Abstract
Boreal waters are typically low in minerals and oligotrophic, and therefore particularly sensitive to changes in mineral composition. We investigated the effects of potassium and the potassium: sodium (K+: Na+) ratio in freshwater on growth performance and oxidative stress in a typical northern species of whitefish, Coregonus lavaretus. Fish were subjected to 0.8 mM Na and 4.4 mM K, which corresponds to the K+:Na+ ratio in a lake contaminated by mining wastes from the Kostomuksha iron mine and ore dressing mill in northwestern Russia. The control group was subjected to water with similar mineralization levels and equal amounts of Na and K (approximately 0.3 mM of each). Potassium excess caused a decrease in fish growth rate and oxidative stress, as indicated by the level of lipid peroxidation product malondialdehyde (MDA). Glutathione-S-transferase (GST) activity and the level of reduced glutathione (GSH) were not affected by cation composition.
Zusammenfassung
Boreale Gewässer sind gewöhnlich schwach mineralisiert und oligotroph, und daher besonders empfindlich gegenüber Änderungen ihrer Ionenzusammensetzung. Wir untersuchten die Einflüsse von Kalium und des Verhältnisses von Kalium zu Natrium (K+ : Na+) im Süßwasser auf die Wachstumsleistung und den oxidativen Stress einer typischen im Norden verbreiteten Maränenart, Coregonus lavaretus. Die Fische wurden Konzentrationen von 0,8 mM Na+ und 4,4 mM K+ ausgesetzt, die dem K+ : Na+ Verhältnis eines Sees entsprachen, der durch Bergbauabfälle der Kostomuksha Eisenzeche und Erzaufbereitunghütte in Nordwestrussland verunreinigt ist. Eine Kontrollgruppe von Versuchstieren wurde in einem Wasser mit ähnlichem Mineralisierungsgrad, jedoch gleichhoher Konzentration von Na+ and K+ (jeweils ungefähr 0,3 mM) gehältert. Der Kaliumüberschuss verursachte einen Rückgang der Wachstumsleistung der Fische und einen oxidativen Stress, der durch den Gehalt des Lipidabbauprodukts Malondialdehyd (MDA) angezeigt wurde. Die Aktivität der Glutathion-S-Transferase (GST) und der Gehalt des reduzierten Glutathions (GSH) wurden durch die Kationenzusammensetzung nicht beeinträchtigt.
Resumen
Las aguas boreales son bajas en minerales y oligotróficas y, debido a ello, son particularmente sensibles a cambios en la composición mineral. Hemos investigado los efectos del potasio y de la relación potasio:sodio (K+: Na+) en agua fresca sobre el crecimiento y el estrés oxidativo en una típica especie de peces del norte: lavareto, Coregonus lavaretus. Los peces fueron expuestos a 0,8 mM de Na y 4,4 mM de K, que corresponde a la relación K+:Na+ en un lago contaminado con residuos mineros de la mina de hierro Kostomuksha en el noroeste de Rusia. El grupo control estuvo expuesto a agua con iguales niveles de mineralización e iguales cantidades de Na y K (aproximadamente 0,3 mM de cada uno). El exceso de K causó un decrecimiento en la velocidad de crecimiento del pez y estrés oxidativo, según indica el nivel de malondialdehido (MDA) que es producto de la peroxidación lipídica. La actividad de la glutatión-S-transferasa (GST) y el nivel de glutatión reducido (GSH) no fueron afectados por la composición catiónica
贝加尔白鲑(突唇白鲑)对钾、钠浓度变化的反应:一种矿井水毒理反应方式
北方针叶林区的湖水以低矿物质和富氧为典型特征,湖水水质类型常因矿物质微弱改变而变化。研究了钾和钾钠比对贝加尔白鲑(突唇白鲑)生长状况和氧化应激的影响。试验组水质为钠0.8 mM和钾4.4 mM,钾钠比模拟了俄罗斯西北部受Kostomuksha铁矿和矿石加工厂污染的湖水环境。对照组水质为钠和钾分别为0.3 mM,具有与试验组相似的矿物质水平。依据脂过氧化作用的产物MDA推断,过量钾使鱼生长速度和氧化应激减慢。谷胱甘肽S-转移酶(GST)的活性和谷胱甘肽(GSH) 水平的降低并未受阳离子成分变化的影响。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freshwater organisms can adapt to changes in the ionic composition of water (Ern et al. 2014; Kwong et al. 2009; Nguyen et al. 2014) and the specific mechanisms of fish osmoregulation at the genetic, molecular, morphological, and behavioral levels have been previously described (Evans 2008; Hiroi et al. 2012; Seale et al. 2012). However, there is insufficient research on the excess of specific ions in water bodies. While this problem is not widespread in nature, it is relevant to isolated bodies of freshwater where the chemistry is largely determined by the composition of the underlying rock or where the water is exposed to industrial effluents.
