Abstract
We examined copper accumulation in the hemolymph, gills and hepatopancreas, and hemolymph osmolality, Na+ and Cl− concentrations, together with gill Na+/K+-ATPase and carbonic anhydrase activities, after dietary copper delivery (0, 100 or 500 Cu µg g−1) for 12 days in a fiddler crab, Minuca rapax. In contaminated crabs, copper concentration decreased in the hemolymph and hepatopancreas, but increased in the gills. Hemolymph osmolality and gill Na+/K+-ATPase activity increased while hemolymph [Na+] and [Cl−] and gill carbonic anhydrase activity decreased. Excretion likely accounts for the decreased hemolymph and hepatopancreas copper titers. Dietary copper clearly affected osmoregulatory ability and hemolymph Na+ and Cl− regulation in M. rapax. Gill copper accumulation decreased carbonic anhydrase activity, suggesting that dietary copper affects acid–base balance. Elevated gill Na+/K+-ATPase activity appears to compensate for the ion-regulatory disturbance. These effects of dietary copper illustrate likely impacts on semi-terrestrial species that feed on metal-contaminated sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Analyses of different routes of metal uptake are essential to understanding bioaccumulation and toxicity in semi-terrestrial organisms, particularly those inhabiting environments under tidal influence. Uptake from the dissolved water phase and from ingested food may both be important routes of metal accumulation (Vitale et al. 1999). Dissolved metals can accumulate by direct adsorption to body surfaces and by absorption across the respiratory epithelia while particulate metals can accumulate following the ingestion and digestion of food (Wang and Fisher, 1999). In crustaceans, most studies have concentrated on metals dissolved in the water phase (see review by Viarengo and Nott 1993), and only a very few investigations have examined the effects of metal contaminated diets (Sá and Zanotto 2008; Sabatini et al. 2009; Bordon et al. 2018). Our current understanding of dietary metal toxicity is inadequate, and consequently, the dietary contamination route is not usually considered in existing regulations regarding environmental contamination or in risk assessments (Borgmann et al. 2005; Schamphelaere et al. 2007).
Copper is an essential micronutrient required by all living organisms for a variety of physiological and biochemical processes. This metal is a co-factor in multiple enzymatic processes but is potentially toxic to aquatic organisms above certain levels (Martins et al. 2011). During exposure to elevated copper titers in the water or diet, cellular detoxification mechanisms may become saturated to a point where protein function is impaired (Grosell et al. 2002). In crustaceans, copper is a component of the respiratory pigment hemocyanin used in oxygen transport (Rainer and Brouwer 1993). However, while high copper titers may cause respiratory disruption in freshwater organisms, the mortality resulting from environmentally relevant waterborne copper contamination usually derives from osmoregulatory disturbances (Grosell et al. 2002). Copper is present in all aquatic environments, and multiple anthropic activities such as industries, agriculture and harbors discharge effluent containing elevated copper, leading to increased exposure and potential toxicity to aquatic organisms (Martins et al. 2011). Complexation of copper with organic and inorganic ligands as well as competition with other cations for binding and uptake pathways greatly influence copper toxicity in fresh water (Grosell et al. 2007).
Although decapod crustaceans are mainly aquatic, many species show varying degrees of terrestriality (Burggren and McMahon 1988). Thus, dietary contamination by heavy metals is particularly relevant in semi-terrestrial crabs where waterborne exposure is episodic and often occurs only during high tide. Nevertheless, very few studies have investigated different routes of metal contamination and chronic toxicity. Dietary metal exposure appears to affect mainly reproduction (Lauer and Bianchini 2010; Bielmyer et al. 2006) and energy metabolism (De Schamphelaere et al. 2007). In contrast, waterborne metals exert a general effect, more related to osmoregulatory disruption (Capparelli et al. 2016, 2017). In aquatic organisms, metal uptake mainly occurs via epithelial surfaces related to gas exchange and ion absorption and secretion such as the gills of crustaceans and fish (Péqueux 1995; Toro et al. 2001). Some metals compete with other cations for binding and active uptake sites in the gills (Paquin et al. 2002; Grosell et al. 2007), and once absorbed, may accumulate in various tissues, particularly the gills, leading to diverse toxic effects (MacRae et al. 1999; Grosell et al 2007).
In aquatic environments, copper and other non-essential trace metals exert their toxic action in a fashion synergistic with salinity variation, and gill Na+/K+-ATPase and carbonic anhydrase activities can be affected in osmoregulating crustaceans during exposure to waterborne metal contamination (Roast et al. 2002; Capparelli et al. 2017). The exact mechanisms of metal toxicity are unclear but many metals cause enzyme kinetic changes that disrupt specific metabolic systems. Na+/K+-ATPase and carbonic anhydrase activities are of special concern owing to their role in ion transport by crustacean gills, and may be particularly vulnerable to waterborne pollutants (Böttcher et al. 1991; Böttcher and Siebers 1993; Kjoss et al. 2005; Bianchini and Wood 2003).
