Abstract
Rhinogobius sp. CB is a common amphidromous goby in Japan. I investigated egg and clutch sizes along a river course and within a spawning season in the Aizu River, Kii Peninsula, Japan. Clutch size within the same female size did not differ along the course of the river, but decreased from early to later in the spawning season. Egg size within the same female size decreased from early to later in the spawning season and from the upper to the lower reaches. These results mean energy resources allocated to the ovary decreased seasonally and locally. Several factors are presented to explain these variations, such as female somatic conditions, a tradeoff between reproduction and growth, food availability, water temperature, and presence of competitive species. Some factors explaining the seasonal and local variations in egg size, such as water temperature and food availability for offspring, are also presented. Egg size correlated well with female size. The trend in egg size variation with female size seems to be an adaptation for their migrating pattern, as larvae from larger female, which tended to inhabit the upper reaches, would have a greater endurance against starvation during their migration to the sea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Egg and clutch sizes in teleost fishes are inversely related and are very important features of female strategies for maximizing reproductive success (e.g., Smith and Fretwell 1974; Einum and Fleming 2000). Egg and clutch sizes are generally species-specific, but these features can vary within and/or among populations in response to environmental conditions, such as female size, food availability, spawning season, or latitudinal distribution (e.g., Wootton 1998; Laptikhovsky 2005).
Rhinogobius sp. CB (cross band type) sensu Kawanabe and Mizuno (1989) is the most common species in the Rhinogobius brunneus species complex in Japan (Mizuno 1989). Rhinogobius sp. CB is amphidromous (McDowall 2007), and its life history involves the following sequence: downstream larval migration to the sea soon after hatching, and then upstream juvenile migration from the sea back into freshwater for further somatic growth, sexual maturation, and reproduction (Mizuno 1960, 1989). Their spawning season ranges from later April to early August (Tamada 2000). Generally, smaller and younger adults spawn in the lower reaches, and larger and older adults spawn in the upper reaches since they migrate upstream as they grow (Tamada 1986, 2008). Because spawning season and vertical distribution in Rhinogobius sp. CB show wide variations, the environmental conditions (e.g., water temperature and food conditions) can also vary greatly. This species therefore provides an interesting case in phenotypic variations in egg and clutch sizes in response to changes in environmental conditions.
Rhinogobius sp. CB exhibits the following inter- and intra-specific variations in egg and clutch sizes: they produce relatively larger eggs and smaller clutch sizes than the sympatric Rhinogogius brunneus complex (Rhinogobius sp. CO and Rhinogobius sp. LD sensu Kawanabe and Mizuno 1989) (Tamada 2001) and produce similar-sized eggs and clutches to Rhinogobius sp. DA sensu Kawanabe and Mizuno (1989) inhabiting small rivers in the same district (Tamada 2005a). Egg size in Rhinogobius sp. CB increases with female size and maternal age, and the 72-h survival rate of starved hatchlings increases with egg size in laboratory conditions (Tamada and Iwata 2005). Females spawn smaller eggs in their second spawning than in their first in laboratory conditions (Tamada and Iwata 2005). In the field, eggs deposited in the nest increase in size in the upper reaches of the river and decrease later in the spawning season, although maternal information for these spawning events is unknown (Tamada and Iwata 2005).
The size of females who deposited egg clusters is difficult to detect in the wild because females depart the nest soon after spawning (Tamada and Iwata 2005). I recently developed a method to resolve the mating pattern and female size from egg density in the nest (Tamada 2008). In the present study, I estimated female size from egg density of egg clusters used by Tamada and Iwata (2005) using Tamada’s (2008) method, and demonstrated variations in egg and clutch sizes along the river course and within the spawning season, including the influence of female size on egg and clutch sizes.
Materials and methods
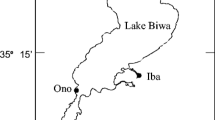
Fieldwork was conducted from May 2003 to July 2003 in the Aizu River, Kii Peninsula, Japan. Three sampling stations were established along the course of the river (see Tamada and Iwata 2005): Station 1 [2 km from the river mouth, <10 m above sea level (a.s.l.)] located near the seaward distribution limit of adults and their spawning ground (Tamada 2000), Station 3 (12 km from the river mouth, 70 m a.s.l.) located near the upper distribution limit of this goby, and Station 2 (8 km from the river mouth, 30 m a.s.l.) located between Station 1 and Station 3.