Since 1982, a natural lake has been used to dispose of tailings from the Kostomuksha iron mine and ore dressing mill (64º61´N, 30º47´E, northwestern Russia). In the 30 years after mining, the hydrochemistry of this lake, now a tailings pond (TP), has drastically changed when compared to the natural background (Table 1). Dissolution of minerals from the tailings has resulted in a 20-fold increase in the concentration of major ions (from 30 to 600 mg/L) as well as a change in the water type, from a regional Ca-HCO3 type to K-SO4-HCO3 (Lozovik et al. 2007). Industrial activity has caused a dramatic decline in biodiversity, as indicated by diminishing plankton, fish, and higher plant communities (Ilmast et al. 2013; Kalinkina et al. 2003; Vlasova 1998). Altered fish growth and reproduction parameters, as well as marked biochemical and histological disorders, were described in fish from the TP and downstream lakes (Borvinskaya et al. 2011, 2012; Churova et al. 2014; Ilmast et al. 2013; Murzina et al. 2011; Nemova et al. 2012; Tkatcheva et al. 2004).
Based on the literature, the high levels of K+ (4 mM) could be extremely challenging for aquatic organisms and a major cause of the TP water toxicity (Lozovik et al. 2007; Tkatcheva et al. 2007). Experimental studies have shown that of the common ions found in freshwater, potassium is arguably the most dangerous. In aquaria experiments, the K > Mg > Ca > Na order of relative toxicity of cations has been established for most water organisms (Fischer et al. 1991; Mount et al. 1997; Trama 1954). Mount et al. (1997) found that the median lethal doses of potassium chloride, potassium sulfate, and potassium bicarbonate for fish and crustaceans were significantly lower than for sodium, magnesium, and calcium salts. Similarly, potassium nitrate is more toxic to aquatic organisms than sodium nitrate (Dowden and Bennett 1965) indicating that potassium ions contribute to toxicity more than the accompanying anions.
Potassium is an essential constituent of the body for intracellular osmotic pressure and buffering, cell permeability, acid-base balance, muscle contraction, and nerve function. Shifting the chemical gradient of K+ between cell cytoplasm and blood plasma disrupts these processes. Its regulation is critical in all vertebrates because a constant K+ concentration is essential for protein and glycogen synthesis, enzyme activities, and cell division and growth (Weiner and Wingo 1998; Opoku-Okrah et al. 2015).
For fish, the physiological level of K+ in cells is 140 to 150 mM in cells and 3.5–5 mM in plasma (Furukawa et al. 2015, 2014; Gardaire et al. 1991). Gills are the main organ of K+ regulation; freshwater fish absorb potassium through the gills and release it through branchial K+ canals (ROMKa) (Furukawa et al. 2014, 2015). Potassium transport is strongly coordinated with Na+, H+, and Cl− transport through specific membrane channels and typically takes place when there are higher concentrations of sodium in both natural waters (Na:K molar = 20–50:1) and blood plasma (Na:K molar = 30–40:1). An imbalance can make normal osmotic regulation problematic. For freshwater catfish Ictalurus punctatus, the LC50 dose is 10 mM of potassium chloride; potassium sulfate and potassium bicarbonate are even more toxic (LC50 doses are 4mM and 5 mM for 96 h, respectively) (Mount et al. 1997). However, potassium tolerance varies between fish species; Furukawa et al. (2014) report that euryhaline tilapia Oreochromis mossambicus placed into freshwater containing 10 mM potassium chloride showed no mortality or changes of physiological levels of plasma potassium, thus demonstrating effective mechanisms to rapidly eliminate excess K+.
In this study, the effects of various K+:Na+ ratios on the whitefish Coregonus lavaretus, a species known to live and reproduce successfully in the Kostomuksha tailings pond, were studied as a simplified model of toxicity associated with mine waste. Levels of tissue oxidation, antioxidant system response, and growth performance were evaluated as stress markers. Other potentially threatening compounds found in the TP were not the focus of this study and will be addressed in later research.
