Abstract
The study of changes in intraspecific habitat use when closely related species coexist aids our understanding of the relationships between intra- and interspecific interactions in fishes. However, evidence of this phenomenon is few and shown in a very limited number of taxa. In particular, the stream goby [Rhinogobius flumineus (Mizuno, 1960)] is known for intraspecific variation in habitat use. We used underwater visual surveys to investigate this species’ size-dependent habitat use, both in isolation and in the presence of a sympatric congener, Rhinogobius nagoyae Jordan & Seale, 1906. A generalized linear mixed model and multiple comparison tests revealed that in the absence of R. nagoyae, R. flumineus body length was positively correlated with river flow velocity. This correlation disappeared when R. flumineus coexisted with R. nagoyae. Additionally, R. nagoyae density increased with flow velocity. Observations of interspecific territorial behavior revealed that larger individuals dominated in male–male competition regardless of species combination. Of the two species, R. nagoyae is significantly larger, so it was likely to exclude even relatively big R. flumineus individuals from fast riffles, the habitat preferred by both species. This study demonstrated that interspecific competition dominates intraspecific competition under sympatric conditions with larger related species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In multiple fish species, individuals exhibit resource use variation, likely as a consequence of intraspecific interactions. Such variation may affect food web connectivity or drive evolutionary diversification (Bolnick et al. 2007; Svanbäck et al. 2008; Quevedo et al. 2009). Many stream fishes (e.g., salmonids, cyprinids, cottids, and plecoglossids) have intraspecific differences in habitat use that often relate to body size (e.g., Grant and Noakes 1988; Katano 1990; Nakano 1995; Usio and Nakano 1998; Davey et al. 2005). Intraspecific variation in resource use is primarily caused by intraspecific competition, whereas the variation can be affected by interspecific competition. For example, trophic niche width of threespine sticklebacks narrows or expands depending on the presence or absence of competitors, such as cutthroat trout or prickly sculpin (Bolnick et al. 2010). Likewise, in northern Japanese streams, size-dependent habitat use (SHU) of fish varies in response to seasonal alterations in species composition (Usio and Nakano 1998). These cases suggest that interspecific competition can override intraspecific niche variation. However, this effect has not been sufficiently demonstrated in a field study that factors out confounding variables, such as seasons and growth stages.
Intra- and interspecific niche differentiation, especially in terms of habitat use, are well known in Rhinogobius gobies, such as Rhinogobius flumineus. This species lives in riffle, pool, and shoreline habitats along the middle and upper reaches of rivers. Males compete to acquire and protect nesting sites during the spawning season (Ishino et al. 2005). Larger males of Rhinogobius gobies can tend to pair with large females; size therefore is advantageous in accessing quality spawning grounds regardless of sex (Ito and Yanagisawa 2003). Indeed, large R. flumineus males are more likely to use fast riffles during the spawning season (Ishida et al. 2005). This period also sees an increase in resource competition with sympatric congeners. One major interspecific competitor is Rhinogobius nagoyae, a species with overlapping spawning seasons (R. flumineus from May to August; R. nagoyae from May to July) (Ishino et al. 2005). The two species also deposit their eggs under stones in riffles (Ishino et al. 2005; Hirashima and Tachihara 2006; Yamazaki et al. 2006).
Because R. nagoyae adults are generally larger than R. flumineus, the latter should be at a disadvantage when competing for spawning grounds. Changes in R. flumineus SHU when coexisting with R. nagoyae are thus likely to be an enlightening example of piscine resource use partitioning resulting from interspecific competition. Therefore, this study aimed to characterize such SHU variation in R. flumineus. We first investigated environmental parameters reflecting relevant habitat conditions to quantify SHU in this species. Next, we evaluated R. flumineus SHU with and without R. nagoyae present. Finally, we performed laboratory behavioral experiments to test whether competition between males of different species occurs, and the degree to which its outcome depends on body size.
