Abstract
Global warming can affect biomass accumulation and the dynamics of periphytic communities, potentially altering their role in aquatic ecosystem functioning. We conducted a 38-day mesocosm experiment to investigate the effects of an increase in winter temperature on periphyton biomass accumulation under eutrophic conditions. We evaluated the warming effect on colonization phases, identifying the most affected phase. The experiment had two treatments (control: current winter temperature of 23.5 ℃, warming: + 5.7 ℃ under IPCC scenario). It was carried out in growth chambers under controlled temperature, light, and humidity. Periphyton and water samplings were performed on days 3, 6, 9, 13, 17, 21, 27, and 38. The increase in temperature did not affect the organic matter accrual rate of the periphyton. However, it negatively affected the net and gross accrual rate of the algal biomass. Ash-free dry mass and chlorophyll-a ratio in the periphyton increased at higher temperatures, indicating a decrease in autotrophic components in the periphyton in the warming treatment. We detected losses in algal biomass during the intermediate and advanced colonization phases. Our results showed a decrease in periphytic algal biomass with an increase in average temperature in winter. In conclusion, a warming scenario can negatively influence periphyton biomass in eutrophic ecosystems, where algal growth in the community is generally unfavorable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities are having a growing impact on natural ecosystems, and climate change is a major threat to the structure of biological communities and the functioning of ecosystems (Meerhoff et al. 2012; Hansson et al. 2013; Salk et al. 2021). In the aquatic ecosystems, eutrophication is one of most threatening the environmental problems affecting aquatic ecosystems globally. In eutrophic environments, high nutrient availability increases phytoplankton biomass causing blooms. Phytoplankton blooms reduce the water transparency and euphotic depth zone, reducing light availability and increasing nutrient competition between autotrophs (Vadeboncoeur et al. 2003; Havens et al. 1996). In temperate lakes, studies indicate global warming can intensify eutrophication (Salk et al. 2021). Warming can cause an increase in phytoplanktonic biomass and disrupt water C:N:P ratio, affecting the ability of phytoplankton and herbivores to use nutrient use efficiently, with possible consequences for the entire aquatic food chain (Domis 2014; Pacheco et al. 2021). There is still little knowledge on the warming effects on eutrophication in subtropical and tropical lentic ecosystems. Recent studies suggest that temperature rise alone is insufficient to increase phytoplanktonic biomass (Gomes et al. 2020; Pacheco et al. 2021). However, the combined effect of warming and enrichment seems to increase cyanobacterial bloom in phytoplankton (Gomes et al. 2020). Understanding of how environmental warming effects on aquatic communities can support monitoring strategies and predictive methods to minimize impacts, especially in tropical and subtropical ecosystems, where knowledge is still limited.
In aquatic communities, periphyton is a crucial primary producer and participates in nutrient cycling and the food web, contributing significantly to ecosystem functioning (Vadeboncoeur and Steinman 2002; Dodds 2003). The periphyton can adhere to the most diverse types of substrates (e.g., sediment, macrophytes, rocks) and is typically abundant in shallow lakes. In these ecosystems, periphyton can contribute from 99 to less than 1% of primary production (Vadeboncoeur and Steinman 2002; Vadeboncoeur et al. 2003). In addition to their crucial ecological role, periphyton can remediate eutrophic ecosystems by removing phosphorus (Wu et al. 2014) and can act as an indicator of environmental changes (De Nicola and Kelly 2014; Dunck et al. 2016).
Several environmental factors, such as light and nutrient availability, can affect periphyton structure and functioning (Vadeboncoeur and Steinman 2002; Meerhoff et al. 2012; Hansson et al. 2020). Some abiotic factors can act as modulators, such as temperature, pH, and salinity (Stevenson 1996; Lambert et al. 2016). While high temperature has physiological effects on periphyton, such as denaturation of proteins and nucleic acids, and degradation of photosystem (Wahid et al. 2007), it can affect the periphyton community structure (Hao et al. 2020). In the ecosystem, global warming has a direct effect on the physical properties of water, such as stratification and mixing processes, and indirect on light and nutrients (Jeppesen et al. 2009; Rühland et al. 2015), which may influence periphyton growth. Furthermore, studies have shown that warming favors the water brownification process, which also can also negatively impact benthic and pelagic algal biomass and primary production (Vasconcelos et al. 2016). Thus, the global warming can influence primary production, abundance, and algal composition of the periphyton (Mahdy et al. 2015; Bondar-Kunze et al. 2021; Silva et al. 2019; Hao et al. 2020). The ideal temperature for periphyton growth ranges from 10 to 30 ℃ and higher temperatures lead to thermal stress, reducing community growth (De Nicola 1996). Increased temperature can cause changes in periphyton, which can negatively affect biomass availability for primary consumers (Bondar-Kunze et al. 2021), leading to changes in the food chains (Pacheco et al. 2021). Despite some advances in knowledge of the effects of warming on the periphyton, gaps in knowledge still exist, such as the effect on the community colonization process.
