Abstract
The pseudoscorpion Paratemnoides nidificator is a generalist predator that captures large arthropods that live on tree trunks. Few pseudoscorpions species show some degree of sociality. We investigated how colonies of the pseudoscorpion P. nidificator adjust their cooperative capture behavior under a situation of changing prey types as a simulation of variation in prey availability. We hypothesized that colonies would be more efficient at prey capture under repeated exposure to the same prey, and that the change in the availability of prey would be followed by new behavioral adjustments to adequately exploit the new prey. Eight experimental colonies housed in the laboratory received repetitions of three different ant species as prey. The number of pseudoscorpions attacking the prey, the number of behavioral acts, and the time expended subduing prey were evaluated as measures of prey capture performance, in relation to repetitive exposure to the same prey and also in relation to prey type changes. However, only individuals’ recruitment significantly responded to prey type exposure. Prey capture behavior was heterogeneous among colonies, resulting in highly variable behavioral responses. Colonies showed a tendency toward increasing capture success through repeated prey type exposure. However, 50% of the colonies were unable to capture the new prey type and died of starvation. Although it is a generalist predator, prey capture behavior could depend on different coordination components for subduing and handling large prey. Therefore, changes in prey availability could cause the attenuation of a cooperative relationship in some colonies, making them more prone to failure during capture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social behavior is rare among invertebrates, although it is common and diversified among hymenopterans (Wilson 1971). If social behavior is unusual in insects, it is even less frequent among arachnids and may occur in very distinct forms (Costa 2006). All social arachnids (some spiders, harvestmen, and scorpions, for example) have a gregarious life, benefiting from communal use of shelters and collective defense against natural predators, generally without specialized cooperative behaviors (Choe and Crespi 1997; Costa 2006). However, in Araneae, a few species form dense colonies mediated by complex cooperative behaviors, such as alloparental care, cooperative defense, and communal web building (Avilés 1997; Lubin and Bilde 2007).
Cooperative prey capture allows predator groups to combine efforts to catch large prey, consequently reducing individual energy investment and increasing the capture success ratio (Krebs and Davies 1997; Alcock 2009; Davies et al. 2012). Large vertebrates, such as wolves, lions, and orcas, are classic examples of species adapted to establishing complex social relationships for the cooperative capture of prey (Krebs and Davies 1997; Davies et al. 2012), enabling the development of models regarding the evolution of cooperative strategies and reciprocity (Packer and Ruttan 1988).
Cooperative capture has been identified as an important feature in the maintenance of social life in spiders (Whitehouse and Lubin 2005; Lubin and Bilde 2007). Coordination among individuals for cooperative attack and food sharing generates dual benefits: the procurement of large amounts of food (initial benefit; a result of communal living) and the suppression of intraspecific aggressiveness (causal benefit: by the reduction of hunger) (Whitehouse and Lubin 2005; Lubin and Bilde 2007). Similar complex social behavior is also found among pseudoscorpions.
Pseudoscorpions are small arachnids (2-8 mm) that live mainly in leaf litter, tree trunk, and cave walls. These predators are mainly solitary animals and feed on small insects and other arthropods (Harvey 1986; Zeh and Zeh 1997; Adis and Mahnert 2002). However, such species, like Paratemnoides nidificator (Balzan 1888), form colonies with dozens or hundreds of individuals in a complex system that involves different cooperative behaviors (Brach 1978; Zeh and Zeh 1990; Del-Claro and Tizo-Pedroso 2009).
Recently published studies have drawn attention to the social system exhibited by populations of P. nidificator living in the Brazilian Cerrado under the bark of trees (families Caesalpiniaceae and Fabaceae). These pseudoscorpions constitute a cooperative system involving cooperative hunting and food sharing, collective colony maintenance, and cooperative parental care (Tizo-Pedroso and Del-Claro 2005, 2007, 2014). A detailed analysis of P. nidificator’s social system revealed that the evolution of sociality is closely related to the origin of their complex parental behaviors and collective hunting (Del-Claro and Tizo-Pedroso 2009). This species also shows an elaborate system of division of labor, in which the adults of both sexes, as well as the nymphs, have distinct tasks in colonies (Tizo-Pedroso and Del-Claro 2011).
