Abstract
The extensive use of plastics has led to the widespread presence of a new type of pollutant called “microplastics (MPs)” in aquatic environments. MPs have large specific surface areas and strong hydrophobicity. In particular, MPs provide a new ecological niche for microorganisms in aquatic environments, which attach to and subsequently form biofilms on microplastic (MP) surfaces. This paper reviews the factors affecting biofilm growth on MP surfaces and the effect of biofilms on the adsorption of other environmental pollutants onto MPs as well as difference analysis. Biofilm formation is influenced by many factors related to the environment, MPs (e.g., type, particle size, and additives), and properties of microorganisms; environmental factors play an especially important role. Crucially, biofilms change the density of MPs and hydrophobicity of the surface of MPs and can attach new functional groups, charged sites, and other additives to MP surfaces. Primarily owing to this, biofilms affect the adsorption of environmental pollutants such as heavy metals, POPs, and pathogenic microorganisms. Notably, such adsorption is affected by MP particle size and additives. In particular, biofilms have a considerable effect on the interactions between MPs and pollutants. Further, this article suggests directions for revealing the influence of biofilms on pollutant adsorption to MPs. This review provides a reference for studying the formation of biofilms on MPs surfaces in aquatic environments and the effect of biofilms on contaminant adsorption onto MPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microplastics (MPs) are plastics smaller than 5 mm in size [1]. The term “MPs” was introduced in 2004 by Thompson et al. in an article published at Plymouth University, UK [2]. MPs can be classified into two categories with different formation processes. Primary MPs are directly released into the environment at microscopic sizes [3], whereas secondary MPs are derived from the physical, chemical, and biological breakdown of large pieces of plastics in the ocean and on land. Breakdown processes destroy the integrity of plastic items and fragment them into plastic debris [3]. Since their invention, plastics have been extensively produced and utilized due to their excellent properties [4]. The global production of plastics is expected to increase to 390.7 million tons by 2021 [5]. By 2030, approximately 90 million tons of plastic waste is expected to enter the aquatic environment annually [6], resulting in a large amount of environmental MPs. The widespread and persistent presence of MPs compromises the health of plants, animals, and humans [7, 8, 120].
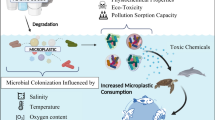
In addition to affecting animals and plants, MPs are carriers of other pollutants in the environment [8]. Because they are strongly hydrophobic, they interact with organic pollutants primarily through sorption–desorption behaviors [9]. As MPs migrate in the environment, they collect pollutants such as heavy metals, POPs (Persistent Organic Pollutants), and pathogenic microorganisms, which migrate with the MPs [10]. The large surface area of MPs facilitates the adhesion of various microorganisms such as bacteria, fungi, and algae [11] (Table 1). These microorganisms quickly colonize the surfaces of MPs in aquatic environments, forming biofilms [12]. Microbial colonization and biofilm formation are promoted by the hydrophobic characteristics of MPs [13]. Biofilm formation is a dynamic process that alters the surface roughness, density, and functional groups of MPs [14]. Driven by physical forces (e.g., Van der Waals forces), microorganisms move to the surface of MPs [31, 32]. Subsequently, they adhere to the surface of MPs by secreting substances such as extracellular polymeric substances (EPS), and through continuous proliferation and development, they form biofilms attached to the surface of MPs [32, 33]. The functional groups in biofilms influence the adsorption and release of contaminants to/from MPs, thereby changing the role of MPs as contaminant carriers [15].
Biofilm formation enhances the sorption of harmful pollutants on MPs [16]. For instance, Wang et al. [78] demonstrated higher adsorption rates of metal ions on biofilm-covered polystyrene (PS) than on bare PS. He et al. [16] cultured biofilms on the surfaces of polyvinyl chloride (PVC), polyamide (PA), and high-density polyethylene (HDPE) MPs and investigated their adsorption capacities to norfloxacin (NOR). The biofilms on the three MPs improved NOR adsorption by varying degrees. However, biofilms do not always enhance the sorption of contaminants into MPs. In field-exposure experiments, Zhang et al. [17] found that biofilms on three MPs unequally contributed to the sorption of nine emerging contaminants. In fact, the sorption of most compounds was inhibited by the biofilm. Therefore, the role of biofilms in the sorption of contaminants into MPs is not absolute. Zhang et al. [17] suggested that although biofilm influences the adsorption of contaminants by MPs, the amount of material adsorbed depends on the nature of the contaminant itself. Although MPs have been extensively researched, the effects of microplastic surfaces on biofilm production and the effects of biofilms on contaminant adsorption to microplastic (MP) surfaces have not been comprehensively reviewed. This paper aims to address the following three aspects: (1) the underlying mechanisms and influencing factors of biofilm production on microplastic surfaces; (2) the influence of biofilms on pollutant adsorption to MPs; and (3) difference analysis and future direction suggestions.
