pebbles and clay-bearing sandy gravels. The upper Pleistocene ice accumulation layer covers the whole high plain area with a thickness of 1–42 m, which mostly consists of yellow silty soil and medium fine-grained sand.
Porous-medium aquifers in unconsolidated Quaternary sediments and pore-fissure aquifers in Cretaceous bedrock are the major sources of groundwater in the area. The groundwater in the Quaternary shallow aquifer is used mainly for domestic and irrigation purposes, whereas that in the Cretaceous deep aquifer is not used. Groundwater in this area generally flows from northeast to southwest and is recharged primarily by atmospheric precipitation.
Water sampling and laboratory analysis
Twenty-one groundwater samples (G1–G21, seven samples each from the three landforms with different surface elevations) were collected from different locations in the shallow aquifers in July 2017. Pre-existing wells were used, as these are easy to sample. The exact sampling locations were recorded utilizing a portable GPS device (Fig. 1). Groundwater samples were collected after pumping wells of 10–80 m depth for 2–3 min to remove the stale water. The sampled water was stored in 1 L white plastic bottles. They were rinsed 2–3 times using the fresh groundwater to be sampled before sampling. Then, the sampling bottles were sealed, labeled, and delivered to the laboratory for physicochemical analysis within 24 h. All groundwater samples were measured and analyzed for pH, electric conductivity (EC), total hardness (TH), total dissolved solids (TDS), major ions (K+, Na+, Ca2+, Mg2+, Cl−, SO42−, HCO3−, F−, NO3−, NO2−), trace metals (Cu, Mn, Cr, Hg, Pb, Cd, As), and chemical oxygen demand (CODMn). TDS and pH were measured in situ utilizing a portable meter (Shanghai Sanxin Company, Shanghai, China). Before testing, all groundwater samples were filtered through a 0.45 μm membrane filter in the laboratory to remove suspended particulates. Major anions (SO42−, Cl−, NO3−, NO2−, and F−) were measured employing anion chromatography with anion columns (Thermo Company, Waltham, USA), while major cations (K+, Na+, Ca2+, and Mg2+) were analyzed using cation chromatography with cation columns. The EDTA titrimetric method was used for TH analysis and CODMn was measured by titrating with potassium permanganate. Inductively coupled plasma mass spectrometry (ICP-MS) was utilized to measure the trace metals content of the samples. The charge balance errors for all the groundwater samples were calculated to check the analytical accuracies. The calculated charge balance errors (%CBE) for all groundwater samples were within the upper error limit of 5% (Wu et al. 2014).
Results and discussion
General hydrochemistry
The analysis results of physicochemical parameters of the groundwater samples from the three landforms are given in Table 1.
The table shows that the pH of all groundwater samples ranged from 6.52 (slightly acidic) to 7.78 (slightly alkaline), with a mean of 7.38. The TDS of the groundwater samples ranged between 108.85 and 2240.34 mg/L with a mean of 754.69 mg/L, and the EC of the groundwater samples varied from 168.19 to 3540.00 μS/cm with an average value of 1081.65 μS/cm. Both TDS and EC were highest in the high plain area, where the groundwater is brackish (defined as having a TDS within the range of 1000–10,000 mg/L) and most of the groundwater has unacceptable quality for irrigation, with EC > 2250 μS/cm. In the plain and river valley areas, all groundwater samples could be classified as being fresh water (TDS < 1000 mg/L), which indicates that the groundwater is suitable for domestic and irrigation uses according to the WHO (2017) guidelines. The TH of groundwater in this study varied from 75.33 to 831.05 mg/L with a higher value in the high plain compared to the plain and river valley areas. Consequently, according to the Chinese Standards for Drinking Water Quality (2006), this indicates that the groundwater is soft (TH < 150 mg/L)-to-slightly hard (TH within the range of 150–300 mg/L) in the plain and river valley areas and is very hard (TH > 450 mg/L) in the high plain area (Li et al. 2010). Long-term drinking of hard or very hard water may lead to ailments such as cardiovascular disease, anencephaly, and other health problems (Chabukdhara et al. 2017). Therefore, the groundwater in the high plain area is not considered to be suitable for potable use. The variations in the EC, TH, and TDS concentrations in the different groundwater samples may be attributed to variations in the lithology and hydrogeological processes in the study area (Mahato et al. 2018).