Although its cooperative behaviors enable P. nidificator to subdue prey like beetles, stinkbugs, ants, spiders, and other invertebrates (Garcia et al. 2016; Tizo-Pedroso and Del-Claro 2007), the mechanisms promoting these behaviors have not yet been thoroughly described. Unlike most cooperative spiders (Gonzaga 2007; Lubin and Bilde 2007), pseudoscorpions do not forage using webs to catch their prey. Instead, P. nidificator ambushes invertebrates that occasionally walk over the tree bark. This behavior generates a component of random encounter of prey, in which pseudoscorpions may attack a wide range of arthropods (Tizo-Pedroso and Del-Claro 2007, 2018; Moura et al. 2018). Only after the beginning of the attack can pseudoscorpions evaluate the prey and decide to evade (in case of risky prey) or persist in the attack. Thus, we predict that to capture a wide variety of prey in a rich environment, colonies of P. nidificator must adjust their capture behavior to adequately subdue each type of prey in accordance with its size, body mass, aggressiveness, and defensive behaviors. Alternatively, if some kind of prey predominates in the habitat, colonies might present behaviors to specialize in its capture, becoming more efficient in their attempts. Thus, the social pseudoscorpion P. nidificator becomes a good model for experimentation and for testing hypotheses about the evolution of cooperative foraging.
This study aimed to investigate cooperative prey capture in P. nidificator, analyzing how pseudoscorpion colonies adjust their prey capture behaviors under a situation of changing prey types as a simulation of changes in prey availability. We hypothesized that colonies would be more efficient at prey capture under repeated exposure to the same prey, and that the change in the availability of prey would be followed by new behavioral adjustments to adequately exploit the new prey. To evaluate prey capture efficiency, we expected that consecutive captures of the same prey would be followed by (i) a reduction in time required to subdue prey (individuals would spend less time to capture it); (ii) an increase in number of pseudoscorpions attacking the prey (familiarization with prey would stimulate the engagement of more individuals); and (iii) a reduction in number of behavioral acts required to capture and manipulate prey (optimized prey capture would demand fewer behavioral acts).
Material and methods
For this study, eight colonies of P. nidificator (with numbers of individuals ranging from 15 to 30 adult pseudoscorpions) were collected from the region of Morrinhos municipality, Goiás State, Brazil (17° 44′ 20.39″ S and 49° 7′ 42.43″ W) (Cerrado biome). The colonies used here were collected under the bark of sibipiruna trees (Caesalpinia peltophoroides Benth.; Caesalpiniaceae). A group of individuals living under the same piece of bark and sharing the silk chambers was considered a colony. Only one colony per tree was collected.
Pseudoscorpions were collected using thin brushes and temporarily housed in plastic boxes containing bark fragments. Later, the colonies were transported to the Laboratory of Behavioral Ecology of Arachnids at the State University of Goiás, Morrinhos. Adult pseudoscorpions were marked using a procedure applied in previous study (Tizo-Pedroso and Del-Claro 2018). Each colony was housed on a glass plate (20 cm × 10 cm and 2 mm) containing fragments of bark fixed with hot glue and small pieces of moistened cotton. Each plate of glass was housed inside a transparent plastic box, and the boxes were maintained in an incubator chamber (under conditions of 12-h light and 12-h dark and constant temperature of 23 °C).
