Abstract
Periphyton is a key primary producer in shallow lakes, sensitive to global warming and changes in nutrient balances. Reduced nitrogen availability due to accelerated denitrification at higher temperatures or in response to reduced N loadings aimed to reduce the eutrophication may affect periphyton biomass and composition, to compensate for the low N availability (e.g. promoting N2-fixing). We analysed periphyton responses to N decline in 12 eutrophic shallow lake mesocosms during one year of low N compared to high N, under three temperature scenarios: ambient, A2 IPCC scenario and A2 increased by 50%. We used two submerged macrophytes (Potamogeton crispus and Elodea canadensis) and artificial imitations of these as substrates for periphyton growth. Nitrogen decline increased periphyton biomass and induced compositional changes irrespective of season, plant type, and temperature. Periphyton biomass was negatively associated to phytoplankton and positively to plant complexity. Warmer scenarios negatively affected periphyton exclusively at high N loadings. Low N conditions were associated with lower periphyton taxonomic richness, lower N2-fixing cyanobacteria biovolume and increased biovolume of large-sized chlorophytes and non-N2-fixing cyanobacteria. Our results suggest that low N conditions promoted periphyton due to a more efficient use of nutrients and improved light conditions resulting from lower phytoplankton biomass and contrasting effects of temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The periphyton (attached algae and cyanobacteria) is often a key primary producer community of shallow lakes (Vadeboncoeur & Steinman, 2002; Liboriussen & Jeppesen, 2003), regulating the biogeochemical cycles and food webs (Dodds, 2003; Vander Zanden & Vadeboncoeur, 2020). In shallow lakes, benthic-littoral production is comparatively higher than in deep systems, and therefore, periphyton production and its interactions with phytoplankton and macrophytes may have a strong influence on ecosystem structure and functioning (Phillips et al., 2016; Vander Zanden & Vadeboncoeur, 2020). Periphyton competes with phytoplankton for light and nutrients and sequesters large amounts of nutrients from the water column (Hansson, 1990, 1992; Havens et al., 1999; Rodusky et al., 2001). It has been estimated that periphyton is responsible for approximately 60% of the phosphorus captured in vegetated areas in freshwaters (Dodds, 2003; Richardson & Marshall, 1986).
Climate change drives profound changes in freshwater ecosystem structure and functioning, affecting hydrological and thermal regimes, biogeochemical processes, and primary production in shallow lakes (Jeppesen et al., 2010; Moss et al., 2011). Predicted scenarios of global surface air temperature indicate an increase between 1.1 – 6.4 °C to 2090–2099 (IPCC, 2014) which may affect the primary producers of freshwater ecosystems with different effects depending on the community (Meis et al., 2009; Moss et al., 2013; McDowell et al., 2020; Woolway et al., 2020). The effects of increased temperatures on the periphyton are still under debate (e.g. Mahdy et al., 2015; Pacheco et al., 2021). Some studies have found that higher temperatures favour periphyton growth (Tarkowska-Kukuryk & Mieczan, 2012; Mahdy et al., 2015). Others have seen negative responses (e.g. Hao et al., 2020; Shurin et al., 2012) and, supporting these latter experiments, latitudinal studies have evidenced that higher periphyton biomasses are often seen in shallow lakes in colder climates (Bécares et al., 2008; Meerhoff et al., 2007, 2012).
Eutrophication and climate change can drive drastic changes in nitrogen levels, influencing its availability and ratio relative to other nutrients, which can affect primary production in shallow lakes (González-Madina et al., 2019; Hungate et al., 2003; Khan & Ansari 2005; Moss et al., 2013; Pacheco et al., 2010; Trochine et al., 2014). Human-derived nutrient inputs to freshwaters, from agricultural, industrial, and domestic runoff are often particularly N-enriched which may affect N to P balances (Arocena et al., 2018; Boyer et al., 2002; Moss et al., 2013; Paerl et al., 2020). Strategies of re-oligotrophication of shallow lakes may affect the nutrient balance in different ways and induce changes in production patterns (Jeppesen et al., 2005). Furthermore, warming increases denitrification (Pinay et al., 2007; Weyhenmeyer et al., 2007) and it has been estimated that a warming of 3 °C doubles the denitrification rate in shallow lakes (Veraart et al., 2011) adding to the aforementioned processes that alter the N balances in freshwaters.
Warming and low N levels may result in compositional changes with a positive selection for diazotrophic (N2-fixing) cyanobacteria (González-Madina et al., 2019; Wagner & Adrian, 2009) that might compensate for the relative N deficiency in the environment by atmospheric N2-fixation (Schindler, 1977; Schindler et al., 2008). However, several experimental and field studies have pointed out that N2-fixation by cyanobacteria cannot compensate for low N levels, since the N2-fixation is a metabolically expensive process (Reynolds, 2006; Camacho et al., 2003; Díaz et al., 2007; Moss et al., 2013) and, in addition, denitrification can exceed the N2-fixation rates (Paerl et al., 2020; Scott et al., 2019). Besides, many other studies have emphasized the relevance of N as limiting or co-limiting the primary production in shallow lakes (Havens et al., 1999; Elser et al., 2007; Paerl et al., 2016; Trochine et al., 2017). Therefore, evidence exists that N availability may control primary production in shallow lakes. Hence, mitigation measures targeting lowering N seem to be valid to limit the production and to mitigate negative consequences of eutrophication, such as harmful algal blooms (Chorus & Spijkerman, 2020; Paerl et al., 2016, 2020) particularly at high P concentrations (Søndergaard et al., 2017). Furthermore, compositional changes in algae and cyanobacteria in response to low N availability are not necessarily related to a higher representation of N2-fixing groups. Under low N and high P availability, non-N2-fixing groups can persist and outcompete with N2-fixers due to other adaptive characteristics, such as the superior capacity of cellular N storage in large-sized groups (Paerl et al., 2014). However, although many studies have focussed on the effects of N availability and oligotrophication on phytoplankton production (e.g. Schindler 1977; Jeppesen et al., 2005; Paerl et al., 2016; Søndergaard et al., 2017; Schindler et al., 2008), the effects on periphyton are less known, particularly under global warming scenarios.
Global warming and eutrophication are considered to promote planktonic over benthic production in shallow lakes (Vander Zanden & Vadeboncoeur, 2020; Vadeboncoeur et al., 2003), while oligotrophication will likely favour the periphytic over planktonic production (Liboriussen & Jeppesen, 2003; Vadeboncoeur & Steinman, 2002; Vadeboncoeur et al., 2003). However, the effects of oligotrophication, along with global warming scenarios, are still unclear, particularly so for the effects of a decline in N availability on periphyton biomass and composition. In this study, we analysed the effects of a dissolved nitrogen decline during one year in eutrophic shallow lake mesocosms under three temperature scenarios, ambient, A2 IPCC scenario, and A2 increased by 50%, comparing with the same experiments performed during the previous year when the availability of N was high (Hao et al., 2020).