Periphyton colonization involves a sequence of steps that lead to the formation of a structurally complex and very dynamic microbiota. During colonization, there is an exponential accrual phase of biomass that reaches a maximum and can remain at a plateau for some time, usually days or weeks, after a loss phase begins (Biggs 1996). The accrual phase dominates immigration/colonization and growth, and the loss phase dominates the loss by death, emigration, sloughing, and grazing (Biggs 1996). However, physical or chemical disturbances can alter colonization phases, increase losses, and/or restart community development (Stevenson 1996). A better understanding of the warming effect on periphyton colonization allows the identifying changes in successional trajectory, biomass accumulation rates, and community structure.
Considering that global warming can intensify eutrophication (Moss et al. 2011; Salk et al. 2021) impairing periphyton biomass accumulation (Vadeboncoeur et al. 2001), we investigated the warming effects on this community under eutrophic conditions. Specifically, we evaluated the changes in periphyton biomass accumulation during colonization under controlled environmental conditions in response to warming in the winter. In eutrophic lakes and reservoirs, intense phytoplankton blooms can promote shading and strong competition for nutrients, impairing primary production, biomass accumulation and algal growth in the periphyton (Vadeboncoeur et al. 2001; Zhang et al. 2015; Borduqui and Ferragut 2012). Thus, the peak of periphyton biomass commonly occurs in phases of low phytoplankton biomass in eutrophic lake ecosystems, as observed in the winter of the studied reservoir (Borduqui and Ferragut 2012). Thus, we hypothesized that a temperature increase in winter would decrease the periphyton biomass peak and accrual rate, affecting the colonization phases under eutrophic conditions.
Materials and methods
Experimental design
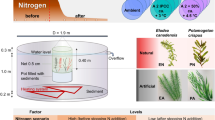
We conducted a mesocosm experiment in growth chambers (2) with controlled environmental conditions in two temperature scenarios to determine the effects of global warming on the periphyton (Fig. 1). For the current scenario, we use the average winter temperature (dry season) of the last ten years in the study area as the basis for the current scenario (http://www.estacao.iag.usp.br/boletim.php). To simulate the warming scenario, we established the higher air temperature based on the worst-case scenario proposed by the Intergovernmental Panel on Climate Change (IPCC 2021), which predicts a potential increase of 5.7 ℃ in 100 years. The treatments inserted inside the growth chambers were as follows: control (current scenario) with a maximum air temperature of 23.5 ℃ in winter; and warming with a maximum air temperature of 28.8 ℃ simulating the future scenario in winter. Both treatments were performed in triplicates. In the study area, periphyton biomass tends to be higher in autumn–winter than in other seasons, see Borduqui and Ferragut 2012 for details, and the community is dominant in algal biomass (Santos et al. 2020). For this reason, the winter temperature was used as a baseline.
The photosynthetically active radiation (PAR) was 488 µmol m−2 s−1 for a 12-h photoperiod in the two growth chambers. The mesocosms were positioned beneath the light source at a fixed distance of 1.2 m (1200 C3 luminaires full spectrum, Apogee model Spectrum Quantum Sensor). To simulate the daily thermal oscillation, the air temperature in chambers was programmed to gradually increase from 1 am to 9 pm and gradually decrease from 9 pm to 1 am every day, maintaining the difference of 5.7 ℃ between treatments. The temperature settings for each time were based on the average of the last five years in the study area. A full spectrum quantum sensor connected to a digital electronic microprocessor measured the PAR (µmol m−2 s−1). A digital electronic microprocessor regulated the temperature with a precision of ± 0.5 ℃. The air relative humidity was maintained at 80%, controlled by a digital electronic microprocessor with variation between 60 and 90% and a precision of ± 8%. Air temperature, humidity, and irradiation were recorded every 15 min.