The species used as a model for copper contamination in the present study is the semi-terrestrial, mudflat fiddler crab, Minuca rapax. This crab is distributed from Florida, throughout the Gulf of Mexico, the Antilles and Venezuela to the Atlantic coast of Brazil where it occurs from Pará to Santa Catarina States (Thurman et al. 2013), inhabiting burrows in muddy sand in estuarine mangrove environments. Minuca rapax is a strong euryhaline osmoregulator and maintains its hemolymph osmolality at around 780 mOsm kg−1 H2O over a wide range of environmental and experimental salinities (Thurman et al. 2017). Populations of this crab are affected by waterborne copper contamination, showing tissue accumulation and impaired osmotic and ionic regulatory ability (Capparelli et al. 2017) and metabolic and oxidative stress (Capparelli et al. 2019).
Fiddler crabs are common inhabitants of mangrove biotopes and are important sediment bioturbators, feeding avidly on sediment grains from which they glean organic matter, algae, micro-organisms and bacteria, which are ingested as food, together with small inorganic particles (Crane 2015; Christy 1978; Kristensen 2008). Given that M. rapax spends much of its time feeding on sediment particles, evaluating the impacts of long-term dietary contamination should provide important insights into copper accumulation and toxicity. We address two questions in the present study: (i) does dietary copper contamination lead to greater tissue bioaccumulation than does waterborne exposure; and (ii) does dietary copper accumulate in the gills and affect osmotic and ionic regulatory ability?
Materials and Methods
Adult, intermolt specimens of Minuca rapax of either sex were collected from Virginia Key Beach (25° 44′ 28.17″ N, 80° 0.8′ 50.74″ W), Virginia Key, Miami, Florida, USA and transported in plastic boxes containing sponge cubes moistened with water from the collection site to the Laboratory of Environmental Physiology and Toxicology at the University of Miami, Florida. Only non-ovigerous, intermolt crabs of carapace width greater than 10 mm were used. To acclimatize to laboratory conditions before use, the crabs were maintained unfed for three days at 25 °C, with free access to a dry surface, in plastic boxes containing water from the collection site.
An artificial diet containing 10% dry mass was prepared by mixing 2 g agar, 3 g sucrose and 5 g fish food (which contains 18% protein, 16% fiber and 2.5% crude fat, calorie content 2300 to 2500 kcal/kg, and 8,000, 1,000, and 50 IU/kg vitamins A, D3 and E, respectively) with solutions containing the requisite concentrations of copper chloride (100 or 500 µg g−1) to give 100 mL of agar medium (Swaileh and Ezzughayyar 2000). 100 mL of each copper-containing medium was divided equally among four petri dishes (25 mL/dish), which after cooling, were kept in a refrigerator at 4 °C. During the experiments, agar cubes were cut out, adjusted to 0.15 g weight and placed in the plastic boxes containing the individual crabs. A control food source without copper chloride (0 µg g−1) was prepared in exactly the same way.
The crabs were kept for 12 days (N = 10 per trial) in the laboratory during the experiments. They were sorted randomly into individual plastic boxes containing dilute seawater (25 ‰ salinity, 750 mOsm kg−1H2O, 350 mmol Na+ L−1, 400 mmol Cl− L−1) in one section and the 0.15-g cube of artificial food in a dry section. During the 12-day experimental period, the crabs were fed with a known amount of food to which either 0, 100 or 500 µg g−1 CuCl2 agar had been added. At the end of each day, any leftover food remnants were removed, the seawater was changed and a new food cube was offered. Usually, all food was consumed within the 24-h period.
Following the 12-day period of dietary copper contamination, the crabs were cryo-anesthetized in crushed ice for 5 min each. A hemolymph sample was drawn into a 1 mL syringe using a needle inserted into the arthrodial membrane at the base of the 3rd or 4th pereiopod and frozen at − 20 °C. The crabs were then killed by removing the carapace, and the anterior and posterior gill pairs and hepatopancreas were dissected from each animal (N = 10). The hemolymph and tissues were frozen at − 80 °C for later use.
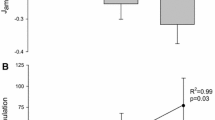
After thawing, hemolymph osmolality was measured in 10 µL aliquots using a vapor pressure micro-osmometer (Wescor Model 5500, Wescor Inc., Logan, UT, USA). Hemolymph [Na+] was measured in 10 μL hemolymph aliquots, diluted 1: 20,000 (v/v) in distilled water, using a Varian FS220Z atomic absorption flame spectrophotometer (Varian, Mulgrave, Victoria, Australia). A standard curve was generated using certified reference standards of 25, 50 and 75 µmol Na+ L−1 (Merck TraceCERT, Merck KGaA, Darmstadt, Germany), R2 = 0.99 being the minimum acceptable coefficient. Hemolymph [Cl−] was quantified employing anion chromatography (Dionex DX-120, fitted with an AS40 automated sampler, Dionex Corp., Sunnyvale, CA, USA).