We offered three different prey species to the experimental colonies maintained under laboratory conditions, simulating an effect of change in prey type to evaluate the responses in terms of capture behaviors. The ants are natural and common prey for P. nidificator, being the most abundant food resource during the dry season (Tizo-Pedroso and Del-Claro 2007). Individuals of Camponotus mus Roger, 1863 (prey I), Cephalotes clypeatus (Fabricius, 1804) (prey II), and Camponotus crassus Mayr, 1862 (prey III) were used as prey (Fig. 1). These three ant species were chosen because they have an arboreal habit, co-occur with pseudoscorpions, and are active throughout the year, ensuring the continuity of experiments. All three ant species are larger than adult pseudoscorpions. However, both Camponotus species are aggressive and more active than C. clypeatus. Cephalotes clypeatus is the largest of the three species, although it is more docile and slow. Although the three species of ants are similar, they can be considered morphologically and behaviorally different. Prey II shows some morphological differences in relation to other two species. Cephalotes clypeatus shows smooth exoskeleton and short mandibles and does not have formic acid.
Each colony was exposed to independent predation events for each type of prey (following the sequence of prey I, II, and III; species were determined previously by a draw). First, we made 10 predation events with prey type I, then we changed to prey II, with 10 events, and finally prey III, with 10 events. An interval of 3 days was allowed for each predation event (both for successful or incomplete capture events). In each predation event, the plastic box containing the glass plate was positioned on an observational support containing a mirror (Fig. 2). After waiting 5 min, the prey was inserted onto the plastic box. The pseudoscorpion colonies were observed by recording the prey capture behaviors. A period of 20 min was granted to evaluate whether predation would occur. When pseudoscorpions did not respond, the observer removed the prey and the observation was considered incomplete.
When captures were successful, the observations followed the ad libitum sampling method (Altmann 1974), which allows the recording of all behaviors in continuous and sequential mode during a maximum time of 60 min. This sampling is highly applicable to colonies of P. nidificator, because individuals are relatively slow and could be easily followed by the observer (Tizo-Pedroso and Del-Claro 2011). This period of observation was sufficient to record the immobilization process, prey subduing process (which usually takes 15–25 min; Tizo-Pedroso and Del-Claro 2005, 2007), and the beginning of the feeding process. At the end, the plastic box was returned to the incubator chamber. Ten complete assays were carried out for each of the three prey types. In the case of failed events, the ants were freed to avoid reuse of the same individuals. The observers recorded the number of successful capture and failed events for each prey type. Additionally, the number of adult pseudoscorpions involved in capture (NPS), the number of behavioral acts performed during capture (NBA) (an estimated mean number of behavioral acts performed per colony per capture event, according to the already known behavioral types (Table 1), as described by Tizo-Pedroso and Del-Claro (2011)), and the time spent subduing the prey (TSP) were recorded. The behavioral acts were categorized according to their function and the phase of the capture.
First, we evaluated differences in the proportion of successes or fails in prey capture using the G test. Second, to evaluate the importance of measured variables in the prey capture behaviors; NPS, NBA, and TSP were compared with the categorical variables (the effect of different colonies, cumulative effect of predation events, and different types of prey) using ANOVA for main effects. We expected that a more efficient prey capture would occur faster, engage more individuals, and require fewer behavioral acts. For these analyses, NPS and NBA were log10 transformed to the assumption of normal distribution.
Lastly, to evaluate if the number of pseudoscorpions engaging in prey capture interfered with the sequences of behaviors and the duration of the capture process, NPS was correlated with NBA and TSP using Spearman’s rank correlation coefficient. Throughout the study period, the change in offered prey was followed by the death of some colonies (these colonies rejected the new prey and probably died of inanition), causing a reduction in sample size. Therefore, to avoid inconsistencies in the comparison of sample groups during statistical analysis, we used the procedure of resampling (based on 1000 resamples) from the original data to complete the treatments for analysis. Original data was submitted to random resampling with replacement. Finally, the tendencies toward increasing capture success over repeated exposure of the same prey were verified using logistic regressions.