We aimed to answer how a decline of the dissolved N under eutrophic conditions affects the biomass and composition of periphyton under different temperature scenarios. We hypothesized that: (1) conditions of low N will increase periphyton biomass, due to less shading from phytoplankton and a greater capacity to store and recirculate nutrients in the periphytic matrix; (2) the decrease in N will promote the selection of large-sized groups in the periphyton such as filamentous green algae, which can store and recirculate resources more efficiently, and this will be reflected in lower taxonomic richness (3) warming will exacerbate the effects of N decrease on the biomass and composition of periphyton due to increased metabolism and denitrification; (4) periphyton at low N will not be associated to a higher biomass of diazotrophic cyanobacteria, not even at warmer scenarios, since atmospheric N2-fixation cannot compensate for N deficiency in periphyton growth.
Materials and methods
Experimental design
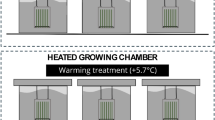
We performed our experiment in the climate change mesocosms facilities of Aquacosms-Aarhus University, in Central Jutland, Denmark (56°14′N, 9°31′E). The mesocosms were designed to study the combined effects of global warming and eutrophication in shallow lakes and have been in continuous operation since August 2003 (Liboriussen et al., 2005). The experiment ran from 2017 to 2019 in the twelve open mesocosms with a diameter of 1.9 m and a depth of 1 m (2800 L) (Fig. 1). They are groundwater-fed and have a surface overflow with a residence time of approximately 2.5 months (Liboriussen et al., 2005). The mesocosms are set at three temperature scenarios: ambient (Amb) without heating, predicted A2 IPCC scenario for the period 2071–2100 (ca. + 3 °C; IPCC 2014) and A2 + 50% (ca. + 4.5 °C; A2 scenario increased to 50%), each replicated four times (total 12 mesocosms). An automatic electrical heating system maintains the temperature of the heated mesocosms using the ambient ones as a reference following the seasonal and daily variation in temperature. Paddles mounted in the tanks continuously mix the water to keep uniform conditions in the water column. All these mesocosms included submerged macrophytes Elodea canadensis Michx. and Potamogeton crispus L. andsmall planktivorous fish (three-spined sticklebacks Gasterosteus aculeatus Linnaeus 1758) stocked in near-natural densities corresponding to Danish lakes (Jeppesen et al., 2002). High nutrient conditions in the mesocosms are maintained with weekly additions of 7 mg P m−2 day−1 and 27.1 mg N m−2 day−1, with Ca(NO3)2 and Na2HPO4 to a constant loading of 54 mg P and 538 mg N per mesocosm as detailed in Liboriussen et al. (2005). Further experimental details are fully described by Liboriussen et al. (2005).The initial average nutrient concentrations were TN 2.40 mg L−1 and TP 165 µg L−1 for the period 2017—2018 (Hao et al., 2020). The N additions in the enriched mesocosms were stopped in June 2018 for one year, to elucidate the effect of N decline on shallow lakes under different temperature scenarios. During this year, the mesocosms kept the P additions and only received low concentrations of N from groundwater (Liboriussen et al., 2005). To analyse the responses of periphyton biomass and composition to the N decline, we conducted identical seasonal experiments: summer (August), autumn (November), winter (February) and spring (May) during one year before stopping the N additions (high N conditions August 2017 to May 2018) and one year after (August 2018 to May 2019). We compared the experiments during the second year, after stopping N additions, with the control conditions before stopping N additions, which partially correspond to the same experiments performed during the same seasons the year before by Hao et al. (2020). The design, seasonality and sample analysis were identical in both experimental phases (high and low levels of N).
Experimental setup, mesocosms and plants used as substrate for periphyton colonization. Temperature scenarios: Ambient, A2 IPCC scenario (ca. 3 °C, IPCC, 2014) and A2 increased by 50% (ca. 4.5 °C). Factorial design, with factors and levels
For each experimental time, we placed in each mesocosm a pot filled with a mix of soil and sediments from the same mesocosm, which included two shoots: one Potamogeton crispus and one Elodea canadensis, together with two artificial plants, morphologically similar (Fig. 1). All these plants, natural and artificial were of similar size and area, but of contrasting morphological complexity, with E. canadensis being more complex than P. crispus (Hao et al., 2020, Fig. 1). We used different natural and artificial plants as substrates for periphyton to elucidate the potential effect of different substrate types and structural complexity (Hao et al., 2020) and potential allelopathy (Hilt, 2006; Pakdel et al., 2013) on periphyton. All the natural plants were collected from one mesocosm, and carefully washed to remove the periphyton at the beginning of each experimental time. The artificial plants were discarded after use, and new plants were used for each experimental time. Thepots with the natural and artificial plants were placed at 30 cm depth and enclosed the plants in a 0.5 cm mesh net surpassing the water surface to avoid grazing by fish and large snails (Fig. 1). The plants were placed for three weeks for each seasonal experimental time (August, November, February and May), to allow periphyton colonization. This was repeated in both phases of high and low levels of N: four experimental times of three weeks each in each condition of high and low N, a total of 8 experiments of 3 weeks each.
At the end of each experiment, we collected phytoplankton samples for compositional analysis and biomass estimation. Then we carefully collected the plants and rinsed the periphyton with a soft brush in 250 ml of tap water. From these samples, we filtered a known fraction through fibreglass GF/C for Chlorophyll-a estimation and saved 50 ml for identification and counting. The filtered volume of Chlorophyll-a varied depending on the concentration of periphyton since we filtered until saturating the GF/C filter, correcting later for the filtered volume. Chlorophyll-a was extracted with cold ethanol and absorbance was measured with a spectrophotometer (UV-1800, Shimadzu, Germany) at 665 nm and 750 nm before and after acidification with 0.1 N HCL to correct by phaeopigments (Nusch, 1980; ISO 1992). Periphyton biomass was standardized by plant area, which was estimated by scanning in Image J. Phytoplankton and periphyton quantitative samples were preserved with 5% Lugol´s iodine acid solution, enumerated in inverted microscope following Utermöhl (1958) and Lund et al. (1958) and estimated biovolume following Hillebrand et al. (1999).
In situ environmental variables temperature, pH and dissolved oxygen were measured every 30 min with different sensors placed at 50 cm depth in each mesocosm (Temperature: PR electronics temperature sensor, pH: OxyGuard pH probe with Manta measurement system, DO: OxyGuard oxygen sensor). Turbidity and conductivity were weekly measured with an YSI650 MDS multiprobe and water samples were collected for nutrient determination in laboratory following the Danish / European standards for total nitrogen (TN, Danish standard no 221, 1975), total phosphorus (TP, Danish standard no 292, 1985), and Danish standard / EN ISO 10304–1 (1996) for orthophosphate (PO4), and total dissolved inorganic nitrogen (TDIN: NH4 + NO3 + NO2) determinations.