Six mesocosms were used in the experiment, each consisting of white plastic boxes made of high-density polyethylene (HDPE) with an average volume of 62 L. The mesocosms were previously sterilized before being placed in growth chambers to prevent contamination. They were filled with 55 L of water from the supereutrophic Garças Reservoir (23°38′40.6″ S and 46º37′28.0″ W), located in the Parque Estadual das Fontes do Ipiranga, São Paulo, Brazil, which is situated at 1 km from the growth chambers. The experiment used unfiltered lake water to simulate natural conditions and retain inoculum for periphyton colonization. In cases in which fish and snails were found in the chamber, they were manually removed throughout the experimental period to avoid any interactions with periphyton. The lake water was collected with previously sterilized polyethylene gallons. After one day of acclimation (T0), two acrylic supports containing ten glass slides (26 × 76 mm) were submerged in the water of each mesocosm to allow for periphyton colonization (Fig. 1). The glass slides were positioned vertically to minimize particle sedimentation and at a depth of 10 cm to avoid photoinhibition.
Water and periphyton samples were collected on days 3, 6, 9, 13, 17, 21, 27, and 38 (07/12 to 08/19/2022). In both treatments, sampled water was replaced with distilled water to avoid the decrease in the water column, which could affect the light availability during the experimental period. The water lost to evaporation was not replaced to maintain the warming effect on the periphyton. Water replacement was carried out carefully to avoid physical disturbance to the developing periphytic community.
Periphyton samples were collected by randomly selecting one colonized glass slide from the mesocosms. The colonized substrates were placed in opaque vials and stored at low temperatures for transport. In the laboratory, the periphyton was removed from the substrate by scraping it with steel blades and jets of distilled water.
Environmental variables
Temperature, relative humidity, and PAR in the growth chambers were monitored daily through sensors coupled to a digital electronic microprocessor. The temperature, electrical conductivity, total dissolved solids (TDS), dissolved oxygen (DO), and pH were measured with a water probe (Horiba U52, HORIBA Corporation, Japan) and underwater radiation (PAR) with Li-Cor 250 (Lincoln, NE, USA). We estimated the total nitrogen and phosphorus concentrations (APHA 2012), and chlorophyll-a concentration (Sartory and Grobbelaar 1984).
Periphyton samples were filtered through a glass fiber filter (Whatman GF/F) previously calcined (500 ℃; 1 h) in a vacuum pump under low pressure (0.3 atm) to measure of the dry mass (DM; g m−2) and ash-free dry mass (AFDM; g m−2), according to APHA (2012). After filtration, the filters were placed in an oven at 105 ℃ and weighed the dry mass 24 h later when the mass reaches constant. Subsequently, the filters were placed in a muffle furnace at 500 ℃ for 1 h to obtain the ash mass and calculate the AFDM. To measure periphyton chlorophyll-a concentration on the glass slide surface (mg m−2), the samples were filtered and posteriorly frozen at – 20 ℃ until analysis within 30 days. Extraction of chlorophyll-a (corrected for pheophytin) from periphyton was performed with 90% ethanol, according to Sartory and Grobbelaar (1984). Chlorophyll-a concentration was used as a proxy for algal biomass in periphyton. To evaluate the trophic nature of the periphyton community, we calculated the ratio of AFDM (mg m−2) to chlorophyll-a (mg m−2) based on APHA (2012). We consider the possibility that nonviable organic material affects this index. We calculated the gross and net accrual rate of periphyton biomass (g m−2 d−1) according to Stevenson (1996).
Trophic State Index (TSI) was calculated using chlorophyll-a (Chloa) and total phosphorus (TP) concentrations, according to Cunha et al. (2013). The TSI categories are: The trophic state classes are ultraoligotrophic (≤ 51.1), oligotrophic (51.2–53.1), mesotrophic (53.2–55.7), eutrophic (55.8–58.1), supereutrophic (58.2–59.0), and hypereutrophic (≥ 59.1).
Data treatment
Two-way repeated measures analysis of variance (two-way RM-ANOVA) was applied to test the significant differences in periphyton biomass and environmental variables between treatments. Tukey’ test was applied for multiple comparisons of means to the determination of the minimum significant difference between treatments. Homogeneity of variance and normality were checked, and data were logarithmized. All analyses were performed using the statistical program Sigma Plot 12.0 (Systat Software, Inc, Germany).
