Abstract
Macrophytes and phytoplankton are recognized as having roles in determining alternative stable states in shallow lakes and reservoirs, while the role of periphyton has been poorly investigated. Temporal and spatial variation of phytoplankton, epipelon and epiphyton was examined in a shallow reservoir with high abundance of aquatic macrophytes. The relationships between algae communities and abiotic factors, macrophyte coverage and zooplankton density were also analyzed. Monthly sampling was performed in three zones of the depth gradient of the reservoir. Two phases of algal dominance were found: a phytoplankton phase and epipelon phase. The phase of phytoplankton dominance was characterized by high macrophyte coverage. Rotifera was the dominant zooplankton group in all the zones. Flagellate algae were dominant in phytoplankton, epipelon and epiphyton. Macrophyte coverage was found to be a predictor for algal biomass. Changes in biomass and species composition were associated with macrophyte cover variation, mainly the Nymphaea. In addition to the abiotic factors, the macrophyte coverage was a determining factor for changes to the algal community, contributing to the alternation between dominance phases of phytoplankton and epipelon. The macrophyte–phytoplankton–periphyton relationship needs to be further known in shallow reservoirs, especially the role of epipelon as an alternate stable state.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Numerous studies have discussed alternate stable states in shallow lakes and reservoirs throughout the world (e.g., Bicudo et al., 2007; Scheffer & van Nes, 2007; O’Farrell et al., 2011; Tezanos-Pinto & O’Farrell, 2014), including tropical ecosystems (Bicudo et al., 2007; Kosten et al., 2009). According to Beisner et al. (2003), the identification of alternate stable states allows a better understanding of the mechanisms associated with ecosystem stability and, from a practical point of view, assists in the elaboration of plans for the conservation or recovery of shallow lakes. Submersed and floating macrophytes and phytoplankton are the autotrophs most directly associated with alternate stable states in shallow lakes (Scheffer & van Nes, 2007). Most studies report that macrophytes are the dominant biomass in the clear water state and phytoplankton in the turbid water state, and that the dominant community has mechanisms to maintain the equilibrium state (e.g., Sayer et al., 2010; Hobbs et al., 2012). Although macrophytes and phytoplankton play important roles in determining alternative stable states, periphytic communities may also make an important contribution in the functioning of shallow lakes and reservoirs (Vadeboncoeur & Steinman, 2002; Vadeboncoeur & Power, 2017). As a component of the food web, periphyton communities (epiphyton, epipelon) can play a significant role in primary production in shallow lakes. Among these, the epipelon seems to contribute significantly to primary productivity and total biomass (Vadeboncoeur et al., 2001; Libouriussen & Jeppesen, 2003; Casco et al., 2009). Research has shown that phototrophic epipelon maintain oligotrophic conditions due to their capacity for nutrient retention, particularly of phosphorus, in restored ecosystems (Genkai-Kato et al., 2012). Other studies have reported that submerged macrophytes can be shaded by epiphyton, which may anticipate the beginning of the turbid state in shallow lakes (Olsen et al., 2015). Thus, the roles of periphytic communities in the context of alternative equilibrium states are still relatively unknown, particularly in tropical reservoirs and lakes.
Regardless of habitat type, algae have different competitive abilities, which depending on environmental conditions, can result in the coexistence or competitive exclusion of species in communities (Passarge et al., 2006). The main relationships between benthic and pelagic systems involve the deposition of planktonic material on sediment, resuspension of epipelic algae and the interception of light and nutrients in the water column before reaching epipelic organisms (Schindler & Scheuerell, 2002; Yang et al., 2009). Studies have shown that phototrophic epipelon can control the release of phosphorus from sediment to the water column, which can control phytoplankton biomass (Liboriussen & Jeppesen, 2003; Genkai-Kato et al., 2012). Autotrophs have close habitats and can often overlap, which can increase competition for resources, especially light and nutrients (Sand-Jensen & Borum, 1991). Thus, changes in associations between autotrophs may have consequences for ecosystem functioning since it directly affects primary productivity, nutrient cycling and the web food (Libouriussen & Jeppesen, 2003; Vadeboncouer et al., 2003). However, the relationships between macrophytes, phytoplankton and periphyton (epiphyton, epipelon) are still poorly understood in tropical lakes/reservoirs, especially when considering phototrophic epipelon (Thomas et al., 2000).