Materials and methods
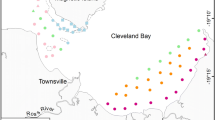
Study sites. Habitat use surveys were conducted at three stream reaches, including riffle and pool habitats, of the Isazu River, northern Kyoto Prefecture, which flows into the Sea of Japan (Fig. 1). At site 1, Rhinogobius flumineus is numerically dominant to Rhinogobius nagoyae, but both species occur regularly. At sites 2 and 3, R. nagoyae is either very rare or does not occur, probably because the sites are far from the river mouth and weirs are present, hindering their amphidromous migration.
Field observation with underwater visual surveys. Underwater visual surveys were conducted during the spawning season from May to July 2015, either on a single day or over two consecutive days; five separate surveys were conducted for each site. At site 1, six transects measuring 7–30 × 2 m were set in select riffles and pools to maximize observation, as shallow areas and obstacles (e.g., fallen trees) often hampered visibility. At sites 2 and 3, where large riffles and pools were adjacent to each other, the five and six transects (10 × 2 m), respectively, were placed at random. All transects were over 10 m apart. Transect position per site was determined by randomly changing the distance between river sides (lateral direction) and the most upstream point (longitudinal direction). During underwater observations, R. flumineus and R. nagoyae were counted and their standard lengths visually estimated to within 1 cm (e.g., 4–5 cm). One-year-old R. flumineus measuring approximately 3 cm are on the threshold of maturity (Miyaji et al. 1976), and individuals measuring ≥ 3 cm were categorized as adults. Only one observer recorded data to minimize errors in estimating fish size. Visual assessments were periodically calibrated through capturing and measuring fish. The observer approached transect lines from downstream and moved upstream to avoid disturbing fishes. Because both species often hide among boulders or cobbles, rocks were turned over to locate as many individuals as possible.
Abiotic data. In each transect, boulder density, flow velocity, and water depth were measured as abiotic variables. Boulders (diameter ≥ 256 mm; Wentworth 1922) per transect were counted unless they were more than half-buried, as such cases could not be used for nesting or shelter. Flow velocity in the upper water column was measured using the float method (e.g., Dobriyal et al. 2017); this involved submerging a styrene foam board (10 × 10 × 5 cm) with a lead weight and averaging turbulence patterns caused by obstacles in riffles. Flow velocity per transect was measured five successive times to minimize error (Dobriyal et al. 2017). Flow velocities < 10 m/min were regarded as 0 m/min, because wind effects were likely to increase in the upper water column at low velocities. Water depth was measured at one or more points every 5 m along the central line parallel to the longest side of the transect. Because site-1 transects were fixed, five random 10 × 2 m plots were set up per survey day to better characterize overall stream structure.
Observation of territorial behavior in laboratory. Experiments were conducted to observe intra- and interspecific male–male competition between R. flumineus and R. nagoyae males exhibiting breeding coloration. Fish were captured in the Isazu River and Saburi River (Fukui Prefecture) during July 2016 and transported to Yoshida Campus, Kyoto University. Aeration was provided during the 3 h transport to the laboratory. Fish were kept in three 150 L tanks at a density of ≤ 0.24 individuals L−1 and fed bloodworms (chironomid larvae; Sanmi, Hyogo, Japan) at approximately 2.9 g tank−1 daily. The room temperature was 22 °C.
Experimental tanks measured 30 × 18 × 24 cm, with the water level at 10.5 cm. Three sides of the tanks were covered with opaque blue sheets. Removable opaque partitions (height 14.5 cm) divided the tanks into two. For behavioral experiments, two tanks were placed in a compartment covered with a dark curtain and illuminated with aquarium lighting (1,070 lm × 2). Two observation holes were cut in the fabric for video cameras that recorded fish interactions (HDR-XR550V, SONY, Tokyo, Japan). One male per species (n = 19 each) was separately introduced into the two partitioned areas of the tanks and given 2 h to acclimatize. Subsequently, the partition was removed to allow interspecific interactions. Individuals were considered aggressive if they displayed either intimidation or attack behavior (or both). Intimidating behavior occurred before attack behavior. Intimidation involved simultaneously spreading fins and opening the mouth. Fin spreading or mouth opening that occurred separately from each other were not considered intimidation. Attack behaviors were biting and subsequent chasing of opponents. These behaviors were recorded until one male became the primary chaser; this male was considered dominant. Once chasing occurred, there was no case where the individual being chased made a retaliation. If no chasing behavior was initiated after 30 min, the experiment was terminated and no data were recorded.