Results
Environmental variables
Throughout the daily air temperature profile, we maintained a consistent temperature difference of 5.7 ℃ between the control and warming treatment. The air temperature ranged from 13.8 to 23.5 ℃ in control, following the average daily winter air temperature drop for the study area (tropical altitude). Temperature variation ranged from 13.5 to 23.5 ℃ in the warming treatment, allowing us to evaluate periphyton biomass responses. Despite the warming treatment experiencing three times greater evaporation, both were classified as hypereutrophic. TSI ranged the 59.3–64.5 in the control and 60.0–63.9 in the warming treatment. Furthermore, underwater radiation availability did not differ significantly between treatments during the experimental period.
The growth chambers maintained an air temperature difference of 5.7 ℃ between treatments throughout the experiment (Fig. 2). Based on average (n = 3514), light irradiation was 419.2 μmol m−2 s−1 (SD = 48.6) in the control and 460.4 μmol m−2 s−1 (SD = 52.4) in the warming treatment. The air humidity ranged from 48.6% to 92% (SD = 8.7) in the control and, in the warming treatment ranged from 38.6% to 87.3% (SD = 12.3).
Considering the variables of mesocosm water, conductivity, DO, free CO2, temperature, and TDS differed between treatments and time (Table 1). The pH, TN, and TP concentrations differed only among days. The interactions between treatment and time factors were significant for TN and TP. The chlorophyll-a concentration and light did not differ between treatments. The water temperature was higher in the warming treatment, reflecting the environmental temperature increase (Fig. 3A). In the warming treatment, we observed an increase in conductivity and free CO2 and chlorophyll-a concentrations and a decrease in DO and TP (Fig. 3A–F).
Boxplot of temperature (A), conductivity (B), free CO2 (C), dissolved oxygen (D; DO), total phosphorus (E; TP), and chlorophyll-a (F) concentrations (n = 24) in the control and warming treatments during the experimental period. In each box, the upper and lower limits indicate the 25th and 75th percentiles, respectively. The vertical bars represent the median, the horizontal bars indicate the standard error, and asterisks highlight potential outliers
Periphyton
Periphyton AFDM increased until day 27 in control and warming treatments (Fig. 4A). However, no difference was found between treatments. Based on AFDM, the net and gross accrual rates were similar between the control and warming treatment (Fig. 4B). During colonization, the temperature increase had a negative effect on periphyton chlorophyll-a (Fig. 5A). Between 17 and 38 days, the periphyton had consecutive losses of chlorophyll-a concentration compared to the control treatment. The temperature increase caused an average decrease of 69.3% in the chlorophyll-a concentration compared to the control. In both treatments, the periphyton chlorophyll-a peak was on 38 days of colonization. Chlorophyll-a differed between treatments (RM-ANOVA: df = 3, F = 9.39, p = 0.039), and the interaction between the factors was significant (df = 4, F = 2.97, p = 0.049). Based on chlorophyll-a concentration, the net and gross accrual rates in the warming treatment were, respectively 9.1 and 5.6 times lower than those of the control (Fig. 5B). The AFDM: chlorophyll-a ratio was higher in the warming treatment than in the control, except on day 13 (Fig. 6). This ratio also differed between the control and warming treatments (RM-ANOVA: df = 3, F = 15.66, p = 0.017).
Discussion
In warming treatment, conductivity, free CO2, and chlorophyll-a concentrations increased, and DO concentration decreased, evidencing that warming during winter can worsen eutrophic conditions. As a result, we found a negative impact on the algal biomass accrual rate in the periphyton. The increase in winter temperature can cause significant losses of periphyton algal biomass. Specifically, our findings suggest that a temperature rise of 5.7 ℃ in winter can decrease photosynthetic periphyton biomass. In the supereutrophic reservoir studied, the highest periphytic biomass occurs in winter, when the intensity of phytoplankton bloom is reduced (Borduqui and Ferragut 2012). The surface water cooling in winter promotes column mixing, contributing to the reduction of phytoplanktonic biomass (Bicudo et al. 2007; Crossetti et al. 2019), which favor periphyton growth. In eutrophic conditions, studies reported that a decline in periphyton biomass accumulation can be associated with nutrient competition among autotrophs (Zhang et al. 2015) and with shading (Borduqui and Ferragut 2012). A long-term experimental study in a temperate channel also has evidenced that warming during the winter reduced the periphyton biomass (Bondar-Kunze et al. 2021). In addition, warming effect on periphyton abundance and species composition can vary with seasonality, trophic state, and host macrophyte species (Hao et al. 2020; Kazanjian et al. 2018). In contrast, Kazanjian et al. (2018) found that warming impaired phytoplankton growth due to competition for resources with periphyton and macrophytes. According to Vasconcelos et al. (2016), lake ecosystem responses to climate change can be mediated by cross-habitat feedback between benthic and pelagic producers. As a result, the response of periphyton algal biomass to warming may be linked to the response of phytoplankton in eutrophic environments.