Results
Predation tests showed that colonies of P. nidificator usually failed to capture at a ratio near 1:1. In total, 176 observation events were performed (totaling 134 h of observation). Ninety-two events (52.27%) were considered successful, and 84 events (47.73%) were incomplete. However, the proportions of success and failure were different when the prey types were analyzed separately. For prey I (C. mus), there were 40 successful events and 33 failures, while for prey II (C. clypeatus), there were 20 successful events and 30 failures. Moreover, for prey III (C. crassus), there were 32 successful events and 21 failures. The analysis showed that a higher tendency toward failure occurred with prey type II (G test = 8.93; DF = 2; p = 0.01; Fig. 3). In total, we evaluated the effects of prey change for eight experimental colonies; however, two colonies did not accept prey II, and later, other two colonies rejected prey III. Thus, four colonies died following the change in the prey, probably because of inanition.
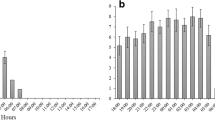
The analysis of main effects showed that for the three prey types, there were significantly different responses for the tested variables (Table 2). First, NPS was significantly affected by colony factor (Fig. 4a) and was markedly variable among the studied colonies. The size of attack groups increased with repetition of prey type, but its effect was not significant (Fig. 4b). However, the size of attack groups varied in relation to prey type (Fig. 4c). Prey II attracted more pseudoscorpions than the other prey.
Analysis of behavioral attributes in relation to colonies, prey type, and repetition. Number of recruited pseudoscorpions in relation to (a) experimental colonies, (b) repeated exposure to the same type of prey, and (c) prey type. Number of behavioral acts performed to capture prey in relation to (d) experimental colonies, (e) repeated exposure to same type of prey, and (f) prey type. Time spent in prey capture in relation to (g) experimental colonies, (h) repeated exposure to same type of prey, and (i) prey type
The number of behavioral acts performed to prey capture was also affected by the colony and type of prey, but it was not affected by repeated exposure to the same kind of prey (Table 2). NBA was markedly different among studied colonies (Fig. 4d). However, NBA did not increase after repeated exposure to a prey type (Fig. 4e), although it was higher during the capture of prey III (Fig. 4f).
The last parameter, the time spent to subdue prey, was also influenced by the colony, the type of prey, and repetition of the same type of prey (Table 2). TSP varied significantly among colonies, with two colonies showing more rapid capture processes and one colony needing more time to subdue its prey (Fig. 4g). TSP was affected by repetition of the same prey. In general, colonies showed a reduction in TSP along with the predation events. The mean TSP tended to be established after the fifth capture event with the same prey (Fig. 4h). Finally, in relation to the kind of prey, colonies spent less time to subdue prey I and needed more time for prey II (Fig. 4i).
The number of pseudoscorpions involved in each successful capture (NPS) was positively correlated with the number of behavioral acts necessary to subdue prey (NBA) and the time spent to subdue prey (TSP). For prey type I (C. mus), NBA was positively correlated with NPS (Rs = 0.56; N = 79; p < 0.05; Fig. 5a). In addition, TSP was positively correlated with NPS, but the relationship can be considered weak (Rs = 0.25; N = 79; p < 0.05; Fig. 5b). For prey type II (C. clypeatus), NBA also was positively correlated with NPS (Rs = 0.48; N = 30; p < 0.05; Fig. 5c). However, there was no relationship between TSP and NPS (Rs = − 0.18; N = 30; p > 0.05; Fig. 5d). Finally, for prey type III (C. mus), NBA was positively related to NPS (Rs = 0.68; N = 17; p < 0.05; Fig. 5e). However, there was no correlation between NPS and TSP (Rs = − 0.11; N = 30; p > 0.05; Fig. 5f).