Based on functioning wetland ecosystems, Goldsborough & Robinson (1996) reported the dominance and persistence of an algal community in different equilibrium states. This conceptual model was analyzed in subtropical lakes of a floodplain, where an algal community was associated with a limnological state, such as open and lake states (Cano et al., 2008). Determining the pattern of alternation between dominance of algal communities may be hampered by the lack of knowledge about the dynamics of the phototrophic epipelon in shallow lakes (e.g., Liboriussen & Jeppesen, 2003; Casco et al., 2009). Considering alteration in algal community dominance due to change in the equilibrium state (Scheffer, 1993; Goldsborough & Robinson, 1996), we investigated temporal and spatial variation in biomass of phytoplankton, epipelon and epiphyton in a shallow reservoir with high abundance of aquatic macrophytes. We also investigated abiotic factors, changes in macrophyte coverage and zooplankton density as factors that could influence changes in the algal community. Specifically, we intended to answer the following questions: (i) Can the biomass of phytoplankton, epipelon and epiphyton characterize limnological phases in a shallow reservoir? (ii) Is there the alternation between dominance phases of phytoplankton and epipelon among limnological phases? (iii) Are changes in algal communities related to macrophyte coverage and zooplankton density? The present study contributes to a better understanding of competitive interactions among autotrophs, including phototrophic epipelon, which is among the least investigated algal communities of lakes and reservoirs.
Materials and methods
Study area
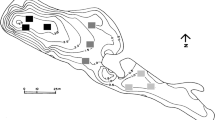
Ninfeias Reservoir is located in the Parque Estadual das Fontes do Ipiranga (23°38′18.95″ S and 46°37′16.3″ W) in the Municipality of São Paulo, State of São Paulo, Brazil. The reservoir is a shallow mesotrophic and polymictic system with a surface area of 5,433 m2, a volume of 7,170 m3, a mean depth of 1.32 m, a maximum depth of 3.6 m, and a mean theoretical residence time of 7 days (Fig. 1, Bicudo et al., 2002). The reservoir has an extensive littoral region with high coverage of two macrophytes, a rooted Nymphaea spp. and a free Utricularia foliosa Linnaeus (Souza et al., 2015). The dry season is characterized by low air temperatures and rainfall during the autumn and winter (April–September), while the rainy season is characterized by higher temperatures and rainfall during spring and summer (October–February). For the rainy and dry seasons during the study period, the mean monthly air temperature was 22.3°C and 17.8°C and the accumulated precipitation 894.8 mm and 322.0 mm, in rainy and dry seasons, respectively (http://estacao.iag.usp.br).

Modified from Bicudo et al. (2007)
Sampling sites location on bathymetric map of Ninfeias Reservoir (black squares pelagic zone, gray squares deep littoral zone; light gray squares shallow littoral zone).
Sampling design
Monthly sampling of water, macrophytes and algae and zooplankton communities was performed in three zones of the depth gradient of the reservoir from January to December 2014: pelagic (2.5–3.5 m deep), deep littoral (1 to 2 m deep) and shallow littoral (< 1 m deep). Three samples from each zone, including the benthic environment, were collected monthly, for a total of 108 water samples, 108 sediment samples and 72 Nymphaea petioles samples. Water samples for chemical analysis and phytoplankton were collected with a Van Dorn bottle at three different depths: subsurface, 1 m (middle) and 3 m (bottom). Water samples were manually integrated for evaluation of the whole water column. Sediment sampling of the first centimeter of sediment (Eaton & Moss, 1966) was performed using a manual corer sampler (Kajak collector; acrylic tube with a 7 cm diameter). The water present in the tube was removed with a hose to minimize inclusion of planktonic algae in the epipelon. Surface sediment samples were adjusted to a constant volume with distilled water. Petioles of Nymphaea spp. were randomly collected at sampling sites. The epiphyton was removed from 30 cm length of the petiole, cut at approximately 5 cm below the leaf for standardization. We sampled petioles with leaves of similar size and did not collect young or senescent plants. Nymphaea petioles were randomly collected at sampling sites. Epiphyton on U. foliosa stem was sampled when present at sampling sites. In both macrophytes, epiphyton was carefully removed by scraping and the use water jets. Total macrophyte coverage was estimated monthly at sampling sites using a 1 × 1 m PVC frame containing 100 smaller squares made with nylon thread (Thomaz et al., 2004). A single observer performed all macrophyte counts to standardize quantification. Quantitative analysis of the zooplankton involved subjecting the samples to narcotization to avoid contraction of the organisms, by adding carbonated water to saturate the sample with CO2 (Fernando, 2002). The samples were stored in glass flasks and fixed with 4% formalin. Samples for quantitative and qualitative analysis of planktonic, epipelic and epiphytic algae community were collected only bi-monthly at all sampling sites (February, April, June, August, October and December 2014).