The predominant cations in the groundwater samples were Ca2+, Mg2+, and Na+. The concentration of Na+ varied from 9.50 to 501.20 mg/L with an average of 163.27 mg/L, the Ca2+ concentration ranged from 22.22 to 194.39 mg/L (avg. 64.46 mg/L), and the Mg2+ concentration varied from 1.70 to 133.46 mg/L (avg. 33.72 mg/L). The concentrations of Ca2+, Mg2+, and Na+ were highest in the high plain area. The order of major cation concentrations in the water samples was Na+ > Ca2+ > Mg2+ in the high plain and plain areas, and Ca2+ > Na+ > Mg2+ in the river valley area. On average, the concentrations of Na+, Ca2+, and Mg2+ accounted for 93.3–99.7% (avg. 98%) of the total cations (TZ+) in the groundwater, and are likely derived from the dissolution of silicates containing minerals such as plagioclase, albite, and anorthite (Singh et al. 2010). The concentrations of Ca2+ and Mg2+ determine the hardness of the groundwater.
The predominant anions in the groundwater samples were HCO3−, Cl−, and SO42− with minor amounts of NO3− and F− also present in the high plain samples. The major anion in the river valley and plain areas was HCO3−. Taking all groundwater samples into account, HCO3− concentrations varied from 115.10 to 741.35 mg/L (avg. 338.77 mg/L), Cl− concentration ranged from 2.00 to 779.77 mg/L (avg. 169.09 mg/L), and SO42− concentration ranged from < 0.75 mg/L to 506.89 mg/L (avg. 133.20 mg/L). The concentrations of HCO3−, Cl−, and SO42− were much higher in the high plain area than in the plain and river valley areas. The order of major anion concentrations in the water samples in the high plain area was Cl− > SO42− > HCO3− and concentration of Cl− accounted for 42.5–57.4% (avg. 51%) of the total anions (TZ−). In the plain and river valley areas, the order was HCO3− > Cl− > SO42− and HCO3− accounted for 72.2–93.7% (avg. 85%) of TZ−. HCO -3 in the groundwater is likely from the dissolution of silicate minerals such as anorthite and albite, SO42− may be from the dissolution of anhydrite (CaSO4) or gypsum (CaSO4·2H2O), and Cl− may be from the dissolution of halite (Singh et al. 2010).
The concentration of F− in the groundwater samples ranged from 0.17 to 1.28 mg/L (avg. 0.53 mg/L). Except for two groundwater samples in the plain area with a higher concentration of F−, its concentration was generally low in this area (Table 1). The NO3− concentration ranged from < 0.018 mg/L to 41.19 mg/L with a mean of 3.78 mg/L and only one water sample located in a village has a very high concentration, indicating that concentrations of this anion easily meet the 50 mg/L threshold for NO3− in potable water recommended by the WHO (2017). Consequently, there appears to be negligible impacts of human land use on groundwater quality in this area.
Hydrogeochemical facies
The Piper trilinear diagram is a graphical representation of hydrochemical data which is helpful in determining connections between different dissolved constituents and for classifying groundwater based on its chemical characteristics (Tiwari et al. 2017). The method was applied to the results of the current study (Fig. 2), and shows that the groundwater samples fall into zones IV, III, and II, demonstrating the dominance of weak acids (HCO3−) over the strong acids (SO42− and Cl−) in the plain and river valley areas and of the alkalis elements (Na+ and K+) over the alkaline earth elements (Ca2+ and Mg2+) in the plain and high plain areas. The combined SO42− and Cl− concentration in the plain and river valley areas is very low compared to groundwater from the high plain area. This suggests that gypsum and halite are not dominant minerals in the lithology. The low combined concentration of these ions may also be due to less human activity in the plain and river valley areas. Groundwater samples were mainly classified as Cl·(HCO3·SO4)–Na·Ca type in the high plain area, and as HCO3–Na·Ca type and HCO3–Ca·Na types in the plain and river valley areas. This indicates that the main processes occurring in the plain and river valley areas are the dissolution of silicate minerals such as albite and anorthite and the dissolution of halite, silicate minerals, and gypsum in the high plain area.