Materials and Methods
Experiment Design
Experimental fish were obtained in April from a local fish farm (The Republic of Karelia, Russia). Juvenile C. lavaretus of uniform length were transferred to 300 L tanks (21 fish per tank) filled with tap water (supplemental Table 1) and maintained at 13 °C by a Hailea HC-250A water chiller. Oxygen saturation was maintained through ceramic oxygen diffusers. A 12L: 12D light regime was provided by 58 W light bulbs (Philips TL-D 58 W/54–765) with a photoperiod controller. The fish were automatically fed once every 24 h using commercial feed (BioMar, 0.5 mm).
After a 2-week acclimation period, fish were randomly assigned into two groups (each of sample size 21); each group was subjected to a specific mineralization regime. Target mineralization values were produced by addition of concentrated solutions of NaCl and KCl and maintained by replacing approximately one-third of the water volume with freshwater with dissolved salts (Table 2) every 24 h.
The experimental fish group was exposed to water with 4.4 mM potassium, 0.8 mM sodium, and a K+:Na+ molar ratio of 5:1; these conditions mimic those recorded in the TP (К5Na1 group). The control fish group was subjected to a solution with K+ and Na+ concentrations equal to that of natural potassium-rich waters (K1Na1 group). The total concentration of major ions in the water for both groups was approximately 400 mg/L, which corresponds to the mineralization in the TP. Experiments were performed in duplicate.
The work was carried out in accordance with the EU Directive 2010/63/EU for animal experiments. Seven individuals from each group were sampled before initiating the experiment (day 0) and on the 5th and 20th day following the exposure onset. At each time point tested, whole fish individuals were weighed and liver and white muscle were then excised and frozen in liquid nitrogen for further analysis.
Hydrochemistry
Measurements of pH, oxygen saturation, and temperature were performed on the fish tank water using a CCO-505 oxygen meter and CPI-505 pH/ion meter (Elmetron). Phosphorus and metal concentrations were determined by quadrupole inductively coupled plasma mass spectrometry (Q-ICP-MS, Thermo Scientific). The certified reference material (CRM) ICP-MS Calibration Standard 21 - IV-STOCK-21-125ML (Inorganic Ventures) was analyzed simultaneously. Chloride and sulfate anion concentrations were determined spectrophotometrically (Utsumi et al. 1978; Kirsten and Lindholm-Franzén 1980). Hydrochemistry analyses were performed daily throughout the experiment period. No significant variations in temperatures, pH, or oxygen saturation were observed between the two tanks (one-way ANOVA) during experimentation. The coefficient of variation in registered ion concentration in each tank did not exceed 25 % during the experiment.
Growth Performance
The specific growth rate (SGR) was calculated according to the following equation:
In this equation, W refers to the mass of the sampled fish in grams, and W 0 and W 1 are the initial and the final mean mass values in grams, respectively.
Biochemical Assay
All chemicals and reagents for biochemical assay were purchased from Sigma–Aldrich. For glutathione S-transferase activity (GST) measurements, fish tissues were individually homogenized in 50 mM Tris–HCl buffer (pH 7.5) containing 5 mM EDTA, followed by 1 h centrifugation at 100,000 g, 4 °C. Supernatant obtained was added to the reaction mixture, which was a 0.125 M phosphate buffer (pH 6.5) with 1 mM 1-chloro-2,4-dinitrobenzene and 1mM GSH (Habig et al. 1974). Enzyme activity was measured by recording any increase in the optical density at 340 nm (ε = 9.6 mM− 1cm− 1). Specific enzymatic activity was defined as the amount of substrate metabolized by the enzyme (in mM/min/mg).
Reduced glutathione (GSH) concentration was determined using a procedure modified from that of Сohn and Lyle (1966) and Hissin and Hilf (1976). Soluble proteins were precipitated from the homogenate by 5 % trichloroacetic acid and removed by centrifugation at 2500 g for 15 min. The supernatant was adjusted to pH 8.5 by 6 M NaOH and diluted by 0.4 M Tris–HCl buffer (pH 8.5) with 5 mM EDTA. Fluorescence of the reaction product was measured after 15 min of incubation with 0.01 % ortho-phthalaldehyde in methanol at room temperature (Em at 420 nm; Ex at 350 nm). Final GSH concentrations were determined in accordance with the standard calibration curve of reduced glutathione in 0.01 % ortho-phthalaldehyde; relative GSH concentration is expressed in micrograms of GSH per milligrams of soluble protein (µg/mg).