Water transparency (Secchi disc depth), subaquatic radiation (PAR, photosynthetically active radiation; Li-Cor LI-250A luximeter), electric conductivity and pH (Horiba W-23 XD) were measured in field. The concentration of dissolved oxygen (azide-modification method), alkalinity (titration method), free CO2, HCO3 (calculated from alkalinity and pH), nitrate (cadmium-reduction method), nitrite (diazotization method), ammonium (phenate method), orthophosphate (ascorbic acid method), total nitrogen (TN) and total phosphorus (TP) (alkaline persulfate method) were determined according to APHA (2005). The concentration of dissolved nitrogen forms was summed to determine dissolved inorganic nitrogen (DIN). The attenuation light coefficient (k) was determined by the expression k = (Ln(I0) − Ln(I))/z, where I0 is the radiation at the surface, I is the radiation at a given depth, z is the depth in meters and Ln is the Neperian logarithm (Kirk, 1994 apud Padial & Thomaz, 2008).
Chlorophyll-a (corrected for pheophytin) concentrations for phytoplankton, epipelon and epiphyton were determined from subsamples filtered through glass-fiber filters (GF/F Whatman, Maidstone, UK), following 24-h extraction with 90% ethanol in the dark (Sartory & Grobbelaar, 1984). Chlorophyll-a concentrations, expressed in mg m−2, of phytoplankton, epipelon and epiphyton were used to determine the dominant community (≥ 50%) in the reservoir. Phytoplankton chlorophyll-a (mg Chl-a m−3) was multiplied by depth to express area biomass (mg Chl-a m−2), according to Robinson et al. (1997). Algal counts were performed under an inverted microscope (Zeiss Axio Observer D1 with Axiovision 4.7 software) according to Utermöhl method. Biovolume data of most algae species were obtained from Fonseca et al. (2014). Biovolume for the other species was determined using the geometric shapes described by Hillebrand et al. (1999). The algae were classified into five groups according to growth form and the presence/absence of motility: colonial, flagellated, filamentous, motile unicellular and non-motility unicellular.
Quantitative analysis of zooplankton was performed in acrylic plate observed under a microscope with × 50 magnification for Cladocera and Copepoda. Counts of Rotifera and Copepoda nauplii were performed in a Sedgewick-Rafter chamber under an optical microscope with a magnification of × 100. The count limit was determined by the rarefaction curve for the species.
Statistical analysis
Environmental conditions were evaluated using principal components analysis (PCA) with a covariance matrix and log-transformed data (x + 1). A PCA was performed for each of three sampled zones of the depth gradient of the reservoir (pelagic, deep and shallow littoral) using PC-ORD 6.0 (McCune & Mefford, 2011). Pearson correlations between variables and PCA scores (axis 1 and 2) were obtained (α < 0.5). Autocorrelation among sampling units and the possible violation of the assumption of their independence was checked by analyzing a correlogram generated by the Moran index with the respective significance of the spatial autocorrelation index (Legendre & Legendre, 2012). The analysis was performed in Past 3.20 (Hammer et al., 2001). Two-way RM-ANOVA was used to detect significant differences in chlorophyll-a concentrations of phytoplankton, epipelon and epiphyton among depth zones and seasons, as well as interactions among factors (α < 0.05; SigmaPlot 11.0). Permutational multivariate analysis of variance (two-way PERMANOVA) was applied to determine the significance of differences in the taxonomic structure of algal communities (α = 0.05). This analysis was performed using Bray–Curtis similarity and 4,999 permutations in PAST 3.01 (Hammer et al., 2001).
The univariate generalized linear model (GLM) was used to examine the relationships between chlorophyll-a concentrations of phytoplankton, epipelon and epiphyton and abiotic variables (temperature, TN, TP, conductivity, pH, suspended particulate matter, water-column depth) and two biotic variables (macrophyte coverage, zooplankton total density). The variables most associated with variation in biomass of the algal communities were identified using GLM (MINITAB Release 14.12.0).
Results
Environmental variables
Limnological conditions in the rainy season were characterized by the highest values for light at the subsurface and TN, TP and PO4-P concentrations (Table 1). The dry season was characterized by the highest value for light at the bottom, Secchi:Zmax ratio and DIN concentration. The depth of the mixing zone (Zmix) of the pelagic zone varied from 0.5 to 1.5 m in the rainy season and from 1.5 to 3.5 in the dry season. PCA axis 1 of abiotic variables of the pelagic, deep littoral and shallow littoral zones explained 71%, 80% and 87.2% of the total variability of the data, respectively (Fig. 2A–C). Monte Carlo randomization revealed that the ordering of axis 1 was significant (P = 0.01). The first ordination axis of the PCAs of the three depth zones revealed that most months of the rainy season were associated with high TN, TP and free CO2 concentrations and temperature (Pearson: r > 0.5). In contrast, the dry season months and some of the late rainy season were correlated with high pH values (Pearson: r > 0.5). PCA axis 1 represented seasonal variation in limnological conditions in the three studied zones. Temporally, the highest values for light at the water subsurface were found in the rainy season in the pelagic and littoral zones (March and November) (Fig. 2). Light attenuation varied little in the pelagic zone throughout the year, which differed from that in the littoral zone, where the lowest attenuation values were in the dry season (deep zone: July, shallow zone: May, Fig. 3).