After chilling briefly on ice, the pooled anterior and posterior gills from each crab were homogenized in dry ice and acetone using a buffer containing (in mmol L−1) imidazole 20, pH 6.8, sucrose 250, EDTA 6 and a protease inhibitor cocktail (Furriel et al. 2001). The hydrolysis of p-nitrophenylphosphate ditris salt (pNPP) (i.e., the K+-phosphatase activity of the Na+/K+-ATPase) by the gill homogenates was assayed continuously for 15 min at 25 °C by monitoring the release of the p-nitrophenolate ion at 410 nm spectrophotometrically (Spectramax Plus 384 microplate reader, Molecular Devices LLC, San Jose, CA, USA) under the standard conditions described by Furriel et al. (2001).
The K+ phosphatase activity of the Na+/K+-ATPase was assayed by adding aliquots of each sample homogenate to a reaction medium containing (in mmol L−1) HEPES buffer 50, pH 7.5, KCl 10, MgCl2 5, pNPP 10 (for total K+-phosphatase activity) or the same medium containing 3 mmol L−1 ouabain, a specific inhibitor of pNPPase activity (for ouabain insensitive activity). The K+ phosphatase activity in each sample was estimated from the difference between the total pNPPase activity and the ouabain insensitive activity.
To assay carbonic anhydrase activity, the pooled anterior and posterior gills from each crab were homogenized using a Potter homogenizer in homogenization buffer (in mmol L−1, mannitol 225, sucrose 75 and Trizma-base 10, pH 7.4). The homogenate was then centrifuged at 100,000×g for 1 h at 4 °C to separate the cytoplasmic and membrane-bound isoforms. Cytoplasmic carbonic anhydrase activity was measured using an electrometric method (Henry 1991).
Measurement of total protein in all homogenates for all assays was performed according to Bradford (1976) employing bovine serum albumin as the standard.
Copper content in the hemolymph, pooled anterior and posterior gills, and in the hepatopancreas was measured by atomic absorption spectroscopy, employing a graphite furnace (Varian FS220Z atomic absorption spectrophotometer, Mulgrave, Victoria, Australia) using certified reference material (SPEX CertiPrep, CAS# Cu[7440-50-8], Thermo Fisher Scientific, Waltham, MA, USA). A standard curve was generated using 15, 30 and 45 µg Cu2+ L−1 standards, R2 = 0.99 being the minimum acceptable coefficient. Matrix interference in the diluted seawater medium (25 ‰S) was resolved using a solvent extraction technique (Blanchard and Grosell 2006). The samples were digested for 24 h in 1 N HNO3 (1: 10 w/v, Trace Metal Grade) at 60 °C, vortexed and centrifuged, and the supernatants were collected for analysis after appropriate dilution. Validation was performed by analyzing percentage copper recovery of the certified reference material (> 80% for all tissues). The calculated limit of detection (LOD) was 0.001 µg g−1.
All data are expressed as mean values ± the standard error of the mean (SEM). After verifying normality of distribution and equality of variance, the physiological and biochemical parameters were analyzed using two-way (effect of copper concentration and tissue on copper accumulation) or one-way (effect of copper concentration on hemolymph osmolality, [Na+] and [Cl−], and gill Na+/K+-ATPase and carbonic anhydrase activities) analyses of variance, followed by the Student–Newman–Keuls post-hoc multiple comparisons procedure. Occasionally, raw data were transformed to meet normal distribution criteria. Differences were considered significant at p = 0.05.
Results and Discussion
No mortality was recorded in M. rapax receiving dietary copper. The crabs did not alter their behavior and they did not avoid the copper-contaminated feed, each crab consuming the entire agar cube offered daily.
The two-way analysis of variance revealed that copper accumulation was affected by both tissue and copper concentration and their interaction (19.2 < F < 98.1, p < 0.001). In the control crabs (no added Cu), [Cu] were highest in the hemolymph (518 µg g−1 Cu, lowest in the gills (40 µg g−1 Cu) and intermediate in the hepatopancreas (360 µg g−1 Cu) (0.001 < p < 0.023) (Fig. 1). Curiously, [Cu] were lower in the hemolymph (p < 0.001) and hepatopancreas (0.017 < p < 0.001) of the crabs receiving the copper-contaminated diets, particularly 500 µg Cu/g, than in the control crabs. In the gills, [Cu] was highest in the crabs receiving 500 µg g−1 Cu (p < 0.001) (Fig. 1).