Lipid peroxidation product malondialdehyde (MDA) was measured by the TBARS method (Bird and Draper 1984; Okhawa et al. 1979) by adding of 0.2 mL of tissue homogenate to 1.5 mL 20 % orthophosphoric acid (pH 3.5) and 1.5 mL of 0.8 % thiobarbituric acid. Samples were then heated in a 95 °C water bath for 1 h. After cooling, 1.0 mL of chilled water and 5.0 mL butanol-pyridine mixture (15:1, v/v) were added and samples were vortexed for 15 s; the flocculent precipitate was then removed by centrifugation at 3000 g for 10 min. Homogenate absorbance was measured at 532 nm using1,1,3,3-tetra-ethoxy-propane as a reference. The MDA level was expressed in nM/g of wet tissue. Protein content in the supernatant was measured spectrophotometrically by recording absorption at 205 nm, using bovine serum albumin as a standard (Noble and Bailey 2009; Sukhovskaya et al. 2010).
Data Analyses
Statistical analyses were performed with Past 3.10 Software. A two-way ANOVA was performed to examine effects of time and treatment; a post-hoc pairwise Tukey HSD test was used to determine significant differences between the datasets. Parameters relations were analyzed using Spearman’s correlation coefficients. Data are presented as the mean ± standard deviation with p ≤ 0.05 in all analyses.
Results
Growth Performance
Growth rates of fish exposed to different modes of mineralization are significantly different. In the К1Na1 group, a linear increase of the body mass was observed during the entire experimental period. However, in the К5Na1 group, the fish did not grow; by the 5th and 20th days, their final weight was similar to the starting condition and significantly lower than in the control group (Table 3).
Biochemical Parameters
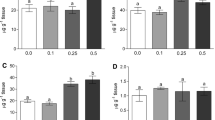
Studied biochemical parameters demonstrated different responses to the exposure conditions. The MDA level variations appear to be tissue-specific (Fig. 1). The effect of potassium on the liver appears to depend on concentration and duration. MDA levels in liver from the К5Na1 group significantly increased by the end of the experiment. Liver MDA in the К1Na1 group also increased over 20 days; however, this elevation was not statistically significant. ANOVA results show that treatment and duration had no effect on the MDA response in muscle tissue; however, interaction between these factors was significant (F2.78=5.0). MDA levels in fish muscles from the К5Na1 group declined over the course of the experiment.
Malondialdehyde (MDA) content, reduced glutathione (GSH) level, and glutathione S-transferase (GST) activity in liver and muscle tissue of C. lavaretus exposed to different mineralization regimes over 20 days. aDifferences were significant compared to the К1Na1 group; *differences were significant compared to the 0 day; #differences are significant compared to the 5th day
The GSH level in both liver and muscle tissue was not affected by different K+:Na+ ratios (Fig. 1). However, GSH concentration in muscle tissue was found to be dependent on the duration of the experiment (F2.78 = 4.3). It decreased in both groups after 20 days compared to the beginning of experiment, and in the K5Na1 group, it was significantly lower from that at day 5.
No significant difference in glutathione S-transferase activity in liver and muscle tissue was detected between the two sample groups and time points through two-way ANOVA and post-hoc testing (Fig. 1).
Discussion
Natural freshwater contains ionic constituents, which are vital for aquatic life. However, many natural and anthropogenic sources can increase ion concentrations to levels toxic to hydrobionts (Mount et al. 1997; Talling et al. 2010). Although potassium is abundant in nature and its common salts are highly soluble, it is seldom found in freshwater at high concentrations (Hem 1985). In relatively fresh water (up to 15 g/L of dissolved salts), potassium has been recorded to range between 0.001 and 2.0 mM; sodium is commonly found at much higher concentrations, approximately 0.1–50 mM (Hem 1985; Meybeck 2003; Talling 1992, 2010). Salt water K+ concentrations can be as high as 10 mM, but K+: Na+ molar ratios are generally less than 0.02 (Hem 1985). In K-rich waters associated with volcanic areas and alkaline lakes, up to 20 % of total cations can be potassium, and sodium concentrations can be equivalent (Mccarraher 1971; Talling 1992). In 1982, Kilham (according to Talling 1992) reported that specific hippo pools accumulated very high concentrations of potassium (2.6 mM K+, with a K+: Na+ ratio of 4.0) as a result of megafaunal activity.
Based on these prior reports, the dissolved potassium concentrations, up to 4 mM and equivalent to 40–53 % of all cations, in the tailing pond of the Kostomuksha mine is unusually high for freshwater environments. It has previously been shown that some freshwater bivalves and unionid mussels are sensitive to potassium and can demonstrate adverse effects at potassium concentrations as low as 0.25 mM (Dietz and Byrne 1990; Fischer et al. 1991; Imlay 1973; Wilcox and Dietz 1995). Freshwater fish are reported to be more tolerant to potassium; median lethal doses range from 10 to 27 mM after exposure times of 96 h (Fischer et al. 1991; Mount et al. 1997). It has also been shown that the toxic effects of potassium are reduced in the presence of high concentrations of other cations including sodium, calcium, and magnesium; this suggests a competitive relationship between these cations (Mount et al. 1997).