PCA of limnological variables at the pelagic (A), deep (B) and shallow littoral (C) zones over the year. Score abbreviations: letters represent months. Vectors: CO2 free CO2, Cond conductivity, DO oxygen dissolved, HCO3 bicarbonate, pH pH, SM suspended material, Temp temperature, TN total nitrogen, TP total phosphorous
Biological variables
The deep littoral and shallow littoral zones had higher macrophyte coverage (average 64%) in the rainy season (Fig. 4A, B). The range of variation in macrophyte coverage was similar between the deep and shallow littoral zones (12–81% and 31–86%, respectively), but average annual coverage was higher in the shallow zone. Total zooplankton density exhibited great temporal variation in the three zones of the reservoir (Fig. 5A–C). Higher zooplankton density was found in June (rainy season) in the pelagic zone and in January in the deep littoral and shallow littoral zones. Total density did not differ significantly among months and zones, but the interaction between the factors was significant (two-way ANOVA: P < 0.05). However, there was a significant difference between the pelagic and shallow littoral zone (Tukey: P < 0.009) and between the dry and rainy seasons (Tukey: P < 0.017). Rotifera were the dominant zooplankton group in the three zones during the study period (65–97%), with the exception of April, when Copepoda density increased (46–57%, Fig. 5D–F). Although the densities of the zooplankton groups did not differ significantly among zones and months, the interaction between the factors was significant (two-way PERMANOVA: F = 67.71; P = 0.0001).
In the pelagic zone, the highest phytoplankton chlorophyll-a was found in the summer (January to February), while for epipelon chlorophyll-a the highest was during the dry season (July) (Fig. 6A). In the driest and coldest month of the year, the lowest phytoplankton chlorophyll-a and the highest epipelon chlorophyll-a were found in the pelagic zone (Fig. 5A). This condition had no clear effect on the littoral zone. Phytoplankton and epipelon chlorophyll-a were higher during the rainy season in both littoral zones, while epiphyton chlorophyll-a was higher during the dry season (April) in both zones (Fig. 6C).
The relative contribution of the phytoplankton, epipelon and epiphyton chlorophyll-a to total photosynthetic biomass per area (mg m2) varied seasonally (Fig. 7A, B). Significant differences were found in the phytoplankton and epipelon chlorophyll-a between the dry and rainy seasons and between the pelagic and littoral zones, while epiphyton chlorophyll-a differed only difference between seasons (two-way RM-ANOVA: P < 0.05). The highest relative contribution of phytoplankton chlorophyll-a for total photosynthetic biomass in the reservoir occurred in the rainy season (55% to 67%). In contrast, epipelon chlorophyll-a had its greatest contribution to the total photosynthetic biomass in the dry season (55% to 93%). The highest relative contribution of epiphyton chlorophyll-a was detected in dry season, but this contribution was always lower than the other communities.
Significant relationships between the biomass of phytoplankton, epipelon and epiphyton and predictor variables are shown in Table 2. The variables TP, TN and particulate matter were predictors for phytoplankton chlorophyll-a in the pelagic zone, while water transparency was the only predictor for epipelon chlorophyll-a. Phytoplankton chlorophyll-a was significantly correlated to macrophyte coverage, TP and TN concentrations in the deep littoral and shallow littoral zones. Epipelon chlorophyll-a was better explained by macrophyte coverage and epiphyton chlorophyll-a was better explained by macrophyte coverage and conductivity in the littoral zones.
Considering groups based on growth forms, flagellated algae were dominant (57 to 94%) in phytoplankton in the three zones throughout the study period, with the exception of June when non-motile unicellular algae dominated (Fig. 8A). For epipelon, flagellated algae were dominant in pelagic zone in both seasons. Colonial algae were predominant during the dry season in deep littoral and shallow littoral, while the filamentous algae increased during the rainy season in both zones (Fig. 8B). For epiphyton, non-motile unicellular algae were predominant during the rainy season at the deep littoral and shallow littoral zones. Although non-motile unicellular algae were abundant during the dry season, there was an increase in motile unicellular forms, mainly at the beginning of the season (Fig. 8C). The descriptor species in the phytoplankton and periphyton (epipelon and epiphyton) and their respective growth forms are shown in Table 3.