Based on AFDM, our findings showed that the organic matter accrual rate in the periphyton did not reflect the increase in temperature in our experiment. Although warming negatively impacted the algal biomass accrual in the periphyton, it did not affect the organic matter accumulation rate. However, AFDM: chlorophyll-a ratio in periphyton, on average, increased with the warming, indicating a decrease in the autotrophic components. The increase in the organic matter could be linked to increased heterotrophs or excess organic debris in the periphyton (Stevenson 1996). Algal biomass was expected to increase during periphyton colonization, especially in the advanced stages, due to the increase of filamentous algae (Biggs 1996), as observed in the community of other lakes (Dunck et al. 2015; Casartelli et al. 2016; Lan et al. 2018). Thus, considering that warming decreased algal biomass and increased AFDM: chlorophyll-a ratio in advanced colonization stages, it is possible that global warming reduces the contribution of autotrophs in the periphyton in eutrophic lakes. Since periphyton plays a crucial role in shallow lakes, contributing up to 99% of primary productivity (Vadeboncoeur and Steinman 2002; Vadeboncoeur et al. 2003), losses in algal biomass can impact the lake ecosystem functioning.
Regarding the colonization period (up to 38 days), we found that the warming affected periphyton algal biomass (chlorophyll-a) from day 17 when the amount in the control was significantly higher than in the warming treatment. Our findings indicate that warming negatively affected biomass accumulation during the intermediate and advanced colonization stages. The initial colonization phase involves the formation of an organic matrix that accommodates bacteria and ruderal algae, which have adaptations that enable them to maintain populations in highly disturbed sites (McCormick 1996; Flemming and Wingender 2010). Consequently, the colonization initial phase of the periphyton may be less affected by warming than the intermediate and advanced phases, as observed in our experiment. However, the most mature phases are commonly characterized by high biomass due to the abundance k-strategist species, especially filamentous species. The loss of algal biomass in the periphyton during the biomass accumulation phase, as observed here, can affect the ecological functions of the community in the ecosystem. Furthermore, warming impacted the biomass accumulation phase, which may affect its ecological functions in the ecosystem.
Other factors may also have influenced changes in algal biomass in the periphyton, such as grazing pressure, photoadaptation, and brownification. Admittedly, grazing can determine periphyton biomass and structure (e.g., Beck et al. 2019). However, some aspects suggested that the grazing pressure must have had little effect on periphyton in our experiment. Firstly, the animals such as fish and snails were manually excluded, reducing grazing during the experiment. Additionally, the grazing pressure exerted by zooplankton is considered low in the studied reservoir, with top-down control not being detected (Amaral et al. 2020). Generally, the traditional control of phytoplankton by large zooplankton does not occur in warm lakes (Jeppesen et al. 2007). In eutrophic environments, the effect of zooplankton herbivory on phytoplankton is size-specific, which may explain the limited control exercised by this community in tropical ecosystems (Wong et al. 2016). Despite the absence of significant difference in light availability in water between treatments, algal photoadaptation may have influenced biomass results, especially chlorophyll-a, due to the difference in ambient light. Microalgae grow in different light intensities and wavelengths and must have specific mechanisms of photoacclimation and photoadaptation, as evidenced in algal species and groups (Richardson et al. 1983; MacIntyre et al. 2002; Huysman et al. 2013). According to Enberg et al. (2015), increased PAR availability, especially with the exclusion of UVR, can cause changes in algal biomass, photosynthetic activity, and community composition. The other aspect not measured in this experiment that can affect biomass accumulation in periphyton is water brownification, which refers to water darkening, often related to increasing organic matter. The brownification can affect algal community growth (Urrutia-Cordero et al. 2017), including the periphytic algae (Puts et al. 2023). Although it was not measured in this study, the warming treatments showed a noticeable change in the color of the water, becoming more brownish, which must have shaded the periphyton. Considering that global warming can affect grazing on the periphyton (Pacheco et al. 2021) and favor the brownification of the waters (Puts et al. 2023), future studies should examine its effects on community colonization.