Hemolymph osmolality was slightly hyper-regulated (845 ± 0.9 mOsm kg−1 H2O, Δ ≈ + 100 mOsm kg−1 H2O) in the control crabs and strongly hyper-regulated (Δ ≈ + 200 mOsm kg−1 H2O) above ambient values in the copper contaminated crabs. Sodium was strongly hyper-regulated (518 ± 1.4 mmol L−1, Δ ≈ + 170 mmol L−1) in the control crabs although less so in the copper-contaminated crabs (Δ ≈ + 40 mmol L−1) while chloride was isocloremic (413 ± 0.9 mmol L−1) in the control crabs and slightly hypo-regulated (Δ ≈ − 35 mmol L−1) in the copper-contaminated crabs.
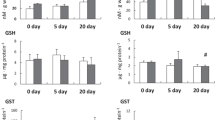
Hemolymph osmolality was higher in the crabs receiving the copper-contaminated diets (0.021 < p < 0.037) (947 mOsm kg−1 H2O) compared to the control crabs (845 mOsm kg−1 H2O). However, hemolymph sodium (p < 0.001) and chloride (0.034 < p < 0.048) concentrations were lower (≈380 mmol L−1) in these same crabs compared to the control crabs (518 mmol Na+ L−1, 413 mmol Cl− L−1) (Fig. 2).
Hemolymph osmolality, sodium and chloride concentrations in Minuca rapax fed a coppercontaminated diet (100 or 500 μg g−1 CuCl2) while held for 12 days with free access to isosmotic seawater (25 ‰ salinity, 750 mOsm kg−1 H2O, 14 mmol L−1 Na+, 16 mmol L−1 Cl−). Data are the mean ± SEM (N = 10). *p ≤ 0.05 compared to control crabs (0 μg g−1 CuCl2)
The gill Na+/K+-ATPase activity increased 1.3-fold in the crabs receiving the copper-contaminated diets (0.012 < p < 0.023) compared to the control crabs (Fig. 3). In contrast, gill carbonic anhydrase activity decreased by 19%–35% in the copper-contaminated (0.004 < p < 0.037) compared to the control crabs (Fig. 3).
The mechanisms of waterborne copper toxicity in crustaceans are starting to be better understood (Rainbow 2002, 2007; Capparelli et al. 2017). However, the uptake and toxicity of dietary-delivered metals have not been well investigated, particularly in semi-terrestrial fiddler crabs for which no data are available.
Our laboratory study of dietary copper delivery for 12 days in Minuca rapax showed that while elevated, copper concentrations of 100 and 500 µg g−1 were not lethal for the crabs, which did not avoid the contaminated diets. Measured copper bioaccumulation was highest in the hemolymph and hepatopancreas, and lowest in the gills in both contaminated and non-contaminated crabs. Interestingly, copper-contaminated crabs showed lower copper concentrations in the hemolymph and hepatopancreas than did control crabs, but higher gill concentrations.
Although delivered by a dietary rather than waterborne route, copper contamination did affect osmotic and ionic regulation in M. rapax. Hemolymph osmolality increased while sodium and chloride concentrations decreased. The increased activity of the gill Na+/K+-ATPase, the main ion-transporting enzyme, may have been induced by the reduced hemolymph Na+ and Cl− concentrations, constituting a compensatory biochemical response. This same response pattern was seen in M. rapax subjected to waterborne copper (Capparelli et al. 2017). In contrast, gill carbonic anhydrase activity decreased, likely reflecting enzyme inhibition by copper accumulated in the gill tissue, as also seen during waterborne copper exposure (Capparelli et al. 2017). The effect on gill carbonic anhydrase suggests that acid–base balance and CO2 excretion also are affected by contamination via dietary copper. While waterborne copper delivered at just 50 µg L−1 disrupts many physiological and biochemical processes (Arnold 2005; Genz et al. 2011), M. rapax appears to possess effective mechanisms for dietary copper detoxification. The crabs are resistant to elevated copper concentrations, showing no mortality, suggesting that tissue specific copper concentrations did not reach lethal threshold levels consequent to dietary exposure at 500 µg g−1 Cu as used here. The reduced copper concentrations seen in the hemolymph and hepatopancreas of the copper-contaminated crabs suggest the activation of copper excretion mechanisms.
Considering the route of contamination and the mechanisms of dietary copper accumulation, higher concentrations and increases would be expected in the hepatopancreas and hemolymph rather than in the gills (Rainbow 2002; Ahearn et al. 2004). However, dietary copper can be absorbed and redistributed from the digestive system to the hemolymph and tissues where it accumulates as insoluble metal-rich granules derived from lysosomes as a result of metallothionein activity (Nassiri et al. 2000; Vogt and Quinitio 1994). The decreased copper concentrations seen in the hepatopancreas of M. rapax at 500 µg g−1 Cu and in the hemolymph at 100 and 500 µg g−1 Cu suggest copper excretion rather than its detoxification. Copper content increased in the gills of M. rapax only at 500 µg g−1 Cu, as also seen in fish (Shaw and Handy 2006; Handy 1996; Kamunde et al. 2001), possibly reflecting systemic copper redistribution via the hemolymph in these dietary contaminated crabs.