High potassium levels and disproportionate concentrations of major ions are potential causes of the ecological disturbance observed in the TP (Lozovik et al. 2007; Tkatcheva et al. 2007). Comparison with downstream water bodies shows that TP fish communities show much lower species diversity; there are only three species that can now be found regularly (C. lavaretus, roach Rutilus rutilus, and pike Esox lucius) and two periodically (burbot Lota lota and bleak Alburnus alburnus).
Previous studies have demonstrated biochemical and histological disorders in fish from the TP and the river system downstream of the mining waste reservoir; oxidative stress and reduced aerobic metabolism can be indicated by lipid dystrophy (consisting of either infiltration or decomposition in the liver), declined protein synthesis in muscles, energy deficiency, and increased proliferation of the mucus, respiratory, and specific osmoregulatory chloride cells in gills (Churova et al. 2014; Tkatcheva et al. 2004). Specifically, the roach and whitefish from the TP were characterized by a retarded growth rate and earlier maturation (Ilmast et al. 2013; Nemova et al. 2012).
The model experiment herein estimates C. lavaretus response to potassium and sodium concentrations relevant to those in the mining waste reservoir TP and natural K+-rich waters. No fish mortalities occurred in this experiment, confirming exposure tolerance of C. lavaretus. However, evidence of altered fish physiological and biochemical parameters was observed in fish exposed to the higher levels of potassium in the water.
Growth Response
Although C. lavaretus showed consistent feeding activity (as evidenced by sampling procedure and gastrointestinal dissection, data not shown), there was no observed weight gain within the К5Na1 group (Table 3), while fish in the К1Na1 group demonstrated a linear body mass increase over a period of 20 days. Results indicate that adverse effects on fish physiology can occur at the TP water mineralization level (approximately 400 mg/L of major ions); however, increasing the proportion of sodium ions while maintaining the mineralization level eliminates this inhibitory effect on fish weight gain.
Previous field studies in the Kostomuksha TP indicated low mean values of fish growth parameters compared to that of fish populations in other lakes in this region. Results obtained in the present work suggest that the growth retardation may be to some extent attributed to the prevalence of potassium in the water.
Oxidative Stress
One method for early detection of harm, such as osmotic stress or anoxia, is the identification of oxidative stress by-products. Oxidation agents attack membrane lipids and generate decomposition products of peroxidized polyunsaturated fatty acids (e.g. malondialdehyde), which are known to be reliable indicators of oxidative stress (Abele et al. 2011; Del Rio et al. 2005; Rahman 2007).
Marked alterations of MDA levels were detected in the liver and muscle tissue of C. lavaretus from the К5Na1 group, indicating the effect of chronic exposure to high potassium concentrations. An intensification of lipid peroxidation in the liver was shown, which is consistent with previous field studies where hepatic MDA levels were much higher in whitefish from the Kostomuksha TP compared to whitefish from an undisturbed lake (Vasilyeva et al. 2012). Thus, enhanced lipid peroxidation in fish liver may indicate a compensatory response to the stress caused by disproportionate cation concentrations. In contrast, muscular MDA levels observed in the present study were much lower in the К5Na1 group; however, field study results showed no significant difference in muscle MDA concentrations of fish from a polluted and a reference site. We can speculate that the observed MDA content decline is likely due to activation of an antioxidant defense system. Induction of antioxidant enzymes, such as catalase and superoxide dismutase, can compensate for lipid peroxidation enhancement, as supported by studies on the influence of polycyclic aromatic hydrocarbons (Ji et al. 2010) and trace metals (Hermenean et al. 2015; Kong et al. 2012). Research performed by Ahmad et al. (2000) also suggests that low lipid peroxidation reflects the protective effects of oxidative enzymes.
Glutathione S-transferase activity and reduced glutathione levels, which can also be used as antioxidant defense system parameters, were shown in the present study to be similar in both К5Na1 and К1Na1 groups. This demonstrates a weak dependence on the proportion of potassium and sodium in the water. In addition, the lack of correlation between studied biomarkers (Spearman rank correlation test, data not shown) was also established, indicating the elimination of peroxidation products not by the glutathione system but by overlapping biochemical pathways. Previous field studies in the Kostomuksha mine area suggest that the GST enzyme is involved in the C. lavaretus adaptation to osmotic conditions in the TP. Activity of hepatic GST in tissues of whitefish from the TP was elevated; however, GST activity in muscles was unaffected (Borvinskaya et al. 2011).