Our findings indicate that warming may negatively impact photosynthetic biomass accumulation in eutrophic environments by impairing algal biomass accumulation in periphyton, particularly during the most favorable seasons for growth, such as winter in the studied reservoir. The warming negative effect on algal biomass in the intermediate and advanced phases of periphyton colonization suggests the persistence of the initial phase, damaging the accumulation phase. We conclude that warming can affect the periphyton colonization process by reducing autotrophic biomass accumulation under eutrophic conditions in a global warming scenario. We emphasize that periphytic algal growth is generally impaired in eutrophic lakes and reservoirs, where warming can further reduce primary producers of this community. Our results suggest that the effects of global warming on periphyton deserve further attention, given the importance of the community for primary production and food webs.
Availability of data and materials
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
References
Amaral LM, de Almeida Castilho MC, Henry R, Ferragut C (2020) Epipelon, phytoplankton and zooplankton responses to the experimental oligotrophication in a eutrophic shallow reservoir. Environ Pollut 263:114603
APHA American Public Health Association (2012) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
Beck WS, Markman DW, Oleksy IA, Lafferty MH, Poff NL (2019) Seasonal shifts in the importance of bottom–up and top–down factors on stream periphyton community structure. Oikos 128:680–691
Bicudo DC, Fonseca BM, Bini LM, Crossetti LO, Bicudo CEM, Araújo-Jesus T (2007) Undesirable side-effects of water hyacinth control in a shallow tropical reservoir. Freshw Biol 52:1120–1133
Biggs BJF (1996) Patterns in benthic algal of streams. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, New York, pp 31–56
Bondar-Kunze E, Kasper V, Hein T (2021) Responses of periphyton communities to abrupt changes in water temperature and velocity, and the relevance of morphology: a mesocosm approach. Sci Total Environ 768:145200
Borduqui M, Ferragut C (2012) Factors determining periphytic algae succession in a tropical hypereutrophic reservoir. Hydrobiologia 683:109–122
Casartelli MR, Lavagnolli GJ, Ferragut C (2016) Periphyton biomass accrual rate changes over the colonization process in a shallow mesotrophic reservoir. Acta Limnol Bras. https://doi.org/10.1590/S2179-975X0116
Crossetti LO, Bicudo DC, Bini LM, Dala-Corte RB, Ferragut C, Bicudo CEM (2019) Phytoplankton species interactions and invasion by Ceratium furcoides are influenced by extreme drought and water-hyacinth removal in a shallow tropical reservoir. Hydrobiologia 831:71–85
Cunha DGF, Calijuri MDC, Lamparelli MC (2013) A trophic state index for tropical/ subtropical reservoirs (TSItsr). Ecol Eng 60:126–134
De Nicola DM (1996) Periphyton responses to temperature at different ecological levels. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, pp 149–181
De Nicola DM, Kelly M (2014) Role of periphyton in ecological assessment of lakes. Freshw Sci 33:619–638
Dodds WK (2003) The role periphyton in phosphorus retention in shallow freshwater aquatic systems. J Phycol 39:840–849
Domis LNS, Van de Waal DB, Helmsing NR, Van Donk E, Mooij WM (2014) Community stoichiometry in a changing world: combined effects of warming and eutrophication on phytoplankton dynamics. Ecology 95:1485–1495
Dunck B, Rodrigues L, Bicudo DC (2015) Functional diversity and functional traits of periphytic algae during a short-term successional process in a Neotropical floodplain lake. Braz J Biol 75:587–597
Dunck B, Algarte VM, Cianciaruso MV, Rodrigues L (2016) Functional diversity and trait–environment relationships of periphytic algae in subtropical floodplain lakes. Ecol Ind 67:257–266
Enberg S, Piiparinen J, Majaneva M, Vähätalo AV, Autio R, Rintala JM (2015) Solar PAR and UVR modify the community composition and photosynthetic activity of sea ice algae. FEMS Microbiol Ecol. 91:fiv102
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Gomes AMDA, Marinho MM, Berjante Mesquita MC, Prestes ACC, Lürling M, Azevedo SM (2020) Warming and eutrophication effects on the phytoplankton communities of two tropical water systems of different trophic states: an experimental approach. Lake Reserv Manag 25:275–282