Spearman’s rank correlation between the number of pseudoscorpions involved in prey capture and the number of behavioral acts for Camponotus mus (a), Cephalotes clypeatus (b) and Camponotus crassus (c); and number of pseudoscorpions involved in capture versus the time required to subdue prey in relation to prey type, for C. mus (d), C. clypeatus (e) and C crassus (f)
Although the colonies used in this study had similar numbers of individuals, the size of the attack group could be influenced by colony size. To confirm this, we compared the number of pseudoscorpions involved in prey capture with the total number of individuals per colony. We found a correlation between NPS and colony size (Rs = 0.07; N = 176; p < 0.05); however, the effect can be considered weak. Thus, we assumed that because of the chosen experimental design, colony size had little influence on the other investigated parameters, and we removed the colony sizes from the analyses to avoid statistical mistakes. Additionally, we analyzed the main effect of colony success over repeated exposure to the same type of prey using logistic regression. Although the colonies showed marked variation in prey capture success, the general effects did not indicate a tendency toward increasing capture success over repeated prey exposure for prey I (χ2 = 2.76; N = 73; p = 0.10) or prey II (χ2 = 0.88; N = 50; p = 0.35); however, there was a significant effect for prey III (χ2 = 5.47; N = 53; p = 0.02).
Discussion
The results showed some interesting effects and could suggest that each colony can behave in a relatively different way when exposed to a new type of prey. Colonies were more able to capture prey when we offered the same type repeatedly. After this improvement, changing the type of prey resulted in a loss of efficiency at the beginning, followed by gradual improvement foraging by some of the colonies. However, the tested effects did not confirm the study hypotheses, because of criteria (i) a reduction in time required to subdue prey (individuals would spend less time to capture it) and (iii) a reduction in number of behavioral acts required to capture and manipulate prey (optimized prey capture would demand fewer behavioral acts.
The ability to adjust feeding behavior is considered an important feature of prey capture performance (Lauder 1981). It is expected that the ability to modulate prey capture acts to increase the use of different food items and feeding performance (Bolnick and Ferry-Graham 2002; Ferry-Graham et al. 2002; Van Wassenbergh et al. 2006). Thus, behavioral adjustments would be likely in generalist predators. Predation tests involving three different prey species showed that although the capture success was around 50%, changes in prey availability resulted in some degree of prey rejection by colonies of P. nidificator. Although all colonies were able to capture prey type I, half of them died after the change of prey. There was a reduction in the number of colonies capturing prey types II and III, consecutively. These colonies did not feed after the change of prey and probably died of starvation. Such evidence suggests that different colonies of P. nidificator do not behave under the same adjustment capabilities when the prey type changes and, eventually, become more abundant in the natural environment during a given period. Alternatively, some colonies may specialize in capture of a more abundant type of prey. This effect can result in mortality of colonies that are unable to adjust their behaviors when prey availability changes.
The type of prey caused significant changes in the number of behavioral acts among the colonies in relation to repetition of the same prey type. Colonies exhibited a higher number of behavioral acts when capturing prey III. Camponotus crassus is more active and fast-moving as compared to the other ants (prey I and II). This difference in ant behaviors might have caused the increase in the number of pseudoscorpion behavioral acts; a more active and agile prey possibly demands more versatility and handling time. In a study with social spiders, there were differences in capture success of two different prey species, considering that one type of prey was winged and more able to escape (Pasquet and Krafft 1992). However, in the case of pseudoscorpions, differences in ant species morphology and behaviors were enough to cause a lack of response by some colonies. Colonies attacked the three prey types; however, failed attack attempts were more frequent with prey II. Moreover, this prey type attracted the largest number of pseudoscorpions and required more time to be subdued, despite being a slower and less active ant. Prey mobility is important in stimulating searching and hunting behaviors in P. nidificator (Tizo-Pedroso and Del-Claro 2007, 2018).