Data source
This review is based on the literature selected from the Elsevier and Web of Science databases. We separately and precisely searched for relevant phrases such as MPs, biofilm, and pollutant adsorption. Under the search topic “MPs,” 12,643 and 18,334 articles were retrieved by Web of Science and Elsevier, respectively, as of June 2023. After adding the search topic “biofilm,” the literature volume decreased to 440 and 3025 articles from Web of Science and Elsevier, respectively. Finally, after adding the search keyword “pollutant adsorption,” 30 and 1359 articles were retrieved from Web of Science and Elsevier, respectively. The filtered literature was related to the growth of MPs and biofilms on the surfaces of MPs in the aquatic environment, along with pollutant adsorption. Among the search results, we selected 355 suitable articles for our records. After screening the abstracts and contents, 162 articles were selected for review. This review comprehensively summarizes the generation of biofilms on microplastic surfaces, identifies the factors influencing biofilm generation, and categorically outlines the effects of biofilms on pollutant adsorption onto MPs. During the writing process, we thoroughly read the selected articles and ultimately selected 45 articles as the data sources in Tables 1, 2 and 3.
Mechanism of biofilm formation on MP surfaces
MPs serve as a substrate for microbial colonization (here, “substrate” is a substance that can be colonized by microorganisms)
Biofilms are dynamic systems of multiple microorganisms commonly found in freshwater environments [18]. They comprise microorganisms and their associated extracellular products and can attach to both biological and nonbiological surfaces [19]. Although MPs are abiotic, they easily become colonized by biofilms after entering the water column [20].
In the aquatic environment, MPs are a unique habitat for microorganisms [21, 22]. MPs enter the water environment and provide a specific ecological niche for the colonization of microorganisms, which is conducive to the aggregation and attachment of various microorganisms [23, 54, 122, 124]. This new niche (sometimes called a “plastic sphere”) is a diverse microbial community including heterotrophs and autotrophs [23, 93, 123]. Moreover, MPs can transport microorganisms and provide carbon for microbial growth and reproduction, which (at least partially) explains why biofilms readily develop on microplastic surfaces [19, 121] (Fig. 1). Bradney et al. [24] concluded that polymers secreted by MPs release organic carbon into the environment, enhancing the activity of biofilm microorganisms. Biofilm formation strongly depends on the hydrophobicity, structure, and roughness of the substrate [25]. Rummel et al. [14] suggested that microorganisms attach more rapidly to MPs and other hydrophobic materials than to hydrophilic materials. Ke and Wigglesworth‐Cooksey [26] also concluded that hydrophobic surfaces are more easily colonized by microbes than hydrophilic surfaces.
Process of biofilm formation
Biofilm formation on MP surfaces is a dynamic process. The high hydrophobicity of the surface of MPs and their large specific surface areas provide good conditions for the attachment of microorganisms. Biofilm formation generally involves a succession of microbial adhesion, extracellular polymer secretion, and microbial proliferation [11]. One study [30] concluded that the entire process of biofilm formation on MP surfaces can be divided into (1) adhesion of microorganisms, (2) proliferation of microorganisms, and (3) partial microbial shedding. The specific formation process is described below and shown in Fig. 2.
-
Adhesion can be reversible or irreversible. Reversibly adhered microbial cells move or are transported to the MP surface through physical forces such as Brownian motion and van der Waals forces [31]. During the reversible adhesion phase, the cells sense and adsorb on the surface through various extracellular organelles and proteins [32]. Irreversibly adhered cells secrete EPS (e.g., DNA, proteins, lipids, and lipopolysaccharides) and extend organelles such as flagella that allow the cells to penetrate the energy barrier. The microorganisms then bind tightly to the surface, facilitating cell cohesion [32, 33].