Hao B, Wu H, Zhen W, Jo H, Cai Y, Jeppesen E, Li W (2020) Warming effects on periphyton community and abundance in different seasons are influenced by nutrient state and plant type: a shallow lake mesocosm study. Front Plant Sci. https://doi.org/10.3389/fpls.2020.00404
Hansson LA, Nicolle A, Granéli W, Hallgren P, Kritzberg E, Persson A, Bjork J, Nilsson A, Brönmark C (2013) Food-chain length alters community responses to global change in aquatic systems. Nat Clim Change 3:228–233
Hansson LA, Ekvall MK, He L, Li Z, Svensson M, Urrutia-Cordero P et al (2020) Different climate scenarios alter dominance patterns among aquatic primary producers in temperate systems. Limnol Oceanogr 65:2328–2336
Havens KE, East TL, Meeker RH, Davis WP, Steinman AD (1996) Phytoplankton and periphyton responses to in situ experimental nutrient enrichment in a shallow subtropical lake. J Plankton Res 18:551–566
Huysman MJJ, Vyverman W, De Veylder L (2014) Molecular regulation of the diatom cell cycle. J Exp Bot 65:2573–2584
IPCC Intergovernmental Panel on Climate Change. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: IPCC, 2021. Available via https://www.ipcc.ch/report/ar6/wg1/. Cited 12 Jun 2023
Jeppesen E, Søndergaard M, Meerhoff M, Lauridsen TL, Jensen JP (2007) Shallow lake restoration by nutrient loading reduction some recent findings and challenges ahead. Hydrobiologia 584:239–252
Jeppesen E, Kronvang B, Meerhoff M, Søndergaard M, Hansen KM, Andersen HE, Olesen JE (2009) Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. J Environ Qual 38:1930–1941
Kazanjian G, Velthuis M, Aben R, Stephan S, Peeters ETHM, Frenken T, Touwen J, Xue F, Kosten S, Van de Waal DB, de Senerpont Domis LN, van Donk E, Hilt S (2018) Impacts of warming on top-down and bottom-up controls of periphyton production. Sci Rep 8:9901
Lambert AS, Dabrin A, Morin S, Gahou J, Foulquier A, Coquery M, Pesce S (2016) Temperature modulates phototrophic periphyton response to chronic copper exposure. Environ Pollut 208:821–829
Lan B, Li H, Xiang X, Lin J, Yin J, Yang S (2018) Spatial-temporal characteristics of epilithic algae succession on artificial substrata in relation to water quality in Erhai Lake, Yunnan Province, China. Biologia 73:821–830
Mahdy A, Hilt S, Filiz N, Beklioğlu M, Hejzlar J, Özkundakci D, Adrian R (2015) Effects of water temperature on summer periphyton biomass in shallow lakes: a pan-European mesocosm experiment. Pan-Am J Aquat Sci 77:499–510
Meerhoff M, Teixeira-de Mello F, Kruk C, Alonso C, Gonzalez-Bergonzoni I, Pacheco JP, Jeppesen E (2012) Environmental warming in shallow lakes: a review of potential changes in community structure as evidenced from space-for-time substitution approaches. Adv Ecol Res 46:259–349
McCormick PV (1996) Resource competition and species coexistence in freshwater benthic algal assemblages. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, USA, pp 229–252
MacIntyre HL, Kana TM, Anning T, Geider RJ (2002) Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria 1. J Phycol 38:17–38
Moss B, Kosten S, Meerhoff M, Battarbee RW, Jeppesen E, Mazzeo N, Havens K, Lacerot G, Liu Z, de Meester L, Paerl H, Scheffer M (2011) Allied attack: climate change and eutrophication. Inland Waters 1:101–105
Pacheco JP, Aznarez C, Meerhoff M, Liu Y, Li W, Baattrup-Pedersen A, Cao Y, Jeppesen E (2021) Small-sized omnivorous fish induce stronger effects on food webs than warming and eutrophication in experimental shallow lakes. Sci Total Environ 797:148998
Puts IC, Ask J, Myrstener M, Bergström AK (2023) Contrasting impacts of warming and browning on periphyton. Limnol Oceanogr Lett 8:1–11
Richardson K, Beardall J, Raven JA (1983) Adaptation of unicellular algae to irradiance: an analysis of strategies. New Phytol 93:157–191
Rühland KM, Paterson AM, Smol JP (2015) Lake diatom responses to warming: reviewing the evidence. J Paleolimnol 54:1–35
Salk KR, Venkiteswaran JJ, Couture RM, Higgins SN, Paterson MJ, Schiff SL (2021) Warming combined with experimental eutrophication intensifies lake phytoplankton blooms. Limnol Oceanogr 67:147–158