The differences in the number of pseudoscorpions attacking all prey types could reflect the variability in colony behavior against a new kind of prey. The change of prey resulted in the reduction of the colonies, also probably reducing the variability of behaviors among the colonies. The results showed marked variation in colonies’ responses to different prey types. This fact may suggest that some colonies are more efficient at catching a new type of prey as compared to other colonies. However, colonies that survived prey changes were more efficient at prey capture with repeated exposure to the prey, reaching a stable time to subdue prey after the fifth or sixth repetition of prey type. These marked behavioral responses among P. nidificator colonies could be related to genetic and plastic variations in populations. Colonies tend to be strongly territorial, with limited dispersal and gene flow and high endogamy (Tizo-Pedroso and Del-Claro 2007, 2011, 2014). Such behavioral and reproductive patterns could restrict the distribution of important alleles among colonies, affecting their behavioral plasticity. More genetically varied colonies might be more plastic and responsive to prey variability.
During prey capture, the involvement of larger number of pseudoscorpions resulted in an increased number of behavioral acts. This was different than the expected outcome, which was that there would be a reduction in the number of behavioral acts as a consequence of prey capture adjustment and optimization. The increased number of pseudoscorpions possibly resulted in the increased prey handling time. In social spiders, the number of spiders involved in an attack favors faster immobilization of prey (Souza et al. 2007). However, in P. nidificator, it is possible that the number of recruited pseudoscorpions could be followed by an increased variety of behavioral acts, increasing the handling time. Colonies of P. nidificator engage in division of labor; mainly in parental care, colony maintenance, and foraging (Tizo-Pedroso and Del-Claro 2011). This element could be involved in the extension of handling time. A preliminary study indicated that the variety of tasks involved in prey capture is higher in large colonies with groups of individuals performing distinct behaviors such as holding prey, injecting venom, and handling prey. This type of task partitioning may cause an increase in the time each individual spent performing a specific group of behaviors. The social spider Anelosimus eximius shows polymorphic behavior, with some individuals tending to defend colonies against predators while others cooperate in colony maintenance (Pruitt et al. 2008; Pruitt and Riechert 2011a). These variations could favor individuals acting in specific tasks in colonies (Pruitt and Riechert 2011b; Grinsted et al. 2013). Social pseudoscorpions might also show variation in individual behaviors that could interfere in a colony’s foraging tendencies.
Behavioral optimization of foraging activities can occur because of learning processes during prey capture events (Byrne and Bates 2006). However, the reduced behavioral complexity could be a consequence of limited diversity of food items (Tinker et al. 2008). In the case of P. nidificator, there is high arthropod species richness associated with tree trunks in the Brazilian tropical savanna, where the pseudoscorpion occurs (Tizo-Pedroso and Del-Claro 2007). Such diversity can have a dilution effect, avoiding the selection of more specific capture strategies. However, the process of selecting behavioral strategies can be measured through behavioral simplification.
Ants constitute the main food item of P. nidificator during the dry season (Tizo-Pedroso and Del-Claro 2007, 2018). However, the seasonal generalist feeding habits of this pseudoscorpion could be associated with the existence of different strategies of prey capture. Behavioral diversity is a component associated with behavioral plasticity, allowing the species to colonize heterogeneous environments. In the premise of this study, it was expected that repetition of the same type of prey would promote increasing prey capture success. Additionally, environmental heterogeneity that reflects better prey availability and a greater diversity of food items can be related to the evolution of different strategies for exploiting resources (Alcock 2009). However, in other contexts, the inverse effect can be found, in which the limitation of food resources tends to encourage increased behavioral diversity (Tinker et al. 2008). Although P. nidificator is recognized as a generalist and opportunistic predator, colonies can experience high levels of mortality when failing to adjust their reactions under severe changes in natural prey availability. Further studies will investigate the behavioral variation among the colonies and its implication for their adaptive value.
References
Adis J, Mahnert V (2002) Pseudoscopiones. In: Adis J (ed) Amazonian Arachnida and Myriapoda: identification keys, to all classes, orders, families, some genera, and lists of known terrestrial species. Pensoft Publ, Sofia, pp 367–380
Alcock J (2009) Animal behavior: an evolutionary approach. 9th ed. Sinauer Associates, Sunderland.