To measure the water temperatures in the three sampling stations, I placed a data logger (Thermo recorder RT-12, Espec mic Corp.), which was set to measure water temperatures in 30-min intervals for 24 h, at each of the three sites on the same day at various times from March (pre-spawning season) to July (late spawning season) 2004. The average and the range of the water temperatures were measured automatically over each 24-h period.
Nest stones containing egg clusters [samples from Tamada and Iwata (2005)] and adult females [May samples from Tamada (2008)] were collected at the three sites. After preserving the adult females in 10% formalin in the field, I measured the standard length (SL), body weight, gonad weight (p), and weight (q) and number (r) of partial eggs in the laboratory. Clutch size was defined as total number of oocytes in the ovaries and was calculated as pr/q. The regression between clutch size and SL was calculated for each month. Since Rhinogobius sp. CB normally discharge all mature eggs at once (Ito and Yanagisawa 2003; Tamada and Iwata 2005; Tamada 2008), this regression was used to estimate female size from egg number deposited in the nest. Egg size (mm3) was calculated from the main and minor axes of the spawned egg [samples from Tamada and Iwata (2005)] using the method by Tamada and Iwata (2005). The mean egg volume from 100 eggs was regarded as egg size in the egg cluster.
In Rhinogobius sp. CB, multiple females often spawn eggs in the same nest simultaneously or sequentially (Ito and Yanagisawa 2003; Tamada 2008). Tamada (2008) discovered that egg density in the nest can be used as an index of mating pattern (samples were the same as those from May in the present study), and egg clusters with egg densities under 106 eggs/cm2 were almost always spawned by a single female, those between 106 and 143 eggs/cm2 almost always spawned by two females, and those over 143 eggs/cm2 almost always spawned by three or more females. I determined female number using Tamada’s (2008) method and regarded egg clusters with egg densities under 106 eggs/cm2 as spawned by a single female. The numbers of eggs in these egg clusters were used for the regression between clutch size and SL in each month to calculate female SL.
Because the egg clusters whose egg densities were over 106 eggs/cm2 were spawned by multiple females (Tamada 2008), they would likely be composed of different sized egg cluster groups. The standard deviation of the distribution of egg size positively correlated with egg density (r = 0.24, P < 0.001, n = 187; K. Tamada, unpublished data). I divided these egg clusters into multiple groups based on their egg size distributions using cohort analysis when the distributional patterns showed two or three clear peaks. Each divided egg cluster group was regarded as spawned by a single female, and the female sizes and egg sizes were estimated using the above-mentioned method.
Differences in egg size and clutch size among sites and months were analyzed using ANCOVA on SL or ANOVA followed by multiple-comparison test.
Results
Water temperature. The average water temperature ranged from 10.0°C (Station 3 on 21 May 2004) to 29.9°C (Station 1 on 28 July 2004) (Fig. 1). The water temperature increased from pre-spawning season to late spawning season in each site and varied along the course of the river, with lower values in the upper reaches. The difference between the average water temperature at Station 1 and at Station 3 ranged from 3.1 to 5.5°C. Averages of the water temperature at Station 2 were between the readings of the two other sites, and generally closer to those at Station 3.
Clutch size. In total, 129 adult females were collected. Female SL and clutch size in each site was shown in Table 1, and both values tended to be larger in the upper reach for each month. To compare clutch size from females of the same SL among 3 months, the regression between clutch size and SL was calculated by pooling the data from the three sites together for each month (Fig. 2). The log10-transformed clutch size correlated well with log10-transformed SL in each month. The regressions significantly differed between months, and clutch size for the same female size showed higher values in May than in June and July (ANCOVA, slope, F 2,123 = 1.26, P = 0.29, intercept, F 2,125 = 8.90, P < 0.001; t test with Bonferroni’s correction, P < 0.05 in May vs. June, P < 0.01 in May vs. July, P = 0.18 in June vs. July).