Discussion
Our results showed the spatial and temporal variation in algal biomass for periphyton (epipelon, epiphyton) and plankton, but no covariation. The highest phytoplankton biomass, for all three zones of the reservoir, was found in the rainy season, while that for epipelon and epiphyton occurred in the dry season. In addition to the high macrophyte coverage, the environmental condition in the rainy season were characterized by high N and P availability in the whole reservoir, while the opposite was found in the dry season. Thus, two limnological phases were found throughout the course of the year: a phase with phytoplankton dominance in the rainy season and a phase with epipelon dominance in the dry season. Water transparency, particulate matter, TP and TN were predictors of phytoplankton biomass in the reservoir, as observed in previous studies (e.g., Fonseca & Bicudo, 2011). In the littoral zone, our findings showed a significant relationship between macrophyte coverage and epiphyton biomass, which, according to previous studies, is due to the dominance of Nymphaea spp. which can significantly shade the community (e.g., Pellegrini & Ferragut, 2018). For epipelon, water transparency was a predictor for algal biomass in the pelagic zone, while macrophyte coverage was the important predictor in the littoral zone. Vadeboncoeur et al. (2014) considered light availability to be the main factor structuring epipelon in temperate lakes of all sizes. Other studies of temperate lakes found the greatest contribution of epipelon during conditions of low nutrient concentration, high light penetration and low abundance of macrophytes (Libouriussen & Jeppensen, 2003; Cano et al., 2008; Genkai-Kato et al., 2012), as presently evidenced. Although different predictors were found for phytoplankton, epipelon and epiphyton biomass, the macrophyte coverage was a common predictor of all three communities in the littoral zones. In the studied reservoir, the dominant macrophyte Nymphaea spp. has large and broad leaves, which promotes strong shading, especially during the rainy season when cover can reach almost 100%. Previous studies have reported that macrophyte communities can influence phytoplankton (Fonseca & Bicudo, 2011) and epiphyton structure (Pellegrini & Ferragut, 2018). Therefore, water transparency and macrophyte coverage assume an important role in temporal changes of algal biomass in different habitats and should influence the change in phytoplankton and epipelon dominance.
Considering the classical theory of alternate stable states for shallow lakes, a gradual reduction in macrophyte abundance may be associated with an increase in phytoplanktonic biomass (Scheffer et al., 1993). Our findings showed the greatest phytoplankton biomass in the period with the greatest macrophyte coverage, that is, there was a positive relationship between the two communities, as also observed in previous studies (Casartelli & Ferragut, 2015). In general, alternate stable state is evaluated in lakes where there is a predominance of submerged macrophytes (e.g., Libouriussen & Jeppessen, 2003; Kosten et al., 2009; Hobbs et al., 2012). In tropical ecosystems, the role of free floating macrophytes in determining alternate stable states has been demonstrated in wetland lakes (O’Farrell et al., 2011; Tezanos-Pinto & O’Farrell, 2014) and a eutrophic reservoir (Bicudo et al., 2007). However, Nymphaea, which is a rooted plant with large floating leaves, is the dominant macrophyte in the present reservoir. In addition, periods of lower macrophyte coverage and phytoplankton biomass may have favored the further development of epipelon and epiphyton, which should play a role in determining a steady state in shallow lakes. According to a review study, the macrophyte community composition seems to influence the dynamics of shallow lakes and, consequently, the associated algal communities (Hilt, 2015).
The high relative density of flagellated algae in the phytoplankton, epipelon and epiphyte structure shows that motility is an important survival strategy in the studied reservoir, particularly by the high Nymphaea cover, which can promote strong shading. The presence of flagellum confers motility to the algae, making it possible to search for better conditions in the water column, especially dissolved nutrients (Sommer, 1988). The presence of the flagellum is an important feature for algae in the sediment (Poulíčková et al., 2014). In phytoplankton, the flagellates Peridinium umbonatum F. Stein, P. gatunense Nygaard, Pseudokephyrion hypermaculatum Ettl and Chromulina sphaerica Bachmann were the descriptor species of higher biomass in the rainy season and, Mallomonas spp. and P. umbonatum during the dry season. These species of Chrysophyceae and Dinophyceae are mixotrophic (Jansson et al., 1996; Olrik, 1998), which is a competitive advantage for resources. In addition, unicellular species with motility increased the contribution to structure, especially the large raphid diatoms (Pinnularia viridis (Nitzsch) Ehrenberg, P. divergens W. Smith and Stauroneis phoenicenteron (Nitzsch) Ehrenberg) in the epipelon in the three zones in both seasons. The presence of motile epipelic algae may be associated with physical characteristics of the sediment (Jones et al., 2014), as well as the amount of mucilage excreted by the algae (extracellular polymeric substance, EPS (Smith and Underwood, 2000). Although Cosmarium contractum O. Kirchner and C. margaritatum (P. Lundell) J. Roy & Bisset were very representative in the epiphyton structure, there was high contribution of flagellate and unicellular species with motility. Thus, environmental conditions favored the participation of flagellate species in algal communities of different habitats.