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 48:227–265
Avilés L (1997) Causes and consequences of cooperation and permanent-sociality in spiders. In: Choe JC, Crespi BJ (eds) The evolution of social behavior in insects and arachnids. Cambridge University Press, Cambridge, pp 476–498
Bolnick DI, Ferry-Graham LA (2002) Optimizing prey-capture behaviour to maximize expected net benefit. Evol Ecol Res 4:843–855
Brach V (1978) Social behavior in the pseudoscorpion Paratemnus elongatus (Pseudoscorpionida: Atemnidae). Insect Soc 25:3–11
Byrne RW, Bates LA (2006) Why are animals cognitive? Curr Biol 16:R445–R448
Choe JC, Crespi BJ (eds) (1997) The evolution of social behaviour in insects and arachnidse. Cambridge University Press, Cambridge
Costa JT (2006) The other insect societies. Belknap Press of Harvard University Press, Cambridge
Davies NB, Krebs JR, West SA (2012) An introduction to behavioural ecology. 4th ed. Wiley-Blackwell, Oxford.
Del-Claro K, Tizo-Pedroso E (2009) Ecological and evolutionary pathways of social behavior in pseudoscorpions (Arachnida: Pseudoscorpiones). Acta Ethol 12:13–22. https://doi.org/10.1007/s10211-009-0052-y
Ferry-Graham LA, Bolnick DI, Wainwright PC (2002) Using functional morphology to examine the ecology and evolution of specialization. Integr Comp Biol 42:265–277. https://doi.org/10.1093/Icb/42.2.265
Garcia LF, Gonzalez-Gomez JC, Valenzuela-Rojas JC, Tizo-Pedroso E, Lacava M, (2016) Diet composition and prey selectivity of Colombian populations of a social pseudoscorpion. Insec Soc 63(4):635–640.
Gonzaga MO (2007) Socialidade e cuidado parental. In: Gonzaga MO, Santos AJ, Japyassú HF (eds) Ecologia e comportamento de aranhas. Editora Interciência, Rio de Janeiro, pp 185–207
Grinsted L, Pruitt JN, Settepani V, Bilde T (2013) Individual personalities shape task differentiation in a social spider. Proc R Soc B Biol Sci 280:20131407. https://doi.org/10.1098/rspb.2013.1407
Harvey MS (1986) The systematics and biology of pseudoscorpions. In: Austin AD, Heather NW (eds) Australian Arachnology. Australian Entomological Society, Brisbane, pp 75–85
Krebs JR, Davies NB (1997) Behavioural ecology: an evolutionary approach. 4th ed. Wiley-Blackwell, Hoboken.
Lauder GV (1981) Intraspecific functional repertoires in the feeding mechanisms of the characoid fishes, Lebiasina, Hoplias, and Chalceus. Copeia 1981:154–168
Lubin Y, Bilde T (2007) The evolution of sociality in spiders. Adv Study Behav 37:83–145. https://doi.org/10.1016/S0065-3454(07)37003-4
Moura RF, Tizo-Pedroso E, Del-Claro K (2018) Colony size, habitat structure, and prey size shape the predation ecology of a social pseudoscorpion from a tropical savanna. Beh Ecol Sociobiol 72(7):103–111.