Clutch sizes in the abdomen plotted against standard length (mm) of the females collected at the three sites along the course of the Aizu River in each month (a May, b June, c July). Solid squares, open squares, and solid triangles indicate Station 1, 2, and 3, respectively. Regression lines are Y = −1.24 + 2.79X (r = 0.97, n = 84, P < 0.0001) in May, Y = −1.58 + 2.98X (r = 0.98, n = 35, P < 0.0001) in June, and Y = −0.08 + 2.52X (r = 0.76, n = 10, P < 0.001) in July
In all sites, the log10-transformed clutch size correlated well with log10-transformed female SL in May (Station 1, r = 0.95, n = 33, P < 0.0001; Station 2, r = 0.93, n = 21, P < 0.0001; Station 3, r = 0.94, n = 30, P < 0.0001) and June (Station 1, r = 0.96, n = 12, P < 0.0001; Station 2, r = 0.96, n = 14, P < 0.0001; Station 3, r = 0.79, n = 9, P < 0.01). The regressions between clutch size and female SL did not significantly differ among the three sites in each month (May, ANCOVA, slope, F 2,78 = 0.59, P = 0.56, intercept, F 2,80 = 0.17, P = 0.84; June, ANCOVA, slope, F 2,29 = 0.63, P = 0.54, intercept, F 2,31 = 1.04, P = 0.36; July, not analyzed because most females did not developed oocytes as this late in the spawning season).
Egg size. A total of 166 of the 187 egg clusters collected were used to estimate female SL (81 egg clusters under 106 eggs/cm2 in egg density; 77 egg clusters between 106 and 143 eggs/cm2 in egg density; 8 egg clusters over 143 eggs/cm2 in egg density). Estimated female SL and egg size in each site were shown in Table 2, and both values tended to be larger in the upper reach for each month. Besides, egg size tended to decrease later in the spawning season.
The relationship between estimated egg size and SL at each site is shown in Fig. 3 for each month. To compare the relationships between estimated female SL and egg size among months, the regression was calculated by pooling the data from the three sites together for each month (Fig. 3). Egg size significantly correlated with the estimated SL in each month. The regressions significantly differed among months, and egg size for the same female size showed higher values in May than in June and July (Table 2).
Spawned egg sizes in the nest plotted against estimated maternal standard length (mm) at the three sites along the course of the Aizu River in each month (a May, b June, c July). Solid squares, open squares, and solid triangles indicate Stations 1, 2, and 3, respectively. Regression lines are Y = 0.67 + 0.008X (r = 0.52, n = 161, P < 0.0001) in May, Y = 0.48 + 0.010X (r = 0.57, n = 64, P < 0.0001) in July, Y = 0.35 + 0.013X (r = 0.65, n = 34, P < 0.0001) in July
In May, significant correlations between egg size and estimated female SL were recognized in all sites (Station 1, r = 0.46, n = 59, P < 0.001; Station 2, r = 0.34, n = 57, P < 0.01; Station 3, r = 0.36, n = 39, P < 0.05). Eggs from females of the same size were significantly larger in the upper reaches in May (ANCOVA, slope, F 2,155 = 0.47, P = 0.63, intercept, F 2,157 = 69.33, P < 0.0001; t test with Bonferroni’s correction, P < 0.0001 in Station 1 vs. Station 2, P < 0.0001 in Station 1 vs. Station 3, P = 0.05 in Station 1 vs. Station 3). In June, significant correlation between egg size and estimated female SL was recognized only in Station 3 (r = 0.70, n = 15, P < 0.01), but not recognized in Station 1 (r = 0.40, n = 21, P = 0.07) and Station 2 (r = 0.24, n = 28, P = 0.21). In July, significant correlations were not recognized in all sites (Station 1, r = 0.14, n = 11, P = 0.70; Station 2, r = 0.43, n = 11, P = 0.20; Station 3, r = 0.17, n = 12, P = 0.17). I used ANOVA to compare egg sizes among sites in June and July. Egg sizes were larger in the upper reach in each month (June, F 2,61 = 20.31, P < 0.0001, Scheffé’s test, P < 0.001 in Station 1 vs. Station 2, P < 0.0001 in Station 1 vs. Station 3 and P < 0.05 in Station 2 vs. Station 3; July, F 2,31 = 45.58, P < 0.0001, Scheffé’s test, P < 0.0001 in Station 1 vs. Station 2, P < 0.0001 in Station 1 vs. Station 3 and P < 0.05 in Station 2 vs. Station 3).