Our results indicated weak relationship between total zooplankton density variation and algal communities. Thus, zooplankton appears to exert weak grazing pressure on algal communities in the pelagic and littoral zones, particularly in the phytoplankton dominance phase (rainy season). However, the decline of phytoplankton coincided with increased density of Copepoda and Cladocera species mainly in the pelagic zone, suggesting a potential influence by the grazers. Rotifers were the dominant zooplankton group in most of the months in the three zones of the reservoir, except in April when Cladocera and Copepoda increased. According to Santos et al. (2018), Polyarthra vulgaris Carlin, 1943 is an important species for the Rotifera structure in the Ninfeias Reservoir. Although abundant in pelagic waters without vegetation, this species is a raptorial rotifer commonly plant-associated and has morphological characteristics and food habit that confers little or no impact on the phytoplankton (Iglesias et al., 2007). In relation to the ichthyofauna, low fish abundance was a characteristic, and the predominant species is detritivorous feeding habits [Geophagus brasiliensis (Quoy & Gaimard, 1824)] and the second most abundant species is predator with a diet of fish [Hoplias malabaricus (Bloch, 1794)] (Castro et al., 2018). Therefore, our results suggest that the top-down effect should not be significant in the studied reservoir, and that herbivory was not a determining factor in the dynamics of algal communities.
Considering the existence of a tenuous threshold between algal communities in aquatic ecosystems (Margalef, 1983), phytoplankton sedimentation can occur and consequently overestimate the epipelic algal biomass (Yang et al., 2009), which may have a close relationship with other communities (Cano et al., 2016). On the other hand, physical disturbances (e.g., precipitation, wind, water-column mix) may cause detachment of epiphytic or epipelic algae (Goldsborough & Robinson, 1996), which may overestimate phytoplankton biomass. Therefore, a structural and biomass overlap between algal communities must certainly exist and should not be ignored, but joint community assessment is necessary. According to Liboriussen and Jeppesen (2006), the integrated assessment of key primary producers in lentic waters allows for a more holistic view of ecosystem functioning, which can significantly improve our understanding of lake dynamics.
In short, we evidenced two phases of algal dominance: phytoplankton and epipelon phases. Our findings showed that phytoplankton dominance phase was characterized by high macrophyte coverage, which contrasts with that expected by the alternate stable state for shallow lakes (Scheffer et al., 1993). Thus, clear phase was characterized by the high abundance of rooted macrophytes, Nymphaea, and phytoplankton biomass and the turbid phase by the greater epipelon and epiphyton biomass. Changes in biomass and species composition of the algal communities were associated with the macrophyte cover variation, mainly the rooted macrophyte Nymphaea. This environmental scenario favored a high abundance of algae species with locomotion structure in phytoplankton, epipelon and epiphyton, as well as weak grazing pressure by zooplankton on algae communities. We conclude that, in addition to the abiotic factors (light and nutrients), the macrophyte coverage was a determining factor for algal community changes, contributing to the alternation of phytoplankton and epipelon dominance phases. Finally, the relationship between macrophyte–phytoplankton–periphyton needs to be better investigated in shallow reservoirs, especially the role of epipelon on alternate stable states.
References
APHA, 2005. Standard Methods for Examination of Water and Wastewater. American Public Health Association WWA, Washington, DC.
Beisner, B. E., D. T. Haydon & K. Cuddington, 2003. Alternative stable states in ecology. Frontiers in Ecology and the Environment 1: 376–382.
Bicudo, D. C., B. M. Fonseca, L. M. Bini, L. O. Crossetti, C. E. M. Bicudo & T. Araújo-Jesus, 2007. Undesirable side-effects of water hyacinth control in a shallow tropical reservoir. Freshwater Biology 52: 1120–1133.
Cano, M. G., M. A. Casco, L. C. Solari, M. E. MacDonagh, N. A. Gabellone & M. C. Claps, 2008. Implications of rapid changes in chlorophyll-a of plankton, epipelon, and epiphyton in a Pampean shallow lake: an interpretation in terms of a conceptual model. Hydrobiologia 614: 33–45.
Casartelli, M. R. & C. Ferragut, 2015. Influence of seasonality and rooted aquatic macrophyte on periphytic algal community on artificial substratum in a shallow tropical reservoir. International Review of Hydrobiology 100: 1–11.
Casco, M. A., M. E. Mac Donagh, M. G. Cano, L. Solari, M. C. Claps & N. Gabellone, 2009. Phytoplankton and epipelon responses to clear and turbid phases in a seepage lake (Buenos Aires, Argentina). International Review of Hydrobiology 94: 153–168.