Data analysis
We compared the periphyton responses for each experiment during the N decline in the mesocosms (August 2018 to May 2019) with the corresponding experiment under normal (high N) conditions (August 2017 to May 2018) (partially from Hao et al., 2020). We analysed periphyton biomass and composition responses to N conditions (high and low N conditions), temperature treatment (Amb, A2, A2 + 50%) and plant type (E. canadensis natural—EN; E. canadensis artificial—EA; P. crispus natural PN and P. crispus artificial PA) by three-way ANOVA followed by post hoc Tukey HSD. We tested the overall effects of the experimental variables on periphyton biomass by general linear models (GLM), checking for normality and homoscedasticity through visual inspection of the residuals. To test the responses of periphyton composition to the experimental variables we performed non-parametric permutational analysis of variance (PERMANOVA; Anderson, 2001). In all cases, we tested the effects of each variable and their interactions and we log-transformed (log(x + 1)) the biomass data when needed.
To analyse the main environmental variables influencing the periphyton composition we performed a canonical correspondence analysis (CCA) testing the individual contribution and significance of each variable from the full set of environmental variables. To decide whether to use CCA we first explored the data structure by detrended correspondence analysis (DCA), and as the length of the gradients were larger than three standard deviations we choose to use CCA (ter Braak & Verdonschot, 1995). We verified the significance of CCA axis and model by unrestricted permutations (Monte Carlo with 999 iterations) and we forward selected the variables to include in the model, eliminating the correlated variables with variance inflation factors (VIF) of 0 or larger than 10 (Oksanen et al., 2019). We performed all the statistical analyses in R (R core team 2019).
Results
Environmental variables
Nitrogen declined in all the mesocosms during the period with low N conditions from June 2018 to June 2019 (Fig. 2). The peak of TN and TDIN observed during the previous winters (2017 and 2018) was not observed for TN and to a lesser extent for TDIN during the period of low N (Fig. 2). Total phosphorus, orthophosphate, turbidity and chlorophyll-a in the water were highly variable during the entire period (2017–2019) regardless of temperature treatment. Only the warmer treatments (A2 + 50%) tended to differ during the first half of the low N period (June to December 2018) with higher orthophosphate and lower chlorophyll-a compared to Amb and A2 treatments (Fig. 2).
Physico-chemical variables in the mesocoms for the period 2017–2019 for each temperature scenario. Total nitrogen (upper left), total dissolved inorganic nitrogen (TDIN: NO3 + NO2 + NH4; upper right), total phosphorus (TP; left), orthophosphate (PO4; right), turbidity (lower left) and phytoplankton chlorophyll-a (lower right). Red dashed line indicates when the nitrogen additions were stopped
Periphyton biomass
Periphyton biomass was higher during the low N period (Table 1; After (Low N) > Before (High N) TukeyHSD P < 0.001) and was in general negatively correlated to TDIN (GLM, R2 = 52.3, F = 69.83, P < 0.0001). This negative response of periphyton biomass to high N was also observed when conducting seasonal comparisons before and after the N decline (Table 1, Fig. 3). Periphyton biomass decreased with increased temperature treatments (Table 1, Amb > A2 > A2 + 50%, Tukey HSD P < 0.03) and was in general negatively correlated with temperature (GLM: R2 = 41.6, F = 13.92, P = 0.0002). This negative response to warming was also observed when separating into seasons for summer, autumn and spring but not for winter (Table 1). These responses were, however, only significant at high N (before stopping the N additions) and there were no differences in periphyton biomass between temperature treatments during the low N period (Table 1). Plant type also influenced periphyton biomass (Table 1) with higher biomass on E. canadensis artificial (EA) than on the less morphologically complex P. crispus (both natural and artificial; Tukey HSD P = 0.0015 and P = 0.0105 ,respectively, for the whole period). Periphyton responses to plant type were similar at both N conditions, with higher biomass in the more complex E. canadensis with different effects depending on the season. At high N conditions (before), periphyton biomass was higher on natural E. canadensis than on the other plants in winter and spring (EN > EA = PN = PA). At low N conditions (after), periphyton biomass was higher on natural and artificial E. canadensis in autumn (EN = EA > PN = PA) and on the artificial E. canadensis in spring (EA > PN = PA) (Table 1). There was no significant interaction between experimental factors analysed and the effects observed on the periphyton biomass were almost exclusively due to the effect of the particular factors in general and when considering by nitrogen conditions or season (Table 1).
During the autumn experiment at low N conditions (after), there was a peak in periphyton biomass on natural P. crispus in the ambient treatments (Fig. 3). Periphyton biomass was negatively associated to phytoplankton chlorophyll-a (R2 = 27.1; F = 2.88, P = 0.046) and did not show any significant responses to the other environmental variables considered.
Periphyton composition
Periphyton taxonomic composition differed between N conditions (before and after) (PERMANOVA: F = 32.76, P = 0.001), temperature (F = 3.45, P = 0.001) and their interactions (F = 1.99, P = 0.002) but showed no differences with respect to the plant type. Periphyton genus richness was higher at high N conditions compared to the low N period (ANOVA: F = 525.62, P < 0.0001) and higher under ambient than in the A2 + 50% scenario (ANOVA: F = 9.06 P = 0.001, Tukey HSD P = 0.001) (Fig. 4).
Periphyton genus richness under high (blue) and low N (red) conditions for the three temperature scenarios: ambient (Amb), A2 IPCC scenario (ca. 3.5 °C) and A2 + 50% (ca. 4.5 °C). Periphyton genus richness was lower at low N (ANOVA: F = 525.62, P < 0.0001) and in the higher temperature scenario (A2 + 50%) compared to ambient (Amb) conditions (ANOVA: F = 9.06 P = 0.001, Tukey HSD P = 0.001)
In the canonical correspondence analysis (CCA), periphyton taxonomic composition was mainly explained by TDIN, TN and phytoplankton biomass (F = 8.9, 375df, P = 0.001; Fig. 5). Turbidity was also selected as an explanatory variable in the CCA model, but was excluded as since it was correlated to phytoplankton biomass (VIF > 10) which explained more variance. Periphyton composition at high N was associated with high N (TN and TDIN) and phytoplankton biomass, as opposed to periphyton under low N conditions (after; Fig. 5). Under high N conditions periphyton was represented by diatoms (Cymbella spp., Diatoma sp., Achnantes sp., Gomphonema spp. and Epithemia spp.), while under low N conditions, the periphyton was characterized by large-sized (linear dimension > 200 µm) filaments of green algae (Rhizoclonium sp., Stigeoclonium sp., Oedogonium spp.) and cyanobacteria,(Lyngbya sp.), as well as by large-sized colonies green algae (Sphaerocystis sp., Botryococcus sp.) and cyanobacteria (Aphanocapsa sp.), together with flagellates (Chlamydomonas sp.) (Fig. 5).