The shore crab Carcinus maenas exposed to sub-lethal or lethal waterborne copper (Rtal and Truchot 1996; Weeks et al. 1993) responds similarly to M. rapax while copper injected into the estuarine crab Scylla serrata is cleared from the hemolymph within 24 h. The bioaccumulation of lead in the blue crab Callinectes danae is greater in response to waterborne than dietary lead delivery (Bordon et al. 2018, 2019), likely owing to gill lead uptake as a consequence of osmoregulatory ion-uptake processes located in the gills.
The pattern of accumulation in dietary copper-contaminated M. rapax contrasts with that encountered in waterborne delivery studies (Capparelli et al. 2017) where the hemolymph and hepatopancreas showed higher titers compared to copper-free crabs. For semi-terrestrial crabs like M. rapax that inhabit burrows in muddy sand in estuarine mangrove environments, food source seems to be an important route of metal exposure. Nevertheless, copper-contaminated water flow over the gills and direct body contact may be as relevant as is exposure via particulate food sources.
With regard to effects on osmotic and ionic regulation, dietary copper increased hemolymph osmolality and decreased Na+ and Cl− in M. rapax held at 25 ‰S, which may reflect toxic copper accumulation in the gills. Minuca rapax is an extremely euryhaline species (Thurman et al. 2017), is an excellent hyper/hypo-osmotic regulator (Capparelli et al. 2016), and is roughly isosmotic at 25 ‰S (780 mOsm kg−1 H2O). The increased osmolality seen in copper-contaminated crabs in an isosmotic salinity may reflect increased K+ and Ca2+ uptake and/or the presence of other osmolytes like NH4+ or free amino acids. Ammonia transport pathways are affected by copper contamination and elevated hemolymph ammonia correlates with increased copper in freshwater crayfish (Allinson et al. 2000). Copper exposure in M. rapax reduced hemolymph Na+ and Cl− concentrations, suggesting impaired ion regulatory capability as seen in species contaminated by waterborne copper (Postel et al. 1998, Handy 2003). Hemolymph Na+ and Cl− concentrations are reduced in mercury-exposed crayfish (Wright and Welbourn 1993) and in copper- and cadmium-exposed amphipods, Gammarus pulex (Brooks and Mills 2003), owing to altered Na+/K+-ATPase activities.
The accumulation of copper in the gills suggests that while M. rapax can tolerate copper at concentrations up to 500 µg g−1 Cu, both acid–base equilibrium and osmoregulatory activities may be affected. Gill Na+/K+-ATPase activity increased in M. rapax fed a copper-contaminated diet in contrast to the decreased activity seen with waterborne contamination at concentrations above 100 µg g−1 Cu (Capparelli et al. 2017) and in response to contamination in situ (Capparelli et al. 2016). In Carcinus maenas, copper inhibits gill Na+/K+-ATPase activity leading to decreased sodium transport into the hemolymph (Handy et al. 2002). These effects derive from changes in enzyme conformation and/or in protein/lipid interactions (Henry et al. 2012). Just how dietary copper affects gill Na+/K+-ATPase activity is uncertain, although oxidative stress also increases concomitantly, and Na+/K+-ATPase activity is modulated by oxidative stress in fish (Hoyle et al. 2007). However, few studies have examined ionic regulation in response to dietary copper contamination, an aspect that requires further study in fiddler and other semi-terrestrial crabs.
Gill carbonic anhydrase activity was inhibited in a concentration-dependent manner in M. rapax contaminated by dietary copper as seen in many crustaceans where copper is a potent inhibitor of carbonic anhydrase (Vitale et al. 1999). A similar disturbance of acid–base regulation by waterborne copper is apparent in M. rapax (Capparelli et al. 2017). Carbonic anhydrase activity in the gills of the estuarine crab Neohelice granulata is likewise inhibited (Bianchini et al. 2008), suggesting a role in copper-induced acid–base disturbance. Sub-lethal copper contamination can cause acid–base balance perturbation even at concentrations that fail to induce or result in only modest osmoregulatory disturbances (Wang et al. 1998). This inhibition suggests that acid–base regulation and CO2 excretion are affected by copper contamination via both dietary and waterborne exposure in M. rapax. Further studies on the effects of copper contamination on acid–base equilibrium and gas exchange in semi-terrestrial crabs are necessary, given that their frequent bimodal respiration in water and air must demand substantial physiological adjustments.