Castro, R. J. D., R. Henry, C. Ferragut & M. Casartelli, 2018. Comparing lacustrine environments: the importance of the kind of habitat on the structure of fishes. Acta Limnologica Brasiliensia. https://doi.org/10.1590/s2179-975x13417.
Eaton, J. W. & B. Moss, 1966. The estimations of numbers and pigment content in epipelic algal populations. Limnology and Oceanography 11: 584–595.
Fernando, C. H., 2002. Guide to tropical freshwater zooplankton: identification, ecology and impact on fisheries. In Guide to Tropical Freshwater Zooplankton: Identification, Ecology and Impact on Fisheries. Backhuys, Leiden.
Fonseca, B. M., C. Ferragut, A. Tucci, L. O. Crossetti, F. Ferrari, D. C. Bicudo, C. L. Sant’Anna & C. E. M. Bicudo, 2014. Biovolume de cianobactérias e algas de reservatórios tropicais do Brasil com diferentes estados tróficos. Hoehnea 41(1): 9–30.
Genkai-Kato, M., Y. Vadeboncoeur, L. Liboriussen & E. Jeppesen, 2012. Benthic–planktonic coupling, regime shifts, and whole-lake primary production in shallow lakes. Ecology 93: 619–631.
Goldsborough, L. G. & G. G. C. Robinson, 1996. Pattern in wetlands. In Stevenson, R. J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology: Freshwater Benthic Ecosystems. Academic, San Diego: 77–117.
Hammer, O., D. A. T. Harper & P. D. Ryan, 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 1–9.
Hillebrand, H., C. D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Hilt, S., 2015. Regime shifts between macrophytes and phytoplankton – concepts beyond shallow lakes, unravelling stabilizing mechanisms and practical consequences. Limnética 34: 467–480.
Hobbs, W. O., J. M. Ramstack Hobbs, T. Lafrançois, K. D. Zimmer, K. M. Theissen, M. B. Edlund, N. Michelutti, M. G. Butler, M. A. Hanson & T. J. Carlson, 2012. A 200-year perspective on alternative stable state theory and lake management from a biomanipulated shallow lake. Ecology Applications 22: 1483–1496.
Iglesias, C., G. Goyenola, N. Mazzeo, M. Meerhoff, E. Rodo & E. Jeppesen, 2007. Horizontal dynamics of zooplankton in subtropical Lake Bianca (Uruguay) hosting multiple zooplankton predators and aquatic plant refuges. Hydrobiologia 584: 179–189.
Jansson, M., P. Blomqvist, A. Jonsson & A. K. Bergström, 1996. Nutrient limitation of bacterioplankton, autotrophic and mixotrophic phytoplankton, and heterotrophic nanoflagellates in Lake Örträsket. Limnology and Oceanography 41: 1552–1559.
Kosten, S. G., E. Jeppesen, D. Motta Marques, E. H. van Nes, N. Mazzeo & M. Scheffer, 2009. Effects of submerged vegetation on water clarity across climates. Ecosystems 12: 1117–1129.
Legendre, P. & L. Legendre, 2012. Numerical Ecology. Elsevier Science Publication, London.
Liboriussen, L. & E. Jeppesen, 2003. Temporal dynamics in epipelic, pelagic and epiphytic algal production in a clear and a turbid shallow lake. Freshwater Biology 48: 418–431.
Margalef, R., 1983. La imprecisa frontera entre el plâncton y otros tipos de comunidades. In Azevedo, M. T. P., Santos, D. P., Sormus, L., Menezes, M., Fujii, M. T., Yokoya, N. S., Senna P. A. C. & Guimarães, S. M. P. B. (eds), Anais do 4º Congresso Latino-Americano, 2ª Reunião Ibero-Americana, 7ª Reunião Brasileira de Ficologia, Caxambu: 319–326.
McCune, B. & M. J. Mefford, 2011. PC-ORD. Multivariate analysis of ecological data.
O’Farrell, I., I. Izaguirre, G. Chaparro, F. Unrein, R. Sinistro, H. Pizarro, P. Rodriguez, P. T. Pinto, R. Lombardo & G. Tell, 2011. Water level as the main driver of the alternation between a free-floating plant and a phytoplankton dominated state: a long-term study in a floodplain lake. Aquatic Sciences 73: 275–287.
Olrik, K., 1998. Ecology of mixotrophic flagellates with special reference to Chrysophyceae in Danish lakes. In Phytoplankton and Trophic Gradients. Springer, Dordrecht: 329–338.