Canonical correspondence analysis of the periphyton and environmental variables before and after the nitrogen decline. Red dots indicate conditions of high N before the decline. Blue dots indicate the conditions of low N after the decline. Colour gradient indicates the different treatment scenarios. TN: total nitrogen, TDIN: total dissolved nitrogen, Phyto_Bm: phytoplankton biomass. We did not include plant type since it did not affect the periphyton composition. The first two axes explained the 57.01 of the constrained variance (λ1 = 33.75%, λ2 = 23.26%; MonteCarlo 999 permutations: F = 8.9, 375df, P = 0.001)
Diazotrophic cyanobacteria biomass was low in general, representing 1–4% of the total biovolume being higher under high N (ANOVA: F = 31.98, P < 0.0001) and in summer and spring than in winter and autumn (summer > spring > winter = autumn, Tukey HSD P = 0.001) (ANOVA: F = 28.94, P < 0.0001).
Discussion
The decrease in N conditions lead to an increase in periphyton biomass, lower genus richness along with a higher biomass of large filaments and colonies particularly of green algae. Periphyton biomass decreased with high N concentration, warming, high phytoplankton biomass, and lower morphological plant complexity, while the composition mostly differed with respect to the N scenario and phytoplankton biomass. This suggests that a decline in N may induce drastic changes in the ecological conditions of shallow lakes, promoting a decay in phytoplankton and increase in periphyton biomass. This change from planktonic to periphytic production is also reflected in compositional changes in the periphyton community to groups with higher capacity to store and recirculate N in conditions of N deficiency. The increased periphyton biomass at low N conditions was not associated with a higher biomass of diazotrophic cyanobacteria, but rather with large-sized groups with effective use of N that were favoured by the decreased phytoplankton biomass that led to improved light conditions.
Periphyton biomass was higher on the morphologically complex plant, E. canadensis, than on P. crispus (both natural and artificial), which coincide with previous findings (Ferreiro et al., 2013; Hao et al., 2017, 2020; Pettit et al., 2016). This has been attributed to the higher surface area available for periphyton colonization in the more complex forms and lower susceptibility to grazers (Taniguchi et al., 2003; Hao et al., 2017; Tramonte et al., 2019). In our experiment, these responses were similar in general with some variation according to N scenario and season. While at high N conditions, periphyton biomass was higher on natural E. canadensis (in winter and spring), at low N conditions the artificial E. canadensis had similar (autumn) or higher (spring) periphyton biomass than the natural. Natural plants can both promote algae growth by nutrient release (Carignan & Kalff, 1982) or inhibit growth by the release of allelopathic compounds (Erhard & Gross, 2006; Hilt, 2006). The secretion of allelopathic compounds may contribute to explain why under high periphyton growth (at low N conditions) periphyton biomass was lower in natural plants. However, the experimental design with all the plants placed together does not allow us to separate the effect of allelopathic substances, as described in other studies (Cao et al., 2019; Liu et al., 2021). Although the periphyton biomass was generally lower on the less complex P. crispus, the highest peak of periphyton biomass was observed on natural P. crispus at ambient temperature and low N conditions during autumn. P. crispus typically decay after summer (Bolduan et al., 1994; Cao et al., 2015; Hao et al., 2017) and its decomposition produces high nutrient release (Wang et al., 2018). Despite we did not observe a drastic decay of P. crispus in the mesocosms, the shoots used for the experiment were in decomposition in autumn at ambient conditions, which may have enhanced the periphyton growth under these conditions.
According to our hypothesis, periphyton biomass responded negatively to warming. Hence, our results support previous findings that warming negatively affects periphyton biomass (Hao et al., 2020; Liu et al., 2021; Shurin et al., 2012) including comparative latitudinal studies (Bécares et al., 2008; Meerhoff et al., 2007) and metanalysis (Meerhoff et al., 2012) while contrasting other findings reporting a positive response of periphyton biomass to high temperatures (e.g. Tarkowska-Kukuryk & Mieczan, 2012; Mahdy et al., 2015).
The response of algae production to temperature also depends on nutrient availability (e.g. Reynolds, 2006; Kosten et al., 2012; Liu et al., 2021; Verbeek et al., 2018) and, additionally, on the combined effect of warming and nutrient availability (Feuchtmayr et al., 2009; Jeppesen et al., 2009, 2011). Accordingly, periphyton biomass decreased with warming at high N loadings, which coincides with other studies (Hao et al., 2020; Liu et al., 2021) but did not change at low N loadings. These responses varied depending on the season and seem to be influenced by phytoplankton responses to warming (Bécares et al., 2008), with contrasting effects depending on the N availability. Verbeek et al. (2018) described that the response of phytoplankton biomass to warming is positive under high nutrient availability, but negative under low nutrient conditions. The effects of phytoplankton biomass could explain this contrasting effect of the temperature depending on the N conditions. At high N conditions, phytoplankton shading restricts periphyton photosynthesis. The metabolic increase imposed by higher temperatures on the periphyton cannot be met due to light restrictions competition by nutrients with the phytoplankton. However, at low N conditions, the phytoplankton biomass decreases and allows an enhanced light environment for the periphyton. Under these conditions, it is likely that the periphyton, with a greater capacity to store and recirculate nutrients, was not adversely affected by temperature since it had enough N reserves and light to cope with the metabolic demands of the higher temperatures. Therefore, the negative effect of warming on the periphyton biomass might have been compensated by higher growth promoted by the enhanced light conditions due to the phytoplankton decline.
In our study, the N decrease induced not only higher periphyton biomass, but drastic compositional changes. According to our hypothesis we observed compositional replacement in response to N decrease, with a higher representation of large-sized filaments and colonies (mostly chlorophytes) along with a decrease in the genus richness. Periphyton can sequester, store and relocate large amounts of nutrients from the water and released by plants and these reserves can be used to compensate for nutrient imbalances (Carignan & Kalff 1982; Blumenshine et al., 1997; Havens et al., 1999). In contrast, phytoplankton can more effectively capture nutrient from the water (Riber & Wetzel, 1987; Reynolds, 2006) but they have a reduced storage capacity than the periphyton (Cattaneo, 1987; Blumenshine et al., 1997; Havens et al., 1999; Rodusky et al., 2001) and they are therefore more sensitive to prolonged periods of low nutrient conditions. Periphyton biomass was enhanced at low N and low phytoplankton biomass, which indicates that high N concentrations together with high P availability promote phytoplankton over periphyton. Furthermore, in these high P availability conditions, low N promoted periphyton biomass, with compositional changes towards large-sized filaments with higher N storage capacity that can effectively outcompete phytoplankton for light and nutrients (Hansson, 1992; Vadeboncoeur & Steinman, 2002; Rodusky et al., 2001). Furthermore, periphyton taxonomic richness was lower at low N conditions and mainly associated with large-sized filaments and colonies, which may indicate a selection of large-sized groups that can compensate for low N: P ratios with high N reserves (Havens et al., 1999). Previous studies suggested that higher nutrient concentrations favour greater taxonomic richness in the periphyton due to a more diversified use of resources. However, when resources become scarce, periphyton richness diminishes in favour of groups with a more efficient use of resources (Passy, 2007; Wu et al., 2017). Temperature also played an important role on periphyton composition, promoting different community composition in the periphyton, particularly in interaction with N scenarios (PERMANOVA). According to our hypothesis, temperature exacerbated the effects of N decrease, decreasing the taxonomic richness of the periphyton. The metabolic increase imposed by higher temperatures, accompanied by low N may have increased the selection pressure of larger groups with greater capacities to store and recirculate N to the detriment of other groups that could not cope with this higher stress under conditions of deficiency of nutrients (Azim et al., 2005).