Most studies of copper toxicity have focused on aquatic organisms and gill contamination while investigations using dietary copper contamination center on digestive enzymes, fecundity and reproduction (De Schamphelaere and Janssen 2007; Kooijman 2000; Nogueira et al. 2004), bioaccumulation (Sá and Zanotto 2008; Bordon et al. 2018), induction of metallothionein-like proteins (Bordon et al. 2018) and oxidative stress (Sabatini et al. 2009). However, our findings show that M. rapax accumulates dietary copper in the gills, and that transport and excretion mechanisms likely lead to decreased hemolymph and hepatopancreas copper titers. Further, dietary copper shows toxic sub-lethal effects, particularly on osmoregulatory processes such as lowered hemolymph Na+ and Cl−, and increased gill Na+/K+-ATPase and decreased carbonic anhydrase activities. Dietary copper does appear to act as an osmoregulatory stressor as seen with waterborne contamination, although a systematic analysis using dietary copper should be conducted. These findings are particularly relevant for semi-terrestrial crabs that spend long periods feeding on sediments, one of the main sources of metal contamination (Chapman and Wang 2001).
References
Ahearn GA, Mandal PK, Mandal A (2004) Mechanisms of heavy-metal sequestration and detoxification in crustaceans: a review. J Comp Physiol B 174(6):439–452
Arnold WR (2005) Effects of dissolved organic carbon on copper toxicity: implications for saltwater copper criteria. Integ Environ Assess Manage 1(1):34–39
Bianchini A, Wood CM (2003) Mechanism of acute silver toxicity in Daphnia magna. Environ Toxicol Chemy 22(6):1361–1367
Bianchini A, Lauer MM, Nery LEM, Colares EP, Monserrat JM, dos Santos Filho EA (2008) Biochemical and physiological adaptations in the estuarine crab Neohelice granulata during salinity acclimation. Comp Biochem Physiol A 151(3):423–436
Bielmyer GK, Grosell M, Brix KV (2006) Toxicity of silver, zinc, copper, and nickel to the copepod Acartia tonsa exposed via a phytoplankton diet. Environ Sci Technol 40(6):2063–2068
Blanchard J, Grosell M (2006) Copper toxicity across salinities from freshwater to seawater in the euryhaline fish Fundulus heteroclitus: is copper an ionoregulatory toxicant in high salinities? Aquat Toxicol 80(2):131–139
Bordon IC, de Campos BG, Gusso-Choueri PK, Miyai CA, de Araujo GS, Emerenciano AK, de Souza Abessa DM (2019) Improvements in metal exposure assays: artificial food to assess bioaccumulation in the blue crab Callinectes danae Smith, 1869 (Crustacea, Decapoda, Portunidae). Int J Environ Res 13(2):431–434
Bordon IC et al (2018) Implications on the Pb bioaccumulation and metallothionein levels due to dietary and waterborne exposures: the Callinectes danae case. Ecotoxicol Environ Saf 162:415–422
Borgmann U, Couillard Y, Doyle P, Dixon DG (2005) Toxicity of sixty-three metals and metalloids to Hyalella azteca at two levels of water hardness. Environ Toxicol Chem 24(3):641–652
Böttcher K, Siebers D (1993) Biochemistry, localization, and physiology of carbonic anhydrase in the gills of euryhaline crabs. J Exp Zool 265(4):397–409
Böttcher K, Siebers D, Becker W, Petrausch G (1991) Physiological role of branchial carbonic anhydrase in the shore crab Carcinus maenas. Mar Biol 110:337–342
Brooks SJ, Mills CL (2003) The effect of copper on osmoregulation in the freshwater amphipod Gammarus pulex. Comp Biochem Physiol A 135(4):527–537
Burggren WW, McMahon BR (eds) (1988) Biology of the land crabs. Cambridge University Press, Cambridge
Capparelli MV, Abessa DM, McNamara JC (2016) Effects of metal contamination in situ on osmoregulation and oxygen consumption in the mudflat fiddler crab Uca rapax (Ocypodidae, Brachyura). Comp Biochem Physiol C 185:102–111
Capparelli MV, Gusso-Choueri PK, de Souza Abessa DM, McNamara JC (2019) Seasonal environmental parameters influence biochemical responses of the fiddler crab Minuca rapax to contamination in situ. Comp Biochem Physiol C 216:93–100
Capparelli MV, McNamara JC, Grosell M (2017) Effects of waterborne copper delivered under two different exposure and salinity regimes on osmotic and ionic regulation in the mudflat fiddler crab, Minuca rapax (Ocypodidae, Brachyura). Ecotoxicol Environ Saf 143:201–209