Discussion
Energy resources allocated to the ovary. In the present study, both clutch size and egg size decreased from early to late in the spawning season and from the upper to the lower reaches in Rhinogobius sp. CB. This means energy resources allocated to the ovary decreased seasonally and locally. Under laboratory conditions [bred with sufficient food and in a constant water temperature (22°C) nearly equal to that in May in the Aizu River], some females spawned twice, with an average interval of 30 days. Eggs later in the spawning season may therefore be deposited by females spawning for the second time or more. The fact that eggs from the second spawning were smaller than those from the first spawning in the laboratory supports this supposition, although there were no differences in clutch sizes between the two spawnings (Tamada and Iwata 2005).
Several factors could explain the seasonal variations in energy resources allocated to the ovary. The liver and body of Rhinogobius sp. CB females are so thin that their somatic conditions are weak. If females spawn twice or more, they would need to save energy resources for the second spawning in order to recover their somatic condition. Water temperature is known to profoundly affect fish growth, and higher water temperatures over optimal ranges increase maintenance requirements (Brett 1971; Wootton 1998), which would reduce the energy resources available to the ovary. Higher water temperature later in the spawning season and in the lower reaches may therefore also reduce energy resources available to the ovary in Rhinogobius sp. CB. Additionally, females later in the spawning season would have to invest energy not only for reproduction, but also for growth in order to have reproductive success for the following year. This may affect energy resources to the ovary later in the spawning season.
For females that spawn twice or more, the interval between successful spawning would likely be longer in the field than under laboratory conditions, because conditions in the field are not as favorable as those used in the laboratory (such as food availability, water temperature, and mating conditions). Delayed spawning may force females to allocate still more energy to growth or maintenance.
Local variations in energy resources allocated to the ovary may also be explained by food availability or water temperature. Generally, food availability affects fecundity in teleost fish (Wootton 1998). In some amphidromous fishes, such as Cottus hangiongensis (Goto 1989), Rhinogobius sp. CB (Tamada 1990), and Rhinogobius sp. LD (Tamada, unpulished data), growth rate or condition factor is higher in the upper reaches than in lower reaches. Goto (1989) suggested this results from the difference in food availability along the river course. The biomass of aquatic insects (the main food for this goby) is higher in the upper reach than in the lower reach in the Aizu River (Anaze et al. 1985), suggesting that food resources are more abundant in the upper reaches. Low food availability in the lower reaches may reduce energy resources available to the ovary in Rhinogobius sp. CB. Water temperature was higher in the lower reaches of the river than in the upper reaches. Higher water temperature in the lower reaches may also affect energy allocated to the ovary in Rhinogobius sp. CB.
Energy investment to the ovary may also be affected by the presence of competitive species. In the Aizu River, the amphidromous gobies Rhinogobius giurinus and Tridentiger brevispinis mainly inhabit the lower reach (Station 1) (Tamada 2000, 2008). Feeding habits of these two gobies resemble those of Rhinogobius sp. CB, and both grow larger than Rhinogobius sp. CB (Kawanabe and Mizuno 1989). Rhinogobius sp. CB is therefore at a disadvantageous in food acquisition. Tridentiger brevispinis (Tamada 2008) and Rhinogobius giurinus (Tamada, unpublished data) also appear to have an advantage in securing nest stones. On the other hand, these two gobies do not inhabit the middle (Station 2) or upper reaches (Station 3) of the river (Tamada 2000, 2008). Two other species, Rhinogobius sp. LD and Rhinogobius sp. CO, inhabit the upper reaches (Station 3), but their population densities are low, and they therefore do not present significant competition (Tamada 2000, 2008). Rhinogobius sp. CB may therefore allocate more energy for competition in the lower reach, resulting in less energy available for the ovaries.