Olsen, S., F. Chan, W. Li, S. Zhao, M. Søndergaard & E. Jeppesen, 2015. Strong impact of nitrogen loading on submerged macrophytes and algae: a long-term mesocosm experiment in a shallow Chinese lake. Freshwater Biology 60: 1525–1536.
Padial, A. A. & S. M. Thomaz, 2008. Prediction of the light attenuation coefficient through the Secchi disk depth: empirical modeling in two large Neotropical ecosystems. Limnology 9: 143–151.
Passarge, J., S. Hol, M. Escher & J. Huisman, 2006. Competition for nutrients and light: stable coexistence, alternative stable states, or competitive exclusion? Ecological Monographs 76: 57–72.
Pellegrini, B. G. & C. Ferragut, 2018. Association between epiphyton species composition and macrophyte diversity in a shallow tropical reservoir. Fundamental and Applied Limnology 191: 111–122.
Poulíčková, A., P. Dvořák, P. Mazalová & P. Hašler, 2014. Epipelic microphototrophs: an overlooked assemblage in lake ecosystems. Freshwater Science 33: 513–523.
Robinson, G. G., S. E. Gurney & L. G. Goldsborough, 1997. Response of benthic and planktonic algal biomass to experimental water-level manipulation in a prairie lakeshore wetland. Wetlands 17: 167–181.
Sand-Jensen, K. & J. Borum, 1991. Interactions among phytoplankton periphyton and macrophytes in temperate freshwaters and estuaries. Aquatic Botany 41: 137–175.
Santos, S. A. M., T. R. Santos, M. S. Furtado, R. Henry & C. Ferragut, 2018. Periphyton nutrient content, biomass and algal community on artificial substrate: response to experimental nutrient enrichment and the effect of its interruption in a tropical reservoir. Limnology 19: 209–218.
Sartory, D. P. & J. U. Grobbelaar, 1984. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114: 177–187.
Sayer, C. D., T. A. Davidson & J. I. Jones, 2010. Seasonal dynamics of macrophytes and phytoplankton in shallow lakes: a eutrophication-driven pathway from plants to plankton? Freshwater Biology 55: 500–513.
Scheffer, M. & E. H. van Nes, 2007. Shallow lakes theory revisited: various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 584: 455–466.
Scheffer, M., S. H. Hosper, M. L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology and Evolution 8: 275–279.
Schindler, D. E. & M. D. Scheuerell, 2002. Habitat coupling in lake ecosystems. Oikos 98: 177–189.
Sommer, U., 1988. Some size relationships in phytoflagellate motility. Hydrobiologia 161: 125–131.
Souza, M. L., B. G. Pellegrini & C. Ferragut, 2015. Periphytic algal community structure in relation to seasonal variation and macrophyte richness in a shallow tropical reservoir. Hydrobiologia 755: 183–196.
Tezanos-Pinto, P. & I. O’Farrell, 2014. Regime shifts between free-floating plants and phytoplankton: a review. Hydrobiologia 740: 13–24.
Thomas, S., P. Cecchi, D. Corbin & J. Lemoalle, 2000. The different primary producers in a small African tropical reservoir during a drought: temporal changes and interactions. Freshwater Biology 45: 43–56.
Thomaz, S. M., L. M. Bini & T. A. Pagioro, 2004. Métodos em Limnologia: macrófitas aquáticas. In Bicudo, C. E. & D. C. Bicudo (eds), Amostragem em Limnologia. Editora Rima, São Carlos: 193–212.
Vadeboncoeur, Y. & M. E. Power, 2017. Attached algae: the cryptic base of inverted trophic pyramids in freshwaters. Annual Review of Ecology, Evolution, and Systematics 48: 255–279.
Vadeboncoeur, Y. & A. D. Steinman, 2002. Periphyton function in lake ecosystems. The World Journal 2: 1–20.
Vadeboncoeur, Y., D. M. Lodge & S. R. Carpenter, 2001. Whole-lake fertilization effects on distribution of primary production between benthic and pelagic habitats. Ecology 82: 1065–1077.
Yang, H., R. J. Flower & R. W. Battarbee, 2009. Influence of environmental and spatial variables on the distribution of surface sediment diatoms in an upland loch, Scotland. Acta Botanica Croatica 68: 367–380.
Acknowledgements
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for doctoral grants for TRS (Grant No. 2013/03130-2) and financial support (Grant No. 2009/52253-4). The authors are very grateful to the students and technicians involved in the laboratory work and in the field.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Alex Elliott
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos, T.R., Castilho, M.C., Henry, R. et al. Relationship between epipelon, epiphyton and phytoplankton in two limnological phases in a shallow tropical reservoir with high Nymphaea coverage. Hydrobiologia 847, 1121–1137 (2020). https://doi.org/10.1007/s10750-019-04172-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04172-2