Statistical analysis. All statistical analyses were conducted in R 3.4.0 (R Core Team 2017). Average abiotic variables were compared across the three stations using pairwise t-tests with Bonferroni correction. Abiotic environmental variables that best explain body size were determined using generalized linear mixed model (GLMM, glmmML function in R). Body size was a discrete variable and assumed to follow a Poisson distribution. The response variable was the standard length of an adult fish (≥ 3 cm), while predictor variables were boulder density, flow velocity, water depth, and R. nagoyae density (the latter for site 1 model only). Survey dates were included as random effects. When standard length was modeled, the ranges of 3–4, 4–5, 5–6, and 6–7 cm were converted into 3.5, 4.5, 5.5, and 6.5 cm, respectively. After factoring in the presence or absence of all response variables and interactions among them, 51 models were generated for site 1, as well as 14 models each at sites 2 and 3. The three best-fit models were selected based on lowest Akaike’s Information Criterion (AIC). Pairwise t-tests with Bonferroni corrections were used to establish whether parameters included in the best-fit models of sites 2 and 3 differed significantly among body length classes at each station. In addition, Spearman rank correlations between the above parameters and densities for both species were calculated.
A binominal test was used to test the effects of size on competition outcome. Separate analyses were performed for R. nagoyae victories and R. flumineus victories. A Fisher’s exact test was then applied to determine whether R. nagoyae won more frequently when they were larger than R. flumineus, compared to when they were smaller.
Results
Abiotic parameters. The average boulder density and flow velocity in transects did not differ significantly among the three stations (Bonferroni pairwise t-test, boulder density: P > 0.06; flow velocity: P > 0.50; Table 1). Water depth was significantly deeper at site 3 than at sites 1 or 2 (P < 0.01).
Size-dependent habitat use. The two subject species were the only Rhinogobius representatives recorded at our study sites. While both were present in site 1, only one Rhinogobius nagoyae was observed at site 2 and none at site 3. Rhinogobius flumineus was significantly smaller than R. nagoyae (t-test, P < 0.01; Fig. 2).
Only flow velocity was included in the best model for sites 2 and 3, with a positive coefficient (Table 2). At site 1, the best model included boulder density and water depth, but not flow velocity.
In multiple comparisons at sites 2 and 3, 4–5 and 5–6 cm fish resided in habitats with significantly greater mean flow velocity than 3–4 cm fish (Bonferroni pairwise t-test, P < 0.01; Fig. 3). No such difference existed at site 1 (P > 0.19). Larger (6–7 cm) individuals did not differ significantly from smaller fish at any station, probably because only 1–8 individuals were 6–7 cm long (P > 0.69).
Flow velocity of transects where Rhinogobius flumineus individuals of different size classes (based on standard length) were observed. Size classes did not differ significantly in their habitat flow velocity at site 1 (triangles), whereas in sites 2 (solid circle) and 3 (open circle), 4–5 and 5–6 cm classes resided in areas with significantly faster flow velocities than the 3–4 cm class (Bonferroni pairwise t-test, P < 0.01). Bars show SEs
At site 1, R. flumineus density was negatively correlated with flow velocity (Spearman rank correlation test, r = –0.44, P = 0.02; Fig. 4); in contrast, R. nagoyae density and flow velocity were positively correlated (r = 0.61, P < 0.01). At sites 2 and 3, R. flumineus density was not correlated with flow velocity (site 2: r = 0.10, P = 0.62; site 3: r = –0.13, P = 0.49).