Egg size. In some teleost fishes, egg size increases with the female's size (e.g., Mann and Mills 1985; Gregersen et al. 2006). Tamada and Iwata (2005) revealed a positive correlation between female size and egg size and between egg size and larval starvation tolerance in Rhinogobius sp. CB under laboratory conditions. In the present study, I confirmed the positive correlation between female size and egg size in the wild. In amphidromous fishes, the distance for downstream larval migration is considered to be an important factor affecting larval survival, because larvae suffer risks of starvation and predation during the migration (Tsukamoto 1991; Moriyama et al. 1998; Iguchi and Mizuno 1999; Maruyama et al. 2003). Tamada and Iwata (2005) suggested that a larger body size is advantageous for the female of this goby because larger females, which tend to spawn in the upper reaches, produce larvae that would have greater endurance against starvation during their migration to the sea. The results from the wild in the present study strongly support Tamada and Iwata’s (2005) suggestion that the trends in egg size variation with female size in Rhinogobius sp. CB are adaptations for their migrating pattern.
Egg size in the same female size also varied along the river course and during spawning season, with females producing larger eggs in the upper reach and early in the spawning season. Egg size varies inter- and intra-specifically with changes in the environmental conditions, such as size and abundance of prey and predators and water temperature (Wootton 1998). In both the seasonal and the local egg size variation, egg sizes were larger in lower water temperatures. This is a common phenomenon in fishes (e.g., Imai and Tanaka 1987; Hinckley 1990; Mihelakakis et al. 1995).
Ware (1975) developed a model showing that the optimal egg size decreases as the incubation period (the time from fertilization to hatching) decreases. According to this model, as incubation time decreases with an increase in temperature, the optimal egg size in this goby should decrease in the lower reaches or later in the spawning season.
Hutchings (1991, 1993) estimated that the production of a small number of large eggs maximizes female fitness under poor food conditions, and a large number of small eggs maximize female fitness under rich food conditions. Generally, the biomass of zooplankton increases late after the so-called “spring bloom” in phytoplankton (Nybakken and Bertness 2005). According to Hutchings’ estimation, optimal egg size in this goby should decrease later in the spawning season when zooplankton is abundant. Decreased egg size within the same maternal size in the lower reaches and later in the spawning season may therefore be adaptations to the changes in water temperature.
Among the amphidromous Rhinogobius species in the Aizu River, Rhinogobius giurinus, whose main spawning season is during the summer, allocates less energy for reproduction and produces smaller eggs than Rhinogobius spp. CB, CO, and LD, whose main spawning season is during the spring (Tamada 2005b). It is interesting that intra-specific variations in clutch and egg sizes from spring to summer in Rhinogobius sp. CB paralleled inter-specific variations in sympatric Rhinogobius species. Similar factors may affect both intra- and inter-specific variations in clutch and egg size in Rhinogobius gobies.
References
Anaze N, Tamai S, Yuba T (1985) Comparison of macro-benthic fauna in the Aizu River (Wakayama Pref.) between 1973 and 1984. The Nanki Seibutu 27:7–13
Brett JR (1971) Energy responses of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon. Am Zool 11:99–113
Einum S, Fleming IA (2000) Highly fecund mothers sacrifice offspring survival to maximize fitness. Nature 405:565–567
Goto A (1989) Growth patterns, and age and size at maturity in female Cottus hangiongensis, with special reference to their life history variation. Jpn J Ichthyol 36:90–99
Gregersen F, Haugen T, Larsen ØN (2006) Egg size differentiation among sympatric demes of brown trout: possible effects of density-dependent interactions among fry. Ecol Freshw Fish 15:237–246
Hinckley S (1990) Variation of egg size of walleye pollock Theragra chalocogramma with a preliminary examination of the effect of egg size on larval size. Fish Bull US 88:471–483