Ion correlation analysis
In the groundwater, different chemical constituents in groundwater are not independent, and correlations among different parameters can be used to explore the sources of the groundwater (Singh et al. 2010). A Pearson correlation matrix of physicochemical parameters of the groundwater samples was calculated (Table 2).
As shown in the table, Ca2+ is strongly correlated with SO42− (with correlation coefficients of 0.866, p < 0.05), suggesting that the dissolution of gypsum is the dominant process controlling the groundwater chemical characteristics in high plain area. Meanwhile, Na+ is also strongly correlated with HCO3− (with correlation coefficients of 0.984 (p < 0.01) and 0.904 (p < 0.01), respectively) in the plain and river valley areas, suggesting that the dissolution of silicate minerals is an important contributing factor to the groundwater chemistry in these areas.
Hierarchical cluster analysis (HCA) of groundwater
Hierarchical cluster analysis (HCA) is a widely used statistical tool for analyzing the origins and influencing factors of groundwater chemistry. There are two HCA modes, known as the R and Q modes. HCA was employed to the data obtained in the present study based on the Ward method (Dolnicar 2002) (Fig. 3). The R-mode HCA was used to classify the nine groundwater quality variables into two clusters. Cluster 1 indicates natural processes of groundwater, which are responsible for the presence of NO2−, F−, NO3−, Ca2+, and Mg2+. Cluster two suggests processes of gypsum, silicate, and halite dissolution, which are responsible for the presence of Na+, Cl−, SO 2-4 , and HCO3−. Overall, it appears that the groundwater chemical constituents in this area are regulated by natural processes based on the R-mode HCA.
The Q-mode HCA was utilized to classify all the groundwater samples into two clusters. Samples G8–G21 belong to cluster 1, and the remaining samples (G1–G7) belong to cluster 2. The concentrations of TDS, TH, Na+, SO42−, and Cl- in cluster 2 are much higher than those in cluster 2. As shown in Fig. 1, the cluster 1 samples were collected from the high plain area with a low groundwater renewal rate, where groundwater flows slowly due to the low permeability of sediments in the area which mostly consist of silts and fine-grained sands. The remaining samples were collected from the plain and river valley areas close to the groundwater discharge area (the Mo River). Groundwater recharge rates are also likely to be much higher in these areas. Therefore, it appears that groundwater evolution is to a large degree controlled by the nature of the geological formations and the local hydrogeological conditions.
Due to the limitation of sampling conditions, the size of groundwater samples is only 21, which is small for such a large area (Formann 1984; Dolnicar 2002). It may affect the accuracy of clustering results due to the high data dimensionality. Therefore, sampling should be continued later if conditions permit.
Water–rock interaction
From the preceding discussion, it may be inferred that groundwater chemistry is mostly controlled by natural processes in the study area. Consequently, a Gibbs diagram was used to investigate the natural factors that influenced the chemical composition of groundwater in the area (Huang et al. 2017; Li et al. 2016). The Gibbs diagram indicates whether the chemical composition of groundwater in an area is dominantly influenced by the effects of evaporation, by water–rock reactions, and or by the effects of precipitation. In addition to the Gibbs diagram, the ratio of Mg2+/Na+ and Mg2+/Ca2+ can also be used to determine whether evaporation and rock interaction are likely to be influencing groundwater quality (Li et al. 2018).