Territorial behavior. We successfully determined competitive outcomes in 19 trials. Of these, nine trials involved mutual intimidation and subsequent attack behavior. In the remaining 10 trials, the battle was one-sided, with one fish finally chasing the other. When R. nagoyae won, they were significantly larger than the losers (binominal test, P < 0.01; Fig. 5), with only one smaller individual winning. When R. flumineus won, they were larger in all cases, but the paucity of trials precluded a significant difference (n = 4, P = 0.13). When R. nagoyae were larger than R. flumineus, they won contests more frequently than when they were smaller (Fisher’s exact test, P < 0.01). Thus, larger body size significantly enhanced the chances of victory regardless of species.
Discussion
This study demonstrated that Rhinogobius flumineus exhibited SHU: large individuals used fast riffles when the species was alone, but were outcompeted for those preferred habitats in the presence of Rhinogobius nagoyae, a larger congener. Rhinogobius exhibit strong intraspecific competition over territory, especially during spawning season, with both males and females prone to aggressive encounters (e.g., Takahashi et al. 2001; Ishino et al. 2005; Ito et al. 2016). Larger individuals usually win male–male competitions and mate with large females, forming a relationship between size and spawning-ground quality regardless of sex (Takahashi et al. 2001; Ito and Yanagisawa 2003).
Clear correlations between flow velocity and body size or density indicate that despite the two subject species being benthic, upper-layer water current provides benefits to them. Indeed, our findings corroborate previous reports of a positive correlation between upper-water-column velocity and R. nagoyae habitat use, whereas bottom velocity had no relation (Sone et al. 2001). Because Rhinogobius eggs require oxygen and are laid under stones (Mizuno 1961; Suk and Choe 2002; Maruyama et al. 2008; Tamada 2011), breeding adults may prefer riffles with rapid flow in the upper to middle layers, a condition that elevates dissolved oxygen levels. These fast-flowing habitats also aid feeding efficiency through increasing the availability of aquatic insects and their larvae (Allan and Russek 1985), the primary prey of Rhinogobius (Sone et al. 2001; Hirashima and Tachihara 2006). Stream-dwelling Rhinogobius are adapted to fast-flowing habitats by possessing an abdominal suction disc that allows them to occupy and feed on river substrates (e.g., stones) without heavy energy expenditure (Ito et al. 2006; Kondo et al. 2013). In addition, the irregularity and high velocity of surface flow in shallow riffles might provide protection from predators, such as waterfowl (e.g., Harvey and Stewart 1991). Therefore, upper/mid-layer water flow is important for stream Rhinogobius. This preference for rapid flow bolsters our findings regarding R. flumineus and R. nagoyae behavior, with large adults preventing smaller individuals from occupying fast riffles regardless of species.
Competition with R. nagoyae removed SHU in R. flumineus, as demonstrated by flow velocity being absent from the best-fit model for R. flumineus SHU when the two species coexisted (at site 1). Flow velocity also did not differ significantly across differently sized R. flumineus at this location. Therefore, while larger R. flumineus tended to use fast-flowing habitats at sites 2 and 3, they did not do so at site 1. Furthermore, R. nagoyae increased in density with increasing flow velocity at the latter location. Consistent with previous research (Mizuno 1961), R. flumineus was smaller on average than R. nagoyae. Thus, the presence of a larger congener negatively influenced R. flumineus SHU, with the species’ density and flow velocity being negatively correlated at site 1. The inability to compete with R. nagoyae for fast riffles led to R. flumineus using inferior habitats with slower currents.
Analysis of territorial behavior in the laboratory supported field observations of SHU, revealing that larger males dominated through intense chasing, regardless of species. Similar size-dependent hierarchy between conspecific or heterospecific individuals has also been observed in salmonid fishes (Nakano and Furukawa-Tanaka 1994; Usio and Nakano 1998). Notably, R. nagoyae body size increases from downstream to upstream habitats (Tamada 2009). Thus, SHU in R. flumineus may be differentially influenced by R. nagoyae depending on how far upstream it is located.
This study has two major limitations. First, flow velocity was measured only for the upper layer, despite the fact that bottom flow would likely play an important role in benthic Rhinogobius. However, because our primary goal was to compare intra- and interspecific differences in habitat, we felt that surface current should be a sufficient reflection of habitat variation. Second, our study was conducted in one stream only, meaning the results may not be generalizable to interspecific interactions between the two Rhinogobius species. Indeed, river size and coastal character affect the density and body size of amphidromous R. nagoyae (Uehara 1996; Tamada 2009). Therefore, the two species may interact differently in other streams.