Herein, in the present work, we did not get the same GST induction as in field studies, perhaps due to oversimplification of field conditions in the experiment. In the field studies, an unpolluted lake with low mineralization concentrations (≈18 mg/L major ions) was used as a reference, so the contribution of osmotic stress to the biological response could not be considered. Additionally, the TP contains a complex combination of cations and anions, which could be acting as an antagonist or could be having a synergetic effect on the biological response that should be taken into account. An analogous laboratory experiment where rainbow trout was subjected to potassium with significant amounts of lithium, calcium, and manganese confirmed some of the effects obseved in the field studies as gills cholesterol decrease; however, other effects, such as changes in the gill epithelium microstructure, were not repeated (Tkatcheva et al. 2007).
Conclusion
The proposed model for study of the Kostomuksha tailing pond was very simple because it did not account for the complex composition of the TP water. Mount et al. (1997) revealed a two or four fold increase of tolerance by water organisms to potassium chloride after adding equal amounts of magnesium and calcium to the water, while a drastic decrease of tolerance was shown when sulfate and bicarbonate salts of potassium were used. Since the TP is rich in both common cations and anions, the actual effect of mine water should reflect the sum of these opposing effects. Since bicarbonate is the dominant anion in the TP, further study of its effect is planned in evaluating TP water toxicity.
Nevertheless, this study showed that applied concentrations of potassium are potentially dangerous to water organisms. The whitefish under study showed altered levels of lipid oxidation by-products in addition to unexpected cessation of growth in the К5Na1 group. The last can be regarded as a severe pathological disorder. The mechanism of the observed reactions remains unclear, as these are non-specific biological responses to stress. These effects appear to occur when potassium ions are at greater concentrations than other cations, suggesting that disproportionate ion concentrations may be toxic to water organisms. Results are consistent with previous studies showing that potassium toxicity is determined by the amount relative to other ions present in the medium as opposed to the absolute concentration of potassium.
References
Ahmad I, Hamid T, Fatima M, Hitendra S, Chand-Jain SK, Athar M, Raisuddin S (2000) Induction of hepatic antioxidants in freshwater catfish (Channa punctatus Bloch) is a biomarker of paper mill effluent exposure. Biochim Biophys Acta 1523(1):37–48
Bird RP, Draper AH (1984) Comparative studies on different methods of malondialdehyde determination. Method Enzymol 90: 105–110
Borvinskaya EV, Nemova NN, Smirnov LP (2011) Glutathione S-transferase in northern freshwater fish: the effect of water mineralization. Doklady Biolog Sci 436 (4):566–568
Borvinskaya EV, Sukhovskaya IV, Murzina SA, Vasilyeva OB, Nazarova MA, Smirnov LP (2012) Biochemical and histological parameters of tissues of pike (Esox lucius L.) from the lake with high mineralization. Comp Biochem Phys A 163:S12. doi:10.1016/j.cbpa.2012.05.040
Churova MV, Murzina SA, Meshcheryakova OV, Nemova NN (2014) Metabolic enzymes activity and histomorphology in the liver of whitefish (Coregonus lavaretus L.) and pike (Esox lucius L.) inhabiting a mineral contaminated lake. Environ Sci Pollut Res Int 21(23):13342–13352. doi:10.1007/s11356-014-3014-5
Cohn VH, Lyle J (1966) A fluorometric assay for glutathione. Anal Biochem 14(3):434–440
Del Rio D, Stewart AJ, Pellegrini N (2005) A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 15(4):316–328
Dietz TH, Byrne RA (1990) Potassium and rubidium uptake in freshwater bivalves. J Exp Biol 150:395–405
Dowden BF, Bennett HJ (1965) Toxicity of selected chemicals to certain animals. J Wat Pollut Control Fed 37:1308–1313
Ern R, Huong DT, Cong NV, Bayley M, Wang T (2014) Effect of salinity on oxygen consumption in fishes: a review. J Fish Biol 84(4):1210–1220. doi:10.1111/jfb.12330