Santos TR, Castilho MC, Henry R, Ferragut C (2020) Relationship between epipelon, epiphyton and phytoplankton in two limnological phases in a shallow tropical reservoir with high Nymphaea coverage. Hydrobiologia 847:1121–1137
Sartory DP, Grobbelaar JU (1984) Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114:177–187
Silva SFM, Torgan LC, Schneck F (2019) Temperature and surface runoff affect the community of periphytic diatoms and have distinct effects on functional groups: evidence of a mesocosms experiment. Hydrobiologia 839:37–50
Stevenson RJ (1996) An introduction to algal ecology in freshwater benthic habitats. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, pp 3–30
Urrutia-Cordero P, Ekvall MK, Ratcovich J, Soares M, Wilken S, Zhang H, Hansson LA (2017) Phytoplankton diversity loss along a gradient of future warming and brownification in freshwater mesocosms. Freshw Biol 62:1869–1878
Vadeboncoeur Y, Steinman AD (2002) Periphyton function in lake ecosystems. T Sci World J 2:1–20
Vadeboncoeur Y, Lodge DM, Carpenter SR (2001) Whole-lake fertilization effects on distribution of primary production between benthic and pelagic habitats. Ecology 82:1065–1077
Vadeboncoeur Y, Jeppesen E, Zanden MJV, Schierup HH, Christoffersen K, Lodge DM (2003) From Greenland to green lakes: cultural eutrophication and the loss of benthic pathways in lakes. Limnol Oceanogr 48:1408–1418
Vadeboncoeur Y, Moore MV, Stewart SD, Chandra S, Atkins KS, Baron JS, Bouma-Gregson K, Brothers S, Francoeur SN, Genzoli L, Higgins SN, Hilt S, Katona LR, Kelly D, Oleksy IA, Ozersky T, Power ME, Roberts D, Smits AP, Timoshkin O, Tromboni F, Zanden MJV, Volkova EA, Waters S, Wood SA, Yamamuro M (2021) Blue waters, green bottoms: benthic filamentous algal blooms are an emerging threat to clear lakes worldwide. Bioscience 71:1011–1027
Vasconcelos FR, Diehl S, Rodríguez P, Hedström P, Karlsson J, Byström P (2016) Asymmetrical competition between aquatic primary producers in a warmer and browner world. Ecology 97:2580–2592
Zhang X, Mei X, Gulati RD, Liu Z (2015) Effects of N and P enrichment on competition between phytoplankton and benthic algae in shallow lakes: a mesocosm study. Environ Sci Pollut Res 22:4418–4424
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Weather Station of the Institute of Astronomy, Geophysics and Atmospheric Sciences of the University of São Paulo (IAG). Weather Bulletin 2022 (In Portuguese). Available via http://www.estacao.iag.usp.br/boletim.php. Cited in June/12/2023
Wong WH, Rabalais NN, Turner RE (2016) Size-dependent top-down control on phytoplankton growth by microzooplankton in eutrophic lakes. Hydrobiologia 763:97–108
Wu Y, Xia L, Yu Z, Shabbir S, Kerr PG (2014) In situ bioremediation of surface waters by periphyton. Bioresour Technol 151:367–372
Acknowledgements
The authors are grateful for the financial support of the Fundação de Amparo à Pesquisa do Estado de São Paulo for the development of the project (FAPESP 2017/50341-0). LDS is grateful for the scientific initiation scholarship granted by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2022/08479-2) and ROC is grateful for the master’s scholarship granted by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and FAPESP (2022/16879-0). All authors would like to thank the technicians and graduate students for their support in collections and laboratory analyses.
Funding
The authors declare that there are no competing financial interests.
Author information
Authors and Affiliations
Contributions
L.D. Sapucaia, R.O. Carneiro, and C. Ferragut: conceptualized experimental sampling, wrote, and edited the original draft; L.D. Sapucaia generated the biotic data; all authors generated the abiotic results. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
Not applicable/not required.
Consent to participate
All the authors consent to participate in this manuscript.
Consent for publication
All the authors consent the publication of this manuscript.
Additional information
Handling Editor: Simon Belle.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sapucaia, L.D., Carneiro, R.O. & Ferragut, C. Effect of increasing temperature on periphyton accrual under controlled environmental conditions. Limnology 25, 255–265 (2024). https://doi.org/10.1007/s10201-024-00749-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-024-00749-6