In conclusion, we successfully described how interspecific competition from R. nagoyae influenced habitat use variation in R. flumineus. Our observations accord with earlier work showing that many stream fishes exhibit intraspecific SHU when feeding or spawning (e.g., Grant and Noakes 1988; Katano 1990; Nakano and Furukawa-Tanaka 1994; Nakano 1995; Usio and Nakano 1998; Davey et al. 2005). Importantly, we found that interspecific competition overrides intraspecific size-dependent habitat differentiation when the competitor’s body size is significantly larger. This study also provided some insight into interspecific interactions between congeners with distinct life histories. Though R. flumineus life history occurs entirely in freshwater, influence from the marine environment can occur via an amphidromous congener. Since R. nagoyae spends the larval period in coastal areas, its recruitment to a stream would heavily depend on marine environmental conditions, such as food availability (Mizuno 1960; Mizuno 1961; Takagi et al. 2012). Our study offers an example of how we can describe dynamic relationships between inter- and intraspecific interactions in stream fishes.
References
Allan JD, Russek E (1985) The quantification of stream drift. Can J Fish Aquat Sci 42:210–215
Bolnick DI, Svanbäck R, Araújo MS, Persson L (2007) Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proc Natl Acad Sci USA 104:10075–10079
Bolnick DI, Ingram T, Stutz WE, Snowberg LK, Lau OL, Paull JS (2010) Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc Roy Soc Lond B 277:1789–1797
Davey AJH, Hawkins SJ, Turner GF, Doncaster CP (2005) Size-dependent microhabitat use and intraspecific competition in Cottus gobio. J Fish Biol 67:428–443
Dobriyal P, Badola R, Tuboi C, Hussain SA (2017) A review of methods for monitoring streamflow for sustainable water resource management. Appl Water Sci 7:2617–2628
Grant JWA, Noakes DLG (1988) Aggressiveness and foraging mode of young-of-the-year brook charr, Salvelinus fontinalis (Pisces, Salmonidae). Behav Ecol Sociobiol 22:435–445
Harvey BC, Stewart AJ (1991) Fish size and habitat depth relationships in headwater streams. Oecologia 87:336–342
Hirashima K, Tachihara K (2006) Longitudinal distribution and dietary habits of the genus Rhinogobius in the Genka River of northern Okinawa Island, Japan. Jpn J Ichthyol 53:71–76
Ishida Y, Takemon Y, Ikebuchi S (2005) Differences in habitat preference of benthic fish among erosional–depositional reaches under different flow regimes. Ann Disas Prev Res Inst Kyoto Univ 48:935–943
Ishino K, Tsuji K, Iwata A, Suzuki T, Mizuno N, Koshikawa T, Kishi Y, Sawara Y, Takahashi S (2005) Gobiidae. In: Kawanabe H, Mizuno N, Hosoya K (eds) Freshwater fishes of Japan, 3rd edn. Yama-to-keikoku-sha, Tokyo, pp 585–623
Ito S, Iwao H, Sakata J, Inoue M, Omori K, Yanagisawa Y (2016) Simultaneous spawning by female stream goby Rhinogobius sp. and the association with brood cannibalism by nesting males. J Fish Biol 89:1592–1602
Ito S, Koike H, Omori K, Inoue M (2006) Comparison of current-velocity tolerance among six stream gobies of the genus Rhinogobius. Ichthyol Res 53:301–305
Ito S, Yanagisawa Y (2003) Mate choice and mating pattern in a stream goby of the genus Rhinogobius. Environ Biol Fish 66:67–73
Katano O (1990) Dynamic relationships between the dominance of male dark chub, Zacco temmincki, and their acquisition of females. Anim Behav 40:1018–1034
Kondo M, Maeda K, Hirashima K, Tachihara K (2013) Comparative larval development of three amphidromous Rhinogobius species, making reference to their habitat preferences and migration biology. Mar Freshwater Res 64:249–266