A Gibbs diagram and Mg/Na versus Mg/Ca diagrams were applied to the data obtained in this study and the results’ area is shown in Fig. 4a, b, respectively. These diagrams suggest that rock weathering is the main mechanism controlling the chemical constituents of groundwater in the plain and river valley areas, whereas groundwater quality in the high plain area appears to be mainly governed by evaporation and rock weathering in some localized areas.
An important process of water–rock interaction is mineral dissolution (Li et al. 2018) and the assessment of ionic milliequivalent ratios can indicate which minerals are likely to have the largest influence on groundwater quality. For example, the dissolution of halite is often indicated by a 1:1 value of the ratio of Na+ vs Cl−, the dissolution of calcite, dolomite, and gypsum is indicated by a 1:1 value of the ratio of (Ca2+ + Mg2+) vs (HCO3− + SO42−), and the dissolution of silicate, especially plagioclase, is indicated by the ratio of ∑ cations (Ca2+ + Mg2+ + Na+ – Cl−) vs HCO3− (Kim 2003).
As shown in Fig. 5a, b, the milliequivalent ratios of Na+ vs Cl− and (Ca2+ + Mg2+) vs (HCO3− + SO42−) in most water samples are close to 1:1, suggesting that the dissolution of halite and gypsum is the main processes that release these ions into groundwater in the high plain area. However, the ratio of Na+ vs Cl– and (Ca2+ + Mg2+) vs (HCO3− + SO42−) in samples deviates from the theoretical 1:1 line, suggesting that silicate dissolution is a possible source of Na+ and HCO3− in the groundwater. Figure 5c suggests that the dissolution of silicates, especially plagioclase, is the dominant reaction that gives rise to the elevated concentrations of Ca2+, Na+, and HCO3− present in groundwater in the area. This is further supported by a mineralogical analysis of sandy sediments in the area which indicate that they are predominantly composed of quartz and plagioclase. In addition, there are some deviations from the 1:1 line, which shows that other processes such as cation exchange reaction may also occur here.
The extent to which groundwater is saturated or undersaturated with respect to a number of key minerals can also influence the chemical composition of groundwater. The saturation indices (SI) of major minerals (Li et al. 2013) were calculated and are shown in Fig. 6. If SI is greater than zero, the minerals in groundwater are oversaturated with respect to that mineral and it may be precipitated from solution. Conversely, if SI is less than zero, the groundwater is unsaturated with respect to the mineral and it may be dissolved (Wang et al. 2017).
Figure 6a shows that the SI values of the dolomite were positive in five of the groundwater samples and the SI values of the calcite were positive in three of the groundwater samples collected from the high plain area, indicating that groundwater is saturated with respect to these mineral phases in the area. However, the corresponding SI values were negative in the remaining groundwater samples, indicating the potential for carbonate minerals to be leached (if present) from sediments elsewhere in the study area. As shown in Fig. 6b, both gypsum and halite are unsaturated in all groundwater samples, showing that these minerals may also continue to dissolve in groundwater if they are ubiquitous in the study area.
Cation exchange
Another important process for controlling the chemical constituents in groundwater is cation exchange (Zhu et al. 2011; Wu and Sun 2016). To study the occurrence of cation exchange, Schoeller (1965) proposed two chloro-alkaline indices: CAI-1 and CAI-2, which can be calculated by the following formula:
Both indices are positive if an exchange between Na+ in the groundwater and Ca2+ in the aquifer materials occurs, as expressed in Eq. (3), whereas negative values indicate reverse cation exchange, as expressed in Eq. (4). As shown in Fig. 7a, the CAI-1 and CAI-2 values of all groundwater samples from the plain and river valley areas were negative, demonstrating that reverse cation exchange between groundwater and rock materials is likely to be another important process influencing groundwater quality in the area:
Furthermore, when cation exchange occurs, the relationship between milliequivalent concentrations of (Na+ + K+ − Cl−) and [(Ca2+ + Mg2+) − (HCO3− + SO42−)] should be linear with a slope of −1 (Wang et al. 2017). Figure 7b shows that their relationship can be fitted to the straight line Y = − 0.9036X – 0.1477 with a high correlation coefficient of 0.9723 (p < 0.01), which strongly suggests the occurrence of cation exchange in this area.