According to out hypothesis the higher periphyton biomass at low N were not associated with a higher biomass of diazotrophic cyanobacteria, since atmospheric N2-fixation cannot compensate for N deficiency in periphyton growth. Instead, we found lower biomass of diazotrophic cyanobacteria at low N conditions even in high temperature scenarios. High nutrient concentrations and low N: P ratios often promote higher cyanobacteria biomass, which include N2-fixing cyanobacteria (Reynolds, 2006; Elser et al., 2007; Kosten et al., 2012; Moss et al., 2013). In addition, warming can also promote the presence of cyanobacteria (Pearl & Huisman, 2008) and decrease the N: P ratio by accelerating denitrification (Veraart et al., 2011). However, N is a key limiting nutrient in lake production that cannot be compensated by cyanobacteria atmospheric N2-fixation (Chorus & Spijkerman, 2020; Elser et al., 2007; Harpole et al., 2011; Pacheco et al., 2010; Paerl et al., 2016; Søndergaard et al., 2017). Recent evidence indicate that N2-fixation is a metabolically expensive process (Agawin et al., 2007; Muggide et al., 2003). It depends on high light intensities, P concentrations and micronutrients among others (Reynolds 2006; De Tezanos Pinto & Litchman 2010) and, therefore cannot compensate for low N: P ratios in lakes (Camacho et al., 2003; Díaz et al., 2007; Moss et al., 2013; Paerl et al., 2020). According to these findings, our study does not support classical theoretical expectations that low N: P ratios favour diazotrophic cyanobacteria (e.g. Schindler, 1977, 2006; Vollenweider 1976).
Conclusions
Overall, our results support previous findings indicating that the N decline in lakes led to changes from a pelagic to periphytic production dominance (Liboriussen & Jeppesen, 2003; Vadeboncoeur et al., 2003). The effect of warming on periphyton depended on N concentrations, with negative effects at high N and no effects at low N, which might be explained by the enhanced light environment by low phytoplankton biomass at low N. Changes in the periphyton taxonomic composition, and a drop in genus richness accompanied the increased periphyton biomass at low N conditions. However, these changes were not related to increased biomass of N2-fixing cyanobacteria, but to large-sized groups with an efficient capacity to store and recirculate nutrients (Havens et al., 1999) favoured by the decreased phytoplankton biomass that led to improved light conditions. Strategies to control N in shallow lakes must consider these potential effects on periphyton growth and their eventual effects on the different ecosystem services and functions.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Agawin, N. S., S. Rabouille, M. J. Veldhuis, L. Servatius, S. Hol, H. M. van Overzee & J. Huisman, 2007. Competition and facilitation between unicellular nitrogen-fixing cyanobacteria and non nitrogen-fixing phytoplankton species. Limnology and Oceanography 52(5): 2233–2248.
Anderson, M. J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46.
Arocena, R., G. Chalar, & J. P. Pacheco, 2018. Agriculture and elevation are the main factors for Pampasic stream habitat and water quality. Environmental Monitoring and Assessment, 190(4): 1–22.
Azim, M. E., Beveridge, M. C. M., Van Dam, A. A. & M. C. J. Verdegem, 2005. Periphyton and aquatic production: an introduction Periphyton: ecology, exploitation and management CABI Wallingford 1–13
Bécares, E., J. Gomá, M. Fernández-Aláez, C. Fernández-Aláez, S. Romo, M. R. Miracle, A. Ståhl-Delbanco, L. A. Hansson, M. Gyllström, W. J. Van de Bund & E. Van Donk, 2008. Effects of nutrients and fish on periphyton and plant biomass across a European latitudinal gradient. Aquatic Ecology 42(4): 561–574.
Blumenshine, S. C., Y. Vadenboncoeur, D. M. Lodge, K. L. Cottingham & S. E. Knight, 1997. Benthic-pelagic links: responses of benthos to water-column nutrient enrichment. Journal of the North American Benthological Society 16: 466–479.
Bolduan, B. R., C. V. Van Eeckhout, H. W. Quade & J. E. Gannon, 1994. Potamogeton crispus - the other invader. Lake and Reservoir Management 10(2): 113–125.
Boyer, E. W., C. Goodale, N. Jaworski & R. W. Howarth, 2002. Anthropogenic nitrogen sources and relationships to riverine nitrogen export in the northeastern USA. Biogeochemistry 57: 267–293.
Camacho, A., W. A. Wurtsbaugh, M. R. Miracle, X. Armengol & E. Vicente, 2003. Nitrogen limitation of phytoplankton in a Spanish karst lake with a deep chlorophyll maximum: a nutrient enrichment bioassay approach. Journal of Plankton Research 25(4): 397–404.
Cao, Y., E. M. Neif, W. Li, J. Coppens, N. Filiz, T. L. Lauridsen, T. A. Davidson, M. Søndergaard & E. Jeppesen, 2015. Heat wave effects on biomass and vegetative growth of macrophytes after long-term adaptation to different temperatures: a mesocosm study. Climate Research 66: 265–274.
Cao, Y., N. Zhang, J. Sun & W. Li, 2019. Responses of periphyton on non-plant substrates to different macrophytes under various nitrogen concentrations: a mesocosm study. Aquatic Botany 154: 53–59.
Carignan, R. & J. Kalff, 1982. Phosphorus release by submerged macrophytes: significance to epiphyton and phytoplankton. Limnology and Oceanography 27: 419–427.
Cattaneo, A., 1987. Periphyton in lakes of different trophy. Canadian Journal of Fisheries and Aquatic Sciences. 44: 296–303.
Chorus, I. & E. Spijkerman, 2020. What Colin Reynolds could tell us about nutrient limitation, N: P ratios and eutrophication control. Hydrobiologia 848: 95–111.
De Tezanos Pinto, P. & E. Litchman, 2009. Interactive effects of N: P ratios and light on nitrogen-fixer abundance. Oikos 119: 567–575.
Diaz, M., F. Pedrozo, C. S. Reynolds & P. Temporettia, 2007. Chemical composition and the nitrogen-regulated trophic state of Patagonian lakes. Limnologica 37: 17–27.
Dodds, W. K., 2003. The role of periphyton in phosphorus retention in shallow freshwater aquatic systems. Journal of Phycology 39(5): 840–849.
Elser, J. J., M. E. S. Bracken, E. A. Cleland, D. S. Gruner, W. S. Harpole, H. Hillebrand, J. T. Ngai, E. W. Seabloom, J. B. Shurin & J. E. Smith, 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10(12): 1135–1142.