Maruyama A, Onoda Y, Yuma M (2008) Variation in behavioural response to oxygen stress by egg‐tending males of parapatric fluvial and lacustrine populations of a landlocked goby. J Fish Biol 72:681–692
Miyaji D, Kawanabe H, Mizuno N (1976) Rhinogobius flumineus. In: Colored illustrations of the freshwater fishes of Japan. Hoiku-sha, Osaka, pp 355–356
Mizuno N (1960) Study on a freshwater goby, Rhinogobius similis Gill, with a proposition on the relationships between land-locking and speciation of some freshwater gobies in Japan. Mem Col Sci Univ Kyoto Ser B 27:97–115
Mizuno N (1961) Study on the gobioid fish, “Yoshinobori” Rhinogobius similis Gill–I. Comparison of life histories of thee ecological types. Bull Jpn Soc Sci Fish 27:6–11
Nakano S (1995) Individual differences in resource use, growth and emigration under the influence of a dominance hierarchy in fluvial red-spotted masu salmon in a natural habitat. J Anim Ecol 64:75–84
Nakano S and Furukawa-Tanaka T (1994) Intra- and interspecific dominance hierarchies and variation in foraging tactics of two species of stream dwelling chars. Ecol Res 9:9–20
Quevedo M, Svanbäck R, Eklöv P (2009) Intrapopulation niche partitioning in a generalist predator limits food web connectivity. Ecology 90:2263–2274
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Sone S, Inoue M, Yanagisawa Y (2001) Habitat use and diet of two stream gobies of the genus Rhinogobius in south-western Shikoku, Japan. Ecol Res 16:205–219
Suk H, Choe JC (2002) The presence of eggs in the nest and female choice in common freshwater gobies (Rhinogobius brunneus). Behav Ecol Sociobiol 52:211–215
Svanbäck R, Eklöv P, Fransson R, Holmgren K (2008) Intraspecific competition drives multiple species resource polymorphism in fish communities. Oikos 117:114–124
Takagi M, Sekiya I, Shibakawa R, Shimizu T, Kawanishi R, Inoue M (2012) Genetic influence of the dam isolations in the Kamo and Nakayama River systems on the common fresh water goby populations Rhinogobius spp. Ecol Civil Eng 15:161–170
Takahashi D, Kohda M, Yanagisawa Y (2001) Male–male competition for large nests as a determinant of male mating success in a Japanese stream goby, Rhinogobius sp. DA. Ichthyol Res 48:91–95
Tamada K (2009) Variations in clutch and egg sizes in the amphidromous goby Rhinogobius sp. CB along a river course and within a spawning season. Ichthyol Res 56:69–75
Tamada K (2011) River bed features affect the riverine distribution of two amphidromous Rhinogobius species. Ecol Freshw Fish 20:23–32
Uehara SI (1996) Distributions of six species of Rhinogobius on the coastal area of Ise Bay, Japan. Jpn J Ichthyol 43:89–99
Usio N, Nakano S (1998) Influences of microhabitat use and foraging mode similarities on intra- and interspecific aggressive interactions in a size-structured stream fish assemblage. Ichthyol Res 45:19–28
Wentworth CK (1922) A scale of grade and class terms for clastic sediments. J Geol 30:377–392
Yamazaki Y, Haramoto S, Fukasawa T (2006) Habitat uses of freshwater fishes on the scale of reach system provided in small streams. Environ Biol Fish 75:333–341
Acknowledgments
We are grateful to K. Watanabe, H. Nakagawa, Y. Yamasaki, and R. Ito (Kyoto University) for constructive comments on the manuscript. Suggestions from laboratory colleagues are also greatly appreciated. Comments from reviewers substantially improved the quality of the manuscript. All experiments in this study complied with the current laws of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Oto, Y., Masuda, R. Size-dependent habitat use in the stream goby Rhinogobius flumineus is affected by a larger sympatric congener. Ichthyol Res 66, 393–399 (2019). https://doi.org/10.1007/s10228-019-00684-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-019-00684-y