Mining activities
Mining activities can affect geochemical characteristics and groundwater quality (Li 2018). The annual output of all the coal mines in the study area is about 50 million tons, one of which is an open pit mine with an annual output of 35 million tons (Fig. 1). Due to the long-term mining of coal mines, the shallow groundwater around them has been basically drained. To analyze the influence of coal mining on groundwater, groundwater samples near the mining area were compared with samples far from mining area. In the plain area, there are four samples (G10, G12, G13, and G14) near the mining area and three samples (G8, G9, and G11) relatively far from the mining area. As shown in Fig. 8, the concentration of Na+, HCO3−, and TDS in groundwater near the mining area is higher than that far from mining area. The groundwater level around the mining area decreases and the thickness of aeration zone increases due to the drainage of groundwater by mining activities. Silicate minerals in the aerated zone dissolve continuously, which increases the concentration of Na+, HCO3− in groundwater under the continuous leaching of atmospheric rainfall. As a result, the TDS concentration in groundwater increased. Therefore, mining activities have a certain impact on groundwater quality.
Water quality assessment
The concentrations of chemical constituents in groundwater samples from this area were compared with the Chinese Standards for Drinking Water Quality (2006) and the WHO (2017) guidelines for drinking water quality. The pH of all groundwater samples was within the desirable limit for drinking (6.5–8.5). For drinking purposes, WHO (2017) guidelines state that the maximum permissible TDS is 1000 mg/L and Chinese national guidelines state that the maximum permissible TH is 450 mg/L. The TDS and TH values of the groundwater samples were above the permissible limits only in the high plain area. The Cl− and SO42− concentrations were very low in the groundwater collected from the plain and river valley areas, and were both within the WHO acceptable limit for drinking purposes (250 mg/L for each ion). In the high plain area, however, these concentrations were well over the limit. F− concentrations were below the Chinese limit (1 mg/L) for all the samples except G12 and G13. The concentrations of trace metals analyzed in all samples were very low and all below the permissible limits. Overall, it is inferred that the groundwater in the plain and river valley areas close to the Mo river is suitable for domestic uses at present, whereas groundwater in the high plain area must be treated before it can be used for drinking. The better quality of the plain and river valley water is most likely due to less human and animal activities in this area.
The electrical conductivity (EC) and Na content play an important role in classifying water for irrigation purposes (Mahato et al. 2018). Irrigation water with a high salt content not only directly affects plant growth, but also indirectly affects plant growth by increasing the osmotic pressure of the soil solution, thus affecting the structure, permeability, and aeration of the soil (Singh et al. 2010).
The sodium adsorption ratio (SAR), or alkali hazard, is determined by the absolute and relative concentration of cations, and is calculated according to the formula:
Percent Na (Na%) is widely utilized for assessing the suitability of groundwater quality for irrigation purpose (Wilcox 1955), and is calculated according to the formula:
The quantity of bicarbonate and carbonate ions (HCO3− and CO32−) in excess of alkaline earths (Ca2+ and Mg2+) also affects the suitability of groundwater for irrigation purpose. Residual sodium carbonate (RSC) can quantify the effects of carbonate and bicarbonate, and is calculated according to the following:
Water quality classification standards for irrigation purpose, based on SAR, EC, Na%, and RSC, are given in Table 3.
In this study, the calculated RSC value was < 2.5 meq/L except G1, G13, and G14, indicating that the groundwater is suitable for irrigation use. The SAR values of all groundwater samples ranged from 0.41 to 10.78, indicating that they are almost of excellent quality. As shown in the USSL diagram (Fig. 9a), groundwater samples fall into zones C1S1, C2S1, and C3S1 in the plain and river valley areas and zones C4S1, C3S2, and C4S2 in the high plain area. The Na% of all groundwater samples except G1, G2, G4, and G14 varied from 18.4 to 58.4%. As shown in the Wilcox diagram (Fig. 9b), the groundwater samples fall within the “excellent”-to-“good” quality zones in the plain and river valley area and within the “doubtful”-to-“unsuitable” quality zones in the high plain. Overall, the groundwater is suitable for irrigation only in the plain and river valley areas and not in the high plain area.