Erhard, D. & E. M. Gross, 2006. Allelopathic activity of Elodea canadensis and Elodea nuttallii against epiphytes and phytoplankton. Aquatic Botany 85: 203–211.
Ferreiro, N., A. Giorgi & C. Feijoo, 2013. Effects of macrophyte architecture and leaf shape complexity on structural parameters of the epiphytic algal community in a Pampean stream. Aquatic Ecology 47: 389–401.
Feuchtmayr, H., R. Moran, K. Hatton, L. Connor, T. Heyes, B. Moss & I. Harvey, 2009. Global warming and eutrophication: effects on water chemistry and autotrophic communities in experimental hypertrophic shallow lake mesocosms. Journal of Applied Ecology 46(3): 713–723.
González-Madina, L., J. P. Pacheco, L. Yema, P. de Tezanos, P. Levrini, J. Clemente, C. Crisci, J. J. Lagomarsino, G. Méndez, C. Fosalba, G. Goyenola & N. Mazzeo, 2019. Drivers of cyanobacteria dominance, composition and nitrogen fixing behavior in a shallow lake with alternative regimes in time and space, Laguna del Sauce (Maldonado, Uruguay). Hydrobiologia, 829(1): 61.
Hansson, L. A., 1990. Quantifying the impact of periphytic algae on nutrient availability for phytoplankton. Freshwater Biology 24(2): 265–273.
Hansson, L. A., 1992. Factors regulating periphytic algal biomass. Limnology and Oceanography 37(2): 322–328.
Hao, B., H. Wu, Y. Cao, W. Xing, E. Jeppesen & W. Li, 2017. Comparison of periphyton communities on natural and artificial macrophytes with contrasting morphological structures. Freshwater Biology 62(10): 1783–1793.
Hao, B., H. Wu, W. Zhen, H. Jo, Y. Cai, E. Jeppesen & W. Li, 2020. Warming effects on periphyton community and abundance in different seasons are influenced by nutrient state and plant type: a shallow lake mesocosm study. Frontiers in Plant Science 11: 404.
Harpole, W. S., J. T. Ngai, E. E. Cleland, E. W. Seabloom, E. T. Borer, M. E. Bracken, J. J. Elser, D. S. Gruner, H. Hillebrand, J. B. Shurin & J. E. Smith, 2011. Nutrient co‐limitation of primary producer communities. Ecology Letters, 14(9): 852–862.
Havens, K. E., T. L. East, A. J. Rodusky & B. Sharfstein, 1999. Littoral periphyton responses to nitrogen and phosphorus: an experimental study in a subtropical lake. Aquatic Botany 63(3–4): 267–290.
Hillebrand, H., C. D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35(2): 403–424.
Hilt, S., 2006. Allelopathic inhibition of epiphytes by submerged macrophytes. Aquatic Botany 85: 252–256.
Hungate, B. A., J. S. Dukes, M. R. Shaw, Y. Luo & C. B. Field, 2003. Nitrogen and climate change. Science 302(5650): 1512–1513.
IPCC, 2014. Climate change 2013: the physical science basis: Working Group I contribution to the Fifth assessment report of the Intergovernmental Panel on Climate Change. Stocker, T. F., Qin, D., Plattner, G. K., Tignor, M., Allen, S. K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P. M., & Midgley, P. M. (eds) Cambridge university press United Kingdom and New York 1535pp.
ISO 10260, 1992. Water quality – Measurement of biochemical parameters – Spectrometric determination of the chlorophyll-a concentration, Int. Org. Standard., Geneva, 1st ed. 1992–07–15, 6 pp.
Jeppesen, E., J. P. Jensen & M. Søndergaard, 2002. Response of phytoplankton, zooplankton, and fish to re-oligotrophication: an 11 year study of 23 Danish lakes. Aquatic Ecosystem Health & Management 5(1): 31–43.
Jeppesen, E., M. Søndergaard, J. P. Jensen, K. E. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. T. Dokulil, B. O. B. Foy & D. Gerdeaux, 2005. Lake responses to reduced nutrient loading–an analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50(10): 1747–1771.
Jeppesen, E., B. Kronvang, M. Meerhoff, M. Søndergaard, K. M. Hansen, H. E. Andersen, T. L. Lauridsen, L. Liboriussen, M. Beklioglu, A. Özen & J. E. Olesen, 2009. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. Journal of Environmental Quality 38(5): 1930–1941.
Jeppesen, E., B. Moss, H. Bennion, L. Carvalho, L. DeMeester, H. Feuchtmayr, N. Friberg, M. O. Gessner, M. Hefting, T. L. Lauridsen & L. Liboriussen, 2010. Interaction of climate change and eutrophication. Climate Change Impacts on Freshwater Ecosystems 17: 119–151.
Jeppesen, E., B. Kronvang, J. E. Olesen, J. Audet, M. Søndergaard, C. C. Hoffmann, H. E. Andersen, T. L. Lauridsen, L. Liboriussen, S. E. Larsen & M. Beklioglu, 2011. Climate change effects on nitrogen loading from cultivated catchments in Europe: implications for nitrogen retention, ecological state of lakes and adaptation. Hydrobiologia 663(1): 1–21.
Khan, F. A. & A. A. Ansari, 2005. Eutrophication: an Ecological Vision. The Botanical Review 71: 449–482.
Kosten, S., V. L. Huszar, E. Bécares, L. S. Costa, E. van Donk, L. A. Hansson, E. Jeppesen, C. Kruk, G. Lacerot, N. Mazzeo & L. De Meester, 2012. Warmer climates boost cyanobacterial dominance in shallow lakes. Global Change Biology, 18(1): 118-126.
Liboriussen, L. & E. Jeppesen, 2003. Temporal dynamics in epipelic, pelagic and epiphytic algal production in a clear and a turbid shallow lake. Freshwater Biology 48(3): 418–431.
Liboriussen, L., F. Landkildehus, M. Meerhoff, M. E. Bramm, M. Søndergaard, K. Christoffersen, K. Richardson, M. Søndergaard, T. L. Lauridsen & E. Jeppesen, 2005. Global warming: Design of a flow-through shallow lake mesocosm climate experiment. Limnology and Oceanography: Methods 3(1): 1–9.
Liu, Y., C. Aznarez, E. Jeppesen, H. He, W. Li, E. E. Levi, J. P. Pacheco & Y. Cao, 2021. Responses of submerged macrophytes and periphyton to warming under two nitrogen scenarios: A microcosm study. Hydrobiologia 848(6): 1333–1346.
Lund, J. W. G., C. Kipling & E. D. Le Cren, 1958. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 11(2): 143–170.
Mahdy, A., S. Hilt, N. Filiz, M. Beklioğlu, J. Hejzlar, D. Özkundakci, E. Papastergiadou, U. Scharfenberger, M. Šorf, K. Stefanidis & L. Tuvikene, 2015. Effects of water temperature on summer periphyton biomass in shallow lakes: a pan-European mesocosm experiment. Aquatic Sciences 77(3): 499–510.