Chapman PM, Wang F (2001) Assessing sediment contamination in estuaries. Environ Toxicol Chem 20:3–22
Christy JH (1978) Adaptive significance of reproductive cycles in the fiddler crab Uca pugilator: a hypothesis. Science 199(4327):453–455
Crane J (2015) Fiddler crabs of the world: Ocypodidae: genus Uca. Princeton University Press, Princeton
De Schamphelaere KAC, Forrez I, Dierckens K, Sorgeloos P, Janssen CR (2007) Chronic toxicity of dietary copper to Daphnia magna. Aquat Toxicol 81(4):409–418
Di Toro DM, Allen HE, Bergman HL, Meyer JS, Paquin PR, Santore RC (2001) Biotic ligand model of the acute toxicity of metals. 1. Technical basis. Environ Toxicol Chem 20(10):2383–2396
Furriel RDPM, McNamara JC, Leone FDA (2001) Nitrophenylphosphate as a tool to characterize gill Na+, K+-ATPase activity in hyperregulating Crustacea. Comp Biochem Physiol A 130(4):665–676
Genz J, Esbaugh AJ, Grosell M (2011) Intestinal transport following transfer to increased salinity in an anadromous fish (Oncorhynchus mykiss). Comp Biochem Physiol A 159(2):150–158
Grosell M, Blanchard J, Brix KV, Gerdes R (2007) Physiology is pivotal for interactions between salinity and acute copper toxicity to fish and invertebrates. Aquat Toxicol 84(2):162–172
Grosell M, Nielsen C, Bianchini A (2002) Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals. Comp Biochem Physiol C 133(1–2):287–303
Hagopian-Schlekat T, Chandler GT, Shaw TJ (2001) Acute toxicity of five sediment-associated metals, individually and in a mixture, to the estuarine meiobenthic harpacticoid copepod Amphiascus tenuiremis. Mar Environ Res 51(3):247–264
Handy RD (1996) Dietary exposure to toxic metals in fish. In Seminar series-society for experimental biology. vol. 57, Cambridge University Press, Cambridge, pp. 29–60
Handy RD (2003) Chronic effects of copper exposure versus endocrine toxicity: two sides of the same toxicological process? Comp Biochem Physiol A 135(1):25–38
Handy RD, Eddy FB, Baines H (2002) Sodium-dependent copper uptake across epithelia: a review of rationale with experimental evidence from gill and intestine. Biochim Biophys Acta 1566:104–115
Henry RP (1991) Techniques for measuring carbonic anhydrase activity in vitro. In The carbonic anhydrases (pp. 119–125). Springer, Boston
Henry RP, Lucu C, Onken H, Weihrauch D (2012) Multiple functions of the crustacean gill: osmotic/ionic regulation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Front Physiol 3:431
Hoyle I, Shaw BJ, Handy RD (2007) Dietary copper exposure in the African Walking Catfish, Clarias gariepinus: transient osmoregulatory disturbances and oxidative stress. Aquat Toxicol 83:62–72
Kamunde CN, Grosell M, Lott JN, Wood CM (2001) Copper metabolism and gut morphology in rainbow trout (Oncorhynchus mykiss) during chronic sublethal dietary copper exposure. Can J Fish Aquat Sci 58(2):293–305
Kjoss VA, Kamunde CN, Niyogi S, Grosell M, Wood CM (2005) Dietary Na does not reduce dietary Cu uptake by juvenile rainbow trout. J Fish Biol 66(2):468–484
Kooijman SA (2000) Dynamic energy and mass budgets in biological systems, 2nd edn. Cambridge University Press, Cambridge
Kristensen E (2008) Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J Sea Res 59(1–2):30–43
Lauer MM, Bianchini A (2010) Chronic copper toxicity in the estuarine copepod Acartia tonsa at different salinities. Environ Toxicol Chem 29(10):2297–2303
MacRae RK, Smith DE, Swoboda-Colberg N, Meyer JS, Bergman HL (1999) Copper binding affinity of rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis) gills: implications for assessing bioavailable metal. Environ Toxicol Chem 18:1180–1189
Martins CM, Menezes EJ, Giacomin MM, Wood CM, Bianchini A (2011) Toxicity and tissue distribution and accumulation of copper in the blue crab Callinectes sapidus acclimated to different salinities: in vivo and in vitro studies. Aquat Toxicol 101:88–99
Nassiri Y, Rainbow PS, Amiard-Triquet C, Rainglet F, Smith BD (2000) Trace-metal detoxification in the ventral caeca of Orchestia gammarellus (Crustacea: Amphipoda). Mar Biol 136(3):477–484
Nogueira AJA, Baird DJ, & Soares AMVM (2004). Testing physiologically-based resource allocation rules in laboratory experiments with Daphnia magna Straus. In Annales De Limnologie-International Journal of Limnology (vol 40(4), pp. 257–267. EDP Sciences.