A high salt content in groundwater is a common phenomenon in arid and semiarid regions, where groundwater loss through evaporation is high. There are plenty of croplands in the high plain area where the groundwater drainage is poor. Long-term irrigation can, therefore, raise the water table close to the surface, which will cause the salts accumulation in the soil by evaporation (Mahato et al. 2018). This is evidenced by the fact that the local climatic conditions with the average annual evaporation being much greater than the average annual rainfall. High EC values can cause saline soils, and high Na concentration can lead to an alkaline soil. Consequently, if such groundwater is used for irrigation, these factors could cause a deterioration in soil quality and a reduction in agricultural production over the long term. In addition, the accumulation of salts can increase the concentrations of TDS and major ions, making the groundwater nonpotable as mentioned previously.
Conclusions
The conclusions drawn from this study can be summarized as follows:
-
1.
Groundwater in the study area ranges from slightly acidic to slightly alkaline. EC, TDS, and TH are much lower in the plain and river valley areas. The predominant ions are Na+, Ca2+, Mg2+, HCO3−, Cl−, and SO42−. The dominant hydrochemical facies in the high plain area is Cl·(HCO3·SO4)–Na·Ca and in the plain and river valley areas is HCO3–Na·Ca type and HCO3–Ca·Na.
-
2.
The use of hierarchical cluster analysis suggests that the evolution of the chemical composition of groundwater is significantly controlled by the nature of local geological and hydrogeological conditions. In addition, more groundwater samples are needed to ensure the accuracy of cluster analysis results.
-
3.
A geochemical assessment of the groundwater quality data suggests that rock weathering, the dissolution of silicates (especially plagioclase), and reverse cation exchange are the main processes regulating the chemical constituents of the groundwater in the plain and river valley areas. In the high plain area, however, evaporation and the dissolution of halite, silicate minerals, and gypsum are the major hydrochemical processes occurring. In addition, mining activities have a certain impact on groundwater quality due to the change of groundwater circulation conditions.
-
4.
The quality assessment indicates that the low values of EC, TDS, TH, and other water quality variables in the groundwater samples collected from the river valley and plain areas make them suitable for human consumption. On the other hand, the groundwater in the high plain area has relatively high values of these variables must be treated before it can be used for drinking. Furthermore, the groundwater in the plain and river valley areas is suitable for irrigation, with low values of SAR, RSC, and Na%. However, high EC and Na concentration result in the groundwater in the high plain area being unsuitable for irrigation.
The results are of great significance to groundwater management in the study area. Although groundwater in the plain and river valley areas is of good quality, it is vulnerable to human contamination; hence, it is strongly recommended that reasonable management measures be taken before undertaking groundwater and coal exploitation.