Meis, S., S. J. Thackeray & I. D. Jones, 2009. Effects of recent climate change on phytoplankton phenology in a temperate lake. Freshwater Biology 54(9): 1888–1898.
McDowell, R. W., A. Noble, P. Pletnyakov, B. E. Haggard & L. M. Mosley, 2020. Global mapping of freshwater nutrient enrichment and periphyton growth potential. Scientific Reports 10(1): 1–13.
Meerhoff, M., J. M. Clemente, F. Teixeira-de Mello, C. Iglesias, A. Pedersen & E., Jeppesen, 2007. Can warm climate-related structure of littoral predator assemblies weaken the clear water state in shallow lakes? Global Change Biology, 13(9): 1888–1897.
Meerhoff, M., F. Teixeira-de Mello, C. Kruk, C. Alonso, I. González-Bergonzoni, J. P. Pacheco, G. Lacerot, M. Arim, M. Beklioğlu, S. Brucet, G. Goyenola, C. Iglesias, N. Mazzeo, S. Kosten & E. Jeppesen, 2012. Environmental warming in shallow lakes. A review of potential changes in community structure as evidenced from space-for-time substitution approaches. Advances in Ecological Research 46: 259–349.
Moss, B., S. Kosten, M. Meerhoff, R. W. Battarbee, E. Jeppesen, N. Mazzeo, K. Havens, G. Lacerot, Z. Liu, L. De Meester & H. Paerl, 2011. Allied attack: climate change and eutrophication. Inland Waters 1(2): 101–105.
Moss, B., E. Jeppesen, M. Søndergaard, T. L. Lauridsen & Z. Liu, 2013. Nitrogen, macrophytes, shallow lakes and nutrient limitation: resolution of a current controversy? Hydrobiologia 710(1): 3–21.
MugiddeHecky, R. R. E., L. L. Hendzel & W. D. Taylor, 2003. Pelagic nitrogen fixation in lake Victoria (East Africa). Journal of Great Lakes Research 29: 76–88.
Nusch, E., 1980. Comparison of different methods for chlorophyll and phaeopigment determination. Archiv Für Hydrobiologie 14: 14–36.
Oksanen, J., F. G. Blanchet, M. Fiendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O´Hara, G. L. Simpson, P. Solymos, M. H. Stevens, E. Szoecs & H. Wagner, 2019. Vegan: community ecology package. R Package Version 2: 5–6.
Pacheco, J. P., C. Iglesias, M. Meerhoff, C. Fosalba, G. Goyenola, F. Teixeira-de Mello, S. García, M. Gelós & F. García-Rodríguez, 2010. Phytoplankton community structure in five subtropical shallow lakes with different trophic status (Uruguay): a morphology-based approach. Hydrobiologia, 646(1): 187–197.
Pacheco, J. P., C. Aznarez, M. Meerhoff, Y. Liu, W. Li, A. Baattrup-Pedersen, Y. Cao & E. Jeppesen, 2021. Small-sized omnivorous fish induce stronger effects on food webs than warming and eutrophication in experimental shallow lakes. Science of the Total Environment, 797: 148998.
Paerl, H. W. & J. Huisman, 2008. Blooms like it hot. Science 320(5872): 57–58.
Paerl, H. W., H. Xu, N. S. Hall, G. Zhu, B. Qin, Y. Wu, K. L. Rossignol, L. Dong, M. J. McCarthy & A. R. Joyner, 2014. Controlling cyanobacterial blooms in hypertrophic Lake Taihu, China: will nitrogen reductions cause replacement of non-N2 fixing by N2 fixing taxa? PloS One 9(11): e113123.
Paerl, H. W., J. T. Scott, M. J. McCarthy, S. E. Newell, W. S. Gardner, K. E. Havens, D. K. Hoffman, S. W. Wilhelm & W. Wurtsbaugh, 2016. It takes two to tango: When and where dual nutrient (N & P) reductions are needed to protect lakes and downstream ecosystems. Environmental Science & Technology 50(20): 10805–10813.
Paerl, H. W., K. E. Havens, H. Xu, G. Zhu, M. J. McCarthy, S. E. Newell, J. T. Scott, N. S. Hall, T. G. Otten & B. Qin, 2020. Mitigating eutrophication and toxic cyanobacterial blooms in large lakes: The evolution of a dual nutrient (N and P) reduction paradigm. Hydrobiologia 847(21): 4359–4375.
Pakdel, F. M., L. Sim, J. Beardall & J. Davis, 2013. Allelopathic inhibition of microalgae by the freshwater stonewort, Chara australis, and a submerged angiosperm, Potamogeton crispus. Aquatic Botany 110: 24–30.
Passy, S. I., 2007. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquatic Botany 86(2): 171–178.
Pettit, N., D. Ward, M. Adame, D. Valdez & S. Bunn, 2016. Influence of aquatic plant architecture on epiphyte biomass on a tropical river floodplain. Aquatic Botany 129: 35–43.
Phillips, G., N. Willby & B. Moss, 2016. Submerged macrophyte decline in shallow lakes: What have we learnt in the last forty years? Aquatic Botany. 135: 37–45.
Pinay, G., B. Gumiero, E. Tabacchi, O. Gimenez, A. M. Tabacchi-Planty, M. M. Hefting, T. P. Burt, V. A. Black, C. Nilsson, V. Iordache & F. Bureau, 2007. Patterns of denitrification rates in European alluvial soils under various hydrological regimes. Freshwater Biology 52(2): 252–266.
R Core Team, 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Reynolds, C. S., 2006. The ecology of phytoplankton, Cambridge University Press:
Richardson, C. J. & P. E. Marshall, 1986. Processes controlling movement, storage, and export of phosphorus in a fen peatland. Ecological Monographs 56(4): 279–302.
Riber, H. H. & R. G. Wetzel, 1987. Boundary-layer and internal diffusion effects on phosphorus fluxes in lake periphyton. Limnology and Oceanography 32: 1181–1194.
Rodusky, A. J., A. D. Steinman, T. L. East, B. Sharfstein & R. H. Meeker, 2001. Periphyton nutrient limitation and other potential growth-controlling factors in Lake Okeechobee, USA. Hydrobiologia 448(1): 27–39.
Schindler, D. W., 1977. Evolution of phosphorus limitation in lakes. Science 195: 260–262.
Schindler, D. W., 2006. Recent advances in the understanding and management of eutrophication. Limnology and Oceanography 51: 356–363.
Schindler, D. W., R. E. Hecky, D. L. Findlay, M. P. Stainton, B. R. Parker, M. J. Paterson, K. G. Beaty, M. Lyng & S. E. M. Kasian, 2008. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proceedings of the National Academy of Sciences 105(32): 11254–11258.
Scott, J. T., M. J. McCarthy & H. W. Paerl, 2019. Nitrogen transformations differentially affect nutrient-limited primary production in lakes of varying trophic state. Limnology and Oceanography Letters 4: 96–104.