Evans DH (2008) Teleost fish osmoregulation: what have we learned since August Krogh, Homer Smith, and Ancel Keys. Am J Physiol-Reg I 295(2):704–713. doi:10.1152/ajpregu.90337.2008
Fischer SW, Stromberg P, Bruner KA, Boulet LD (1991) Molluscicidal activity of potassium to the zebra mussel, Dreissena polymorphia: toxicity and mode of action. Aquat Toxicol 20:219–234
Furukawa F, Watanabe S, Kakumura K, Hiroi J, Kaneko T (2014) Gene expression and cellular localization of ROMKs in the gills and kidney of Mozambique tilapia acclimated to fresh water with high potassium concentration. Am J Physiol Reg I 307(11):R1303–R1312. doi:10.1152/ajpregu.00071.2014
Furukawa F, Watanabe S, Seale AP, Breves JP, Lerner DT, Grau EG, Kaneko T (2015) In vivo and in vitro effects of high-K(+) stress on branchial expression of ROMKa in seawater-acclimated Mozambique tilapia. Comp Biochem Phys A 187:111–1118. doi:10.1016/j.cbpa.2015.05.017
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, 3rd edn. USGS Water-Supply Paper 2254, Alexandria
Hermenean A, Damache G, Albu P, Ardelean A, Ardelean G, Ardelean DP, Horge M, Nagy T, Braun M, Zsuga M, Kéki S, Costache M, Dinischiotu A (2015) Histopatological alterations and oxidative stress in liver and kidney of Leuciscus cephalus following exposure to heavy metals in the Tur River, north western Romania. Ecotox Environ Safe 119:198–205
Hiroi J, McCormick SD (2012) New insights into gill ionocyte and ion transporter function in euryhaline and diadromous fish. Respir Physiol Neurobiol 184(3):257–268. doi:10.1016/j.resp.2012.07.019
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74(1):214–226
Ilmast NV, Sterligova OP, Kuchko YaA, Pavlovskiy SA (2013) Kostomuksha water reservoir hydrobiocenoses (White Sea water basin) in the conditions of technogenic pollution. Isvestia of Samara scientific centre 15(3): 916–920 (in Russian)
Imlay MJ (1973) Effect of potassium on survival and distribution of freshwater mussels. Malacologia 12:97–113
Ji Y, Lu G, Wang C, Zhang J (2012) Biochemical responses of freshwater fish Carassius auratus to polycyclic aromatic hydrocarbons and pesticides. Water Sci Eng 5(2):145–154
Ji K, Kim J, Lee M, Park S, Kwon HJ, Cheong HK, Jang JY, Kim DS, Yu S, Kim YW, Lee KY, Yang SO, Jhung IJ, Yang WH, Paek DH, Hong YC, Choi K (2013) Assessment of exposure to heavy metals and health risks among residents near abandoned metal mines in Goseong, Korea. Environ Pollut 178:322–328
Kalinkina NM, Kulikova TP, Morozov AK, Vlasova LI (2003) Causes of technogenic changes in a freshwater zooplanktonic community. Biol Bull 30(6):627–632
Kirsten WJ, Lindholm-Franzén I (1980) Spectrophotometric determination of chloride, bromide, and iodide with an improved mercury-iron-thiocyanate method. Microchem J 25:240–245
Kong X, Wang S, Jiang H, Nie G, Li X (2012) Responses of acid/alkaline phosphatase, lysozyme, and catalase activities and lipid peroxidation to mercury exposure during the embryonic development of goldfish Carassius auratus. Aquat Toxicol 120–121:119–125
Kwong AK, Ng AH, Leung LY, Man AK, Woo NY (2009) Effect of extracellular osmolality and ionic levels on pituitary prolactin release in euryhaline silver sea bream (Sparus sarba). Gen Comp Endocrinol 160(1):67–75. doi:10.1016/j.ygcen.2008.10.024
Lozovik PA, Kalmykov MV, Dubrovina LV (2007) Chemical composition of industry-generated waters. In: Lozovik PA, Kulikova TP, Martunova NN (eds) Status of water objects in republic of Karelia. According to 1998–2006 monitoring results. Karelian Research Centre of Russian Academy of Sciences, Petrozavodsk, pp 100–106 (in Russian)
Mccarraher DB (1971) Survival of some freshwater fishes in the alkaline eutrophic waters of Nebraska. J Fish Res Board Can 28:1811–1814
Meybeck M (2003) Global occurrence of major elements in rivers treatise on geochemistry. In: Drever JI (ed) Treatise on Geochemistry. Elsevier 5:207–223
Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM (1997) Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magnaand Pimephales promelas (fathead minnows). Environ Toxicol Chem 16(10):2009–2019