References
Agartan E, Yazicigil H (2012) Assessment of water supply impacts for a mine site in western turkey. Mine Water Environ 31(2):112–128
Arkoc O, Ucar S, Ozcan C (2016) Assessment of impact of coal mining on ground and surface waters in Tozaklı coal field, Kırklareli, northeast of Thrace, Turkey. Environmental Earth Science 75(6):514
Chabukdhara M, Gupta SK, Kotecha Y, Nema AK (2017) Groundwater quality in Ghaziabad district, Uttar Pradesh, India: multivariate and health risk assessment. Chemosphere 179:167–178
Chen L, Feng Q (2013) Geostatistical analysis of temporal and spatial variations in groundwater levels and quality in the Minqin oasis, northwest China. Environmental Earth Sciences 70(3):1367–1378
Choubey VD (1991) Hydrogeological and environmental impact of coal mining, Jharia coalfield, India. Environ Geol Water Sci 17(3):185–194
DelgadoOuteiriño I, AraujoNespereira P, CidFernández JA et al (2009) Behaviour of thermal waters through granite rocks based on residence time and inorganic pattern. J Hydrol 373(3):329–336
Dolnicar S (2002) A review of unquestioned standards in using cluster analysis for data-driven market segmentation. Faculty of commerce—papers
Formann AK (1984) Die Latent-Class-Analyse: Einführung in die Theorie und Anwendung. Beltz, Weinheim
Fulazzaky MA, Seong T, Masirin M (2010) Assessment of water quality status for the Selangor River in Malaysia. Water Air Soil Pollut 205:63–77
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Gupta DC (1999) Environmental aspects of selected trace elements associated with coal and natural waters of Pench Valley coalfield of India and their impact on human health. Int J Coal Geol 40(2–3):133–149
Huang XJ, Wang GC, Liang XY et al (2017) Hydrochemical and Stable Isotope (δD and δ18O) Characteristics of Groundwater and hydrogeochemical processes in the Ningtiaota coalfield, Northwest China. Mine Water Environ 37(1):119–136
Hutchins MG, Smith B, Rawlins BG, Lister TR (1999) Temporal and spatial variability of stream waters in wales, the welsh borders and part of the west midlands, UK.1. major ion concentrations. Water Res 33(16):3479–3491
Kim K (2003) Long-term disturbance of ground water chemistry following well installation. Ground Water 41(6):780–789
Kurunc A, Uz BY, Aslan GE et al (2016) Seasonal changes of spatial variation of some groundwater quality variables in a large irrigated coastal mediterranean region of turkey. Sci Total Environ 554–555:53–63
Li P (2018) Mine water problems and solutions in China. Mine Water Environ. https://doi.org/10.1007/s10230-018-0543-z
Li P, Qian H, Wu J (2010) Groundwater quality assessment based on improved water quality index in Pengyang County, Ningxia, northwest China. Journal of Chemistry 7(S1):S209–S216
Li P, Wu J, Qian H (2012) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environmental Earth Sciences 69(7):2211–2225
Li P, Qian H, Wu J, Zhang Y, Zhang H (2013) Major ion chemistry of shallow groundwater in the Dongsheng Coalfield, OrdosBasin, China. Mine Water Environ 32(3):195–206
Li P, Li X, Meng X, Li M, Zhang Y (2016) Appraising groundwater quality and health risks from contamination in a semiarid region of northwest China. Exposure Health 8(3):361–379
Li P, Feng W, Xue C, Tian R, Wang S (2017a) Spatiotemporal variability of contaminants in lake water and their risks to human health: a case study of the Shahu Lake tourist area, northwest China. Exposure Health 9(3):213–225
Li P, Qian H, Zhou W (2017b) Finding harmony between the environment and humanity: an introduction to the thematic issue of the Silk Road. Environ Earth Sciences 76(3):105
Li P, Tian R, Liu R (2018) Solute geochemistry and multivariate analysis of water quality in the Guohua phosphorite mine, Guizhou Province, China. Exposure Health. https://doi.org/10.1007/s12403-018-0277-y
Mahato MK, Singh P, Singh AK, Tiwari AK (2018) Assessment of hydrogeochemical processes and mine water suitability for domestic, irrigation, and industrial purposes in east Bokaro Coalfield. Mine Water and the Environment, India. https://doi.org/10.1007/s10230-017-0508-7
Ministry of Health of the P. R. China, Standardization Administration of the P. R. China (2006) Standards for drinking water quality (GB5749–2006). China Standard Press, Beijing (in Chinese)