Packer C, Ruttan L (1988) The evolution of cooperative hunting. Am Nat 132:159–198
Pasquet A, Krafft B (1992) Cooperation and prey capture efficiency in a social spider, Anelosimus eximius (Araneae, Theridiidae). Ethology 90:121–133
Pruitt JN, Riechert SE (2011a) How within-group behavioural variation and task efficiency enhance fitness in a social group. Proc R Soc B Biol Sci 278:1209–1215. https://doi.org/10.1098/rspb.2010.1700
Pruitt JN, Riechert SE (2011b) Within-group behavioral variation promotes biased task performance and the emergence of a defensive caste in a social spider. Behav Ecol Sociobiol 65:1055–1060. https://doi.org/10.1007/s00265-010-1112-z
Pruitt JN, Riechert SE, Jones TC (2008) Behavioural syndromes and their fitness consequences in a socially polymorphic spider, Anelosimus studiosus. Anim Behav 76:871–879. https://doi.org/10.1016/j.anbehav.2008.05.009
Souza ALT, Gonzaga MO, Vasconcellos-Neto J (2007) Prey capture behaviour in the social spider Anelosimus eximius (Araneae : Theridiidae): responses to prey size and type. Ethology 113:856–861. https://doi.org/10.1111/j.1439-0310.2007.01384.x
Tinker MT, Bentall G, Estes JA (2008) Food limitation leads to behavioral diversification and dietary specialization in sea otters. Proc Natl Acad Sci U S A 105:560–565. https://doi.org/10.1073/pnas.0709263105
Tizo-Pedroso E, Del-Claro K (2005) Matriphagy in the neotropical pseudoscorpion Paratemnoides nidificator (Balzan 1888) (Atemnidae). J Arachnol 33:873–877
Tizo-Pedroso E, Del-Claro K (2007) Cooperation in the neotropical pseudoscorpion, Paratemnoides nidificator (Balzan, 1888): feeding and dispersal behavior. Insect Soc 54:124–131. https://doi.org/10.1007/s00040-007-0931-z
Tizo-Pedroso E, Del-Claro K (2011) Is there division of labor in cooperative pseudoscorpions? An analysis of the behavioral repertoire of a tropical species. Ethology 117:498–507. https://doi.org/10.1111/j.1439-0310.2011.01906.x
Tizo-Pedroso E, Del-Claro K (2014) Social parasitism: emergence of the cuckoo strategy between pseudoscorpions. Behav Ecol 25:335–343
Tizo-Pedroso E, Del-Claro K (2018) Capture of large prey and feeding priority in the cooperative pseudoscorpion Paratemnoides nidificator. Acta Ethol 21:109–117. https://doi.org/10.1007/s10211-018-0288-5
Van Wassenbergh S, Herrel A, Adriaens D, Aerts P (2006) Modulation and variability of prey capture kinematics in clariid catfishes. J Exp Zool A Comp Exp Biol 305A:559–569. https://doi.org/10.1002/Jez.A.293
Whitehouse MEA, Lubin Y (2005) The functions of societies and the evolution of group living: spider societies as a test case. Biol Rev 80:347–361
Wilson EO (1971) The insect societies. Harvard University Press, Cambridge
Zeh JA, Zeh DW (1990) Cooperative foraging for large prey by Paratemnus elongatus (Pseudoscorpionida, Atemnidae). J Arachnol 18:307–311
Zeh DW, Zeh JA (1997) Sex via the substrate: sexual selection and mating systems in pseudoscorpions. In: Choe JC, Crespi BJ (eds) The evolution of mating systems in insects and arachnids. Cambridge University Press, Cambridge, pp 329–339
Acknowledgements
We would like to thank Breendow César Barbosa and Nayanny Gonçalves for their valuable assistance during the implementation of the study and while obtaining the results. We also thank three anonymous reviewers for their significant contributions to the manuscript.
Funding
We thank the Universidade Estadual de Goiás (UEG) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the scholarships granted to the students involved in this study. We also thank the Programa de Bolsa de Incentivo à Pesquisa e Produção Científica (PROBIP-UEG) for their financial support. K. Del-Claro thanks CNPq and FAPEG and E. Tizo-Pedroso thanks CNPq and FAPEG for research grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ribeiro, R.F., Gomes, F.C., Tizo, A.F.S. et al. Cooperative foraging in neotropical pseudoscorpions: effects of prey changes on behavioral adjustments of colonies. acta ethol 21, 153–161 (2018). https://doi.org/10.1007/s10211-018-0294-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10211-018-0294-7