Murzina SA, Nefedova ZA, Nemova NN (2011) Liver dysfunction and pathologies of roach (Rutilus rutilus), pike (Esox lucius) and whitefish (Coregonus lavaretus) under ore-dressing sewages contamination (Lake Kostomukshskoe and Lake Kamennoi, northern Karelia, Russia). Application of histological methods in analyses of liver structure of fishes. J Int Sci Publ Ecol Safe 5(3) (ISSN 13113–2563; on-line)
Nemova NN, Ieshko EP, Meshcheryakova OV, Il’mast NV, Anikieva LV, Lebedeva DI, Churova MV, Sterligova OP, Kuchko YaA (2012) The European whitefish, Coregonus lavaretus (L.), under conditions of technogenic pollution in the Kostomuksha tailing pond. Ekologiya 4:298–302
Nguyen PT, Do HT, Mather PB, Hurwood DA (2014) Experimental assessment of the effects of sublethal salinities on growth performance and stress in cultured tra catfish (Pangasianodon hypophthalmus). Fish Physiol Biochem 40(6):1839–1848. doi:10.1007/s10695-014-9972-1
Noble JE, Bailey MJA (2009) Quantitation of proteins. Method Enzymol 463:73–95
Okhawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Analyt Biochem 95:351–358
Opoku-Okrah C, BK Safo Acquah, EE Dogbe (2015) Changes in potassium and sodium concentrations in stored blood. Pan Afr Med J 20:236, doi:10.11604/pamj.2015.20.236.5851
Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2(2):219–236
Seale AP, Watanabe S, Grau EG (2012) Osmoreception: perspectives on signal transduction and environmental modulation. Gen Comp Endocrinol 176(3):354–360. doi:10.1016/j.ygcen.2011.10.005
Sukhovskaya IV, Borvinskaya EV, Smirnov LP, Nemova NN (2010) Comparative analysis of the methods for determination of protein concentration—spectrophotometry in the 200–220 nm range and the Bradford protein assay. P Karelian Res Centre RAS 2: 68–71 (in Russian)
Talling JF (1992) Environmental regulation in African shallow lakes and wetlands. Rev Hydrobiol Tropic 25:87–144
Talling JF (2010) Potassium - a non-limiting nutrient in fresh waters? Freshwater Rev 3:97–104
Tkatcheva V, Hyvärinen H, Kukkonen J, Ryzhkov LP, Holopainen IJ (2004) Toxic effects of mining effluents on fish gills in a subarctic lake system in NW Russia. Ecotoxicol Environ Safe 57:278–289
Tkatcheva V, Holopainen IJ, Hyvärinen H, Kukkonen JVK (2007) The responses of rainbow trout gills to high lithium and potassium concentrations in water. Ecotoxicol Environ Safe 68:419–425
Trama FB (1954) The acute toxicity of some common salts of sodium, potassiuma nd calcium to the common bluegill (Lepomis macrochirus Rafinesque). P Acad Nat Sci Phil 106:185–205
Utsumi S, Oinuma Yo, Isozaki A (1978) Spectrophotometric determination of the micro amounts of sulfate ion with dimethylsulfonazo III. Bunseki Kagaku 5:278–282
Vasilyeva OB, Nazarova MA, Rippati PO, Nemova NN (2012) Lipid content and lipid peroxidation in fish organs and tissues. In: Nemova NN, Ilmast NV, Ieshko EP, Mescheryakova OV (eds) Biota of the Northern Lakes under anthropogenic transformation. Karelian Research Centre of Russian Academy of Sciences, Petrozavodsk, pp 175–185 (in Russian)
Vlasova LI (1998) Zooplankton. In: Filatov NN, Kulikova TP, Lozovik PA (eds) Current state of water objects in the Republic of Karelia, result of monitoring 1992–1997, Petrozavodsk, pp 134–137 (in Russian, with English summary)
Weiner ID, Wingo CS (1998) Hyperkalemia: a potential silent killer. J Am Soc Nephrobiol 9:1535–1543
Wilcox SJ, Dietz TH (1995) Potassium transport in the freshwater bivalve Dreissena polymorpha. J Exp Biol 198:861–868
Acknowledgements
The research was made in the frame of the budgetary theme No. 0221-2014-0033 and the Program of the Presidium of the Russian Academy of Sciences no. 21 “Biodiversity of Natural Systems. Biological Resources of Russia: State Evaluation and Fundamental Bases of Monitoring,” project no. 0221-2015-0003 “Dynamics of Changes in Ichthyofauna of Freshwater Ecosystems of Russian European North under Climatic and Anthropogenic Influence.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borvinskaya, E., Sukhovskaya, I., Vasil’eva, O. et al. Whitefish (Coregonus lavaretus) Response to Varying Potassium and Sodium Concentrations: A Model of Mining Water Toxic Response. Mine Water Environ 36, 393–400 (2017). https://doi.org/10.1007/s10230-016-0426-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-016-0426-0