Nair HC, Padmalal D, Joseph A et al (2017) Delineation of groundwater potential zones in river basins using geospatial tools-an example from Southern Western Ghats, Kerala, India. Journal of Geovisualization and Spatial Analysis 1(1–2):5
Patel P, Raju NJ, Reddy BCSR et al (2016) Geochemical processes and multivariate statistical analysis for the assessment of groundwater quality in the Swarnamukhi River basin, Andhra Pradesh, India. Environmental Earth Sciences 75(7):1–24
Piper (1944) A graphical procedure in the geochemical interpretation of water analysis. Am Geophys Union Trans 25:914–928
Rajesh R, Brindha K, Elango L (2015) Groundwater quality and its hydrochemical characteristics in a shallow weathered rock aquifer of southern India. Water Quality, Exposure and Health 7(4):515–524
Redwan M, Abdel Moneim AA, Amra MA (2016) Effect of water-rock interaction processes on the hydrogeochemistry of groundwater west of Sohag area, Egypt. Arab J Geosci 9(2):111
Roisenberg C, Loubet M, Formoso ML et al (2016) Tracing the origin and evolution of geochemical characteristics of waters from the Candiota Coal Mine Area (Southern Brazil): part I. Mine Water Environ 35(1):29–43
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigation and development. Water Research, Series-33. UNESCO, Delft, pp 54–83
Singh G (1988) Impact of coal mining on mine water quality. Int J Mine Water 7(3):49–59
Singh AK, Mahato MK, Neogi B et al (2010) Quality assessment of mine water in the Raniganj coalfield area, India. Mine Water Environ 29(4):248–262
Tiwari AK, Singh AK, Singh MP (2017) Hydrogeochemical analysis and evaluation of surface water quality of Pratapgarh district, Uttar Pradesh, India. Applied Water Science 7(4):1609–1623
Tiwary RK (2001) Environmental impact of coal mining on water regime and its management. Water Air Soil Pollution 132(1–2):185–199
Towfiqul Islam ARM, Shen S, Bodrud-Doza MD, Safiur Rahman M (2017) Assessing irrigation water quality in faridpur district of Bangladesh using several indices and statistical approaches. Arab J Geosci 10(19):418
Utom AU, Odoh BI, Egboka BCE (2013) Assessment of hydrogeochemical characteristics of groundwater quality in the vicinity of Okpara coal and Obwetti fireclay mines, near Enugu town, Nigeria. Applied Water Science 3(1):271–283
Wang L, Dong Y, Xu Z et al (2017) Hydrochemical and isotopic characteristics of groundwater in the northeastern Tennger Desert, northern China. Hydrogeol J 25(8):2363
WHO (2017) Guidelines for drinking-water quality: fourth edition incorporating the first addendum. World Health Organization, Geneva, p 631
Wilcox LV (1955) Classification and use of irrigation waters. USDA Circular, 969, Washington, USA
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Exposure Health 8(3):311–329
Wu J, Li P, Hui Q et al (2014) Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: a case study in Laoheba phosphorite mine in Sichuan, China. Arab J Geosci 7(10):3973–3982
Wu J, Wang L, Wang S et al (2017a) Spatiotemporal variation of groundwater quality in an arid area experiencing long-term paper wastewater irrigation, northwest China. Environmental Earth Sciences 76(13):460
Wu J, Xue C, Tian R, Wang S (2017b) Lake water quality assessment: a case study of Shahu Lake in the semi-arid loess area of northwest China. Environmental Earth Science 76(5):232
Zhang DS, Fan GW, Ma LQ et al (2009) Harmony of large-scale underground mining and surface ecological environment protection in desert district-a case study in Shendong mining area, northwest of China. Proctect Earth Planet 1(1):1114–1120
Zhu B, Yang X, Rioual P et al (2011) Hydrogeochemistry of three watersheds (the erlqis, zhungarer and yili) in northern XinJiang NW china. Appl Geochem 26(8):0–1548
Acknowledgements
The research was supported by the National Key R&D Program of China (Grant No. 2016YFC0501104), State Key Laboratory of Water Resource Protection and Utilization in coal mining (Grant No. SHJT-16-30.10), and the National Natural Science Foundation of China (Grant No. 41302214).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Jin, D., Wang, T. et al. Hydrogeochemical processes and quality assessment of shallow groundwater in Chenqi coalfield, Inner Mongolia, China. Environ Earth Sci 78, 347 (2019). https://doi.org/10.1007/s12665-019-8355-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8355-4