Hutchings JA (1991) Fitness consequences of variation in egg size and food abundance in brook trout, Salvelinus fontinalis. Evolution 45:1162–1168
Hutchings JA (1993) Behavioural implications of intraspecific life history variation. Mar Behav Physiol 23:187–203
Iguchi K, Mizuno N (1999) Early starvation limits survival in amphidromous fishes. J Fish Biol 54:705–712
Imai C, Tanaka S (1987) Effect of sea water temperature on egg size of Japanese anchovy. Nissuishi 53:2169–2178
Ito S, Yanagisawa Y (2003) Mate choice and mating pattern in a stream goby of the genus Rhinogobius. Environ Biol Fish 66:67–73
Kawanabe H, Mizuno N (1989) Freshwater fishes of Japan. Yama-kei, Tokyo
Laptikhovsky VV (2005) Latitudinal and bathymetric trends in egg size variation: a new look at Thorson’s and Rass’s rules. Mar Ecol 27:7–14
Mann RHK, Mills CA (1985) Variation in the sizes of gonads, eggs and larvae of the dace, Leuciscus leuciscus. Environ Biol Fish 13:227–287
Maruyama A, Rusuwa B, Yuma M (2003) Interpopulational egg-size variation of a landlocked Rhinogobius goby related to the risk of larval starvation. Environ Biol Fish 67:223–230
McDowall RM (2007) On amphidromy, a distinct form of diadromy in aquatic organisms. Fish Fish 8:1–13
Mihelakakis A, Yoshimatsu T, Kitajima C (1995) Changes in egg size of Japanese flounder during one spawning season. J Fac Agr Kyusyu Univ 40:53–59
Mizuno N (1960) Study on a freshwater goby, Rhinogobius similis Gill, with a proposition on the relationships between land-locking and speciation of some freshwater gobies in Japan. Mem Col Sci Univ Kyoto Ser B 27:97–115
Mizuno N (1989) Shima-yoshinobori, Rhinogobius sp. CB. In: Kawanabe H, Mizuno N (eds) Freshwater fishes of Japan. Yama-kei, Tokyo, pp 586–587
Moriyama A, Yanagisawa Y, Mizuno N, Omori K (1998) Starvation of drifting goby due to retention of free embryos in upstream reaches. Environ Biol Fish 52:321–329
Nybakken JW, Bertness MD (2005) Marine biology. Pearson Education, San Francisco
Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am Nat 108:708–720
Tamada K (1986) Size variation of a freshwater goby, Rhinogobius brunneus, cross-band type, along the river length in terms of age and growth. The Nanki Seibutu 28:22–34
Tamada K (1990) Studies on the comparison of condition factors of the common freshwater goby, Rhinogobius brunneus (Cross-band type), along the stream. The Nanki Seibutu 32:13–19
Tamada K (2000) Distributions of the spawning grounds of four amphidromous gobies (Rhinogobius spp.) in the Aizu River, Wakayama Prefecture, Japan. Jpn J Ichthyol 47:55–59
Tamada K (2001) Clutch size and egg size in the three species of Rhinogobius complex dwelling in a single stream Japan. Jpn J Ichthyol 48:49–52
Tamada K (2005a) Clutch and egg size in Rhinogobius sp. DA inhabiting small rivers. Jpn J Ichthyol 52:17–20
Tamada K (2005b) Egg and clutch sizes of a goby Rhinogobius giurinus in the Aizu River, Kii Peninsula, Japan. Ichthyol Res 52:392–395
Tamada K (2008) Estimate of mating pattern of a paternal nest brooder goby of Rhinogobius, using egg density in the nest. Ichthyol Res 55:191–197
Tamada K, Iwata K (2005) Intra-specific variations of egg size, clutch size and larval survival related to maternal size in amphidromous Rhinogobius goby. Environ Biol Fish 73:379–389
Tsukamoto K (1991) Age and growth of ayu larvae Plecogrossus altivelis collected in the Nagara, Kiso and Tone River during the downstream migration. Bull Jpn Soc Sci Fish 57:2013–2022
Ware DM (1975) Relation between egg size, growth, and natural mortality of larval fish. J Fish Res Board Can 32:2503–2512
Wootton RJ (1998) Ecology of teleost fishes, 2nd edn. Kluwer, London
Acknowledgments
I thank Dr. Katsuya Iwata (Wakayama University), Sigeyuki Yamato (Kyoto University), and Atsushi Maruyama (Ryukoku University) for their helpful advices.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tamada, K. Variations in clutch and egg sizes in the amphidromous goby Rhinogobius sp. CB along a river course and within a spawning season. Ichthyol Res 56, 69–75 (2009). https://doi.org/10.1007/s10228-008-0069-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-008-0069-7