Standard, Danish, 1975. Determination of nitrogen content after oxydation by peroxodisulphate, Dansk Standards Forlag, Charlottenlund, Copenhagen, Denmark:
Standard, Danish, 1985. Water analysis – total phosphor – photometric method, Dansk Standards Forlag, Charlottenlund, Copenhagen Denmark:
Standard, Danish, 1996. Water Quality – Determination of dissolved fluoride, chloride, nitrite, orthophosphate, bromide, nitrate and sulphate ions, using liquid chromatography of ions – Part 1: Method for water with low contamination, Dansk Standards Forlag, Charlottenl- und, Copenhagen Denmark:
Shurin, J. B., J. L. Clasen, H. S. Greig, P. Kratina & P. L. Thompson, 2012. Warming shifts top-down and bottom-up control of pond food web structure and function. Philosophical Transactions of the Royal Society b: Biological Sciences 367(1605): 3008–3017.
Søndergaard, M., T. L. Lauridsen, L. S. Johansson & E. Jeppesen, 2017. Nitrogen or phosphorus limitation in lakes and its impact on phytoplankton biomass and submerged macrophyte cover. Hydrobiologia 795(1): 35–48.
Taniguchi, H., S. Nakano & M. Tokeshi, 2003. Influences of habitat complexity on the diversity and abundance of epiphytic invertebrates on plants. Freshwater Biology 48: 718–728.
Tarkowska-Kukuryk, M. & T. Mieczan, 2012. Effect of substrate on periphyton communities and relationships among food web components in shallow hypertrophic lake. Journal of Limnology 71(2): 279–290.
Ter Braak, C. J. & P. F. Verdonschot, 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquatic Sciences 57(3): 255–289.
Tramonte, R. P., N. C. Osório, F. H. Ragonha, G. D. Pinha, L. Rodrigues & R. P. Mormul, 2019. Periphyton consumption by an invasive snail species is greater in simplified than in complex habitats. Canadian Journal of Zoology 97: 13–21.
Trochine, C., M. Guerrieri, L. Liboriussen, T. L. Lauridsen & E. Jeppesen, 2014. Effects of nutrient loading, temperature regime and grazing pressure on nutrient limitation of periphyton in experimental ponds. Freshwater Biology 59(5): 905–917.
Trochine, C., M. Guerrieri, L. Liboriussen, P. Willems, T. L. Lauridsen, M. Søndergaard & E. Jeppesen, 2017. Factors controlling the stable isotope composition and C: N ratio of seston and periphyton in shallow lake mesocosms with contrasting nutrient loadings and temperatures. Freshwater Biology 62(9): 1596–1613.
Utermöhl, H., 1958. Zur vervollkommnung der quantitativen phytoplankton-methodik: Mit 1 Tabelle und 15 abbildungen im Text und auf 1 Tafel. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Mitteilungen 9(1): 1–38.
Vadeboncoeur, Y. & A. D. Steinman, 2002. Periphyton function in lake ecosystems. The Scientific World Journal 2: 1449–1468.
Vadeboncoeur, Y., E. Jeppesen, M. J. V. Zanden, H. H. Schierup, K. Christoffersen & D. M. Lodge, 2003. From Greenland to green lakes: cultural eutrophication and the loss of benthic pathways in lakes. Limnology and Oceanography 48(4): 1408–1418.
Vander Zanden, M. J. & Y. Vadeboncoeur, 2020. Putting the lake back together 20 years later: what in the benthos have we learned about habitat linkages in lakes? Inland Waters 10(3): 305–321.
Veraart, A. J., J. J. De Klein & M. Scheffer, 2011. Warming can boost denitrification disproportionately due to altered oxygen dynamics. PLoS One 6(3): e18508.
Verbeek, L., A. Gall, H. Hillebrand & M. Striebel, 2018. Warming and oligotrophication cause shifts in freshwater phytoplankton communities. Global Change Biology 24: 4532–4543.
Vollenweider, R. A., 1976. Rotsee, a source, not a sink for phosphorus? A comment to and a plea for nutrient balance studies. Schweizerische Zeitschrift Für Hydrologie 38: 29–34.
Wagner, C. & Adrian, R., 2009. Cyanobacteria dominance: quantifying the effects of climate change. Limnology and Oceanography, 54(6 part 2): 2460–2468.
Wang, L., Q. Liu, C. Hu, R. Liang, J. Qiu & Y. Wang, 2018. Phosphorus release during decomposition of the submerged macrophyte Potamogeton crispus. Limnology 19: 355–366.
Weyhenmeyer, G. A., E. Jeppesen, R. Adrian, L. Arvola, T. Blenckner, T. Jankowski, E. Jennings, P. Noges, T. Noges & D. Straile, 2007. Nitrate-depleted conditions on the increase in shallow northern European lakes. Limnology and Oceanography 52(4): 1346–1353.
Woolway, R. I., B. M. Kraemer, J. D. Lenters, C. J. Merchant, C. M. O’Reilly & S. Sharma, 2020. Global lake responses to climate change. Nature Reviews Earth & Environment 1(8): 388–403.
Wu, N., X. Dong, Y. Liu, C. Wang, A. Baattrup-Pedersen & T. Riis, 2017. Using river microalgae as indicators for freshwater biomonitoring: Review of published research and future directions. Ecological Indicators 81: 124–131.
Acknowledgements
This study was supported by the Sino-Danish Centre – Aarhus University, the University of the Chinese Academy of Sciences and the University of the Republic, Uruguay. E.J. was also supported by the TÜBITAK, BIDEB 2232 program (118C250). C.A. was supported by the Doctoral INPhINIT–INCOMING program, fellowship code (LCF/BQ/DI20/11780004), from “la Caixa” Foundation (ID 100010434). We thank Beibei Hao for her valuable assistance with the experimental design and Ann Lene Vigh and Kathrine Tabermann Uhrenholt for the field and lab assistance.
Author information
Authors and Affiliations
Contributions
JPP conceptualization, methodology, formal analysis, investigation, data curation and writing—original draft; CA methodology, investigation and writing—review & editing; EEL methodology, investigation and writing—review & editing, ABP writing—review & editing and supervision; EJ conceptualization, methodology, funding acquisition, writing—review & editing and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable / not required.
Consent to participate
All the authors consent to participate in this manuscript.
Consent for publication
All the authors consent the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Sidinei Magela Thomaz.
Guest editors: José L. Attayde, Renata F. Panosso, Vanessa Becker, Juliana D. Dias & Erik Jeppesen / Advances in the Ecology of Shallow Lakes
Rights and permissions
About this article
Cite this article
Pacheco, J.P., Aznarez, C., Levi, E.E. et al. Periphyton responses to nitrogen decline and warming in eutrophic shallow lake mesocosms. Hydrobiologia 849, 3889–3904 (2022). https://doi.org/10.1007/s10750-021-04755-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04755-y