Paquin PR, Gorsuch JW, Apte S, Batley GE, Bowles KC, Campbell PG, Gensemer RW (2002) The biotic ligand model: a historical overview. Comp Biochem Physiol C 133(1–2):3–35
Pequeux A (1995) Osmotic regulation in crustaceans. J Crustac Biol 15(1):1–60
Postel U, Petrausch G, Riestenpatt S, Weihrauch D, Malykh J, Becker W, Siebers D (1998) Inhibition of Na+/K+-ATPase and of active ion-transport functions in the gills of the shore crab Carcinus maenas induced by cadmium. Mar Biol 130(3):407–416
Rainbow PS (2002) Trace metal concentrations in aquatic invertebrates: why and so what? Environ Pollut 120(3):497–507
Rainbow PS (2007) Trace metal bioaccumulation: models, metabolic availability and toxicity. Environ Int 33(4):576–582
Rainer J, Brouwer M (1993) Hemocyanin synthesis in the blue crab Callinectes sapidus. Comparat Biochem Physiol Part B 104(1):69–73
Roast SD, Rainbow PS, Smith BD, Nimmo M, Jones MB (2002) Trace metal uptake by the Chinese mitten crab Eriocheir sinensis: the role of osmoregulation. Mar Environ Res 53(5):453–464
Rtal A, Truchot JP (1996) Carcinus maenas. Mar Pollut Bull 32(11):802–811
Sá MG, Zanotto FP (2008) Dietary copper absorption and excretion in three semi-terrestrial grapsoid crabs with different levels of terrestrial adaptation. Comparat Biochem Physiol Part C 148:112–116
Sabatini SE, Chaufan G, Juárez ÁB, Coalova I, Bianchi L, Eppis MR, de Molina MDCR (2009) Dietary copper effects in the estuarine crab, Neohelice (Chasmagnathus) granulata, maintained at two different salinities. Comp Biochem Physiol C 150(4):521–527
Shaw BJ, Handy RD (2006) Oreochromis niloticus. Aquat Toxicol 76(2):111–121
Thurman CL, Faria SC, McNamara JC (2013) The distribution of fiddler crabs (Uca) along the coast of Brazil: implications for biogeography of the western Atlantic Ocean. Mar Biodivers Records 6:1
Thurman CL, Faria SC, McNamara JC (2017) Geographical variation in osmoregulatory abilities among populations of ten species of fiddler crabs from the Atlantic coast of Brazil: a macrophysiological analysis. J Exp Mar Biol Ecol 497:243–253
Viarengo A, Nott JA (1993) Mechanisms of heavy metal cation homeostasis in marine invertebrates. Comparat Biochem Physiol Part C 104(3):355–372
Vitale AM, Monserrat JM, Castilho P, Rodriguez EM (1999) Inhibitory effects of cadmium on carbonic anhydrase activity and ionic regulation of the estuarine crab Chasmagnathus granulata (Decapoda, Grapsidae). Comp Biochem Physiol C 122(1):121–129
Vogt G, Quinitio ET (1994) Accumulation and excretion of metal granules in the prawn, Penaeus monodon, exposed to water-borne copper, lead, iron and calcium. Aquat Toxicol 28(3–4):223–241
Wang T, Knudsen PK, Brauner CJ, Busk M, Vijayan MM, Jensen FB (1998) Copper exposure impairs intra-and extracellular acid-base regulation during hypercapnia in the fresh water rainbow trout (Oncorhynchus mykiss). J Comp Physiol B 168(8):591–599
Wang WX, Fisher NS (1999) Delineating metal accumulation pathways for marine invertebrates. Sci Total Environ 237:459–472
Weeks JM, Jensen FB, Depledge MH (1993) Acid-base status, haemolymph composition and tissue copper accumulation in the shore crab Carcinus maenas exposed to combined copper and salinity stress. Mar Ecol Progress Ser Oldendorf 97(1):91–98
Wright DA, Welbourn PM (1993) Effects of mercury exposure on ionic regulation in the crayfish Orconectes propinquus. Environ Pollut 82(2):139–142
Acknowledgements
This investigation was financed by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2011/22537-0 to JCM) from which MVC received doctoral scholarships (2011/08065-9 and 2013/10672-6). JCM received excellence in research scholarships from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 300662/2009-2, 303613/2017-3). MG is a Maytag Chair of Ichthyology. This study is part of a doctoral dissertation by MVC (Comparative Biology, FFCLRP/USP) and received support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, 33002029031P8, finance code 001 to JCM and MVC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Capparelli, M.V., McNamara, J.C. & Grosell, M.G. Tissue Accumulation and the Effects of Long-Term Dietary Copper Contamination on Osmoregulation in the Mudflat Fiddler Crab Minuca rapax (Crustacea, Ocypodidae). Bull Environ Contam Toxicol 104, 755–762 (2020). https://doi.org/10.1007/s00128-020-02872-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02872-3