-
During the microbial proliferation stage, the adsorbed microorganisms begin replicating and growing. Over time, the microorganisms establish a community and eventually evolve into a biofilm. The cells are protected from the external environment by secreted extracellular polymers [30].
-
The shedding process follows biofilm formation. During this stage, certain biofilm cells regain a transient state of motility and detach from the biofilm [34]. The shed cells can reattach to other surfaces, forming new niches in the environment. This step facilitates cell proliferation and self-protection [32].
As exposure continues, increasing numbers of microorganisms will attach, colonize, and accumulate on the MP surface [11]. Biofilm formation is rapid and changes the properties and future fate of MPs [35]. The unique structure of a biofilm affects the physical and chemical properties of MPs [30]. Biofilms tend to change the microscopic morphology of the colonized MPs, decreasing its hydrophobicity of the surface of MPs and increasing its density [11, 36]. Living biofilms can regulate the interaction between MPs and their surroundings [37]. They can also change the chemical properties and capability of pollutant adsorption on the MPs [30, 38].
Factors affecting microbial colonization on MP surfaces
Microbial colonization of MP surfaces in aquatic environments is a very complex process. The composition and richness of the microbial community change with time in the environment [39]. For example, Li et al. [40] found that the MPs exposed to the natural environment for 2 weeks contained mainly Bacteroides and Pseudomonas. However, after 4 weeks, the abundance of Vibrio bacteria was increased, and after 6 weeks, the number of various autotrophic bacteria was also increased. Using a 44-week MP incubation experiment, De Tender et al. [41] found that the characteristics of the fungal community varied greatly and no core group of fungal organisms was identified, indicating that the fungal community changed over time. In addition to time, the microbial colonization process also involves many other influencing factors, including environmental conditions, MP factors, and microorganisms properties [42]. This subsection discusses the influencing factors in two parts: 1) environmental factors and 2) MPs and microorganism factors.
Environmental factors
Biofilm formation is affected by water environmental conditions. Factors such as geographic location, nutrient variations in the water column, salinity, and pH of the water column, water flow rate, and seasonal variations can affect microbial colonization. Xu et al. [43] found that the microbial-species richness on MP surfaces differs between the Yellow Sea and the South China Sea. Specifically, the number of operational taxonomic units was lower in the South China Sea samples than in the Yellow Sea samples. The predominant groups in the Yellow Sea samples were Glaciecola (0.41%–31.41%), Colvaria (0.93%–30.67%), Moraxellaceae (0.04%–24.32%), Erythrobacteraceae (0.28%–36.08%), and Rhodobacteraceae (1.92%–28.05%). However, Pseudoalteromonas (0.04%–24.32%) and Bizionia (0.11%–43.90%) were the dominant microorganisms in the South China Sea samples. Nutrients such as carbon, nitrogen, and phosphorus also affect biofilm development because they are required for biofilm maturation. Li et al. [40] found that nitrogen, phosphorous, and salinity mainly affect the average growth rate of biofilms. Nitrogen and phosphorus were positively correlated with the average biofilm growth rate, whereas salinity was negatively correlated. They observed a 41% decrease in the surface biomass of MPs from the upper part of the Haihe estuary (salinity: 11.12%) to sites close to the Bohai estuary (salinity: 30.02%); the average biofilm growth rate in the Haihe estuary (total nitrogen (TN) = 3.21 mg/L; total phosphorus (TP) = 0.30 mg/L) was 1.76 that in the Bohai estuary (TN = 0.31 mg/L; TP = 0.06 mg/L). These values showed that biofilm growth was affected by both freshwater and seawater. The pH value changes also affect bacterial growth. Bacterial cells adapt to external pH changes through the proton motility force, but drastic pH changes can destroy this mechanism and induce cell death [44]. Meanwhile, hydrodynamic conditions affect microbial colonization. Biofilm structures differ in different fluid states [44]. In laminar flows, the biofilm is patchy and comprises round cells; in turbulent flows, it comprises wavy and elongated cells [45]. The flow rate affects the density of the biofilm coverage on MP surfaces [46]. The large force at higher shear rates reduces the strength of bacterial attachment [31]. Biofilm formation also responds to seasonal changes. Chen et al. [47] studied the state of biofilm coverage on PP in four seasons and found that biofilm coverage was dense and dark green in summer. In winter, the coverage was less dense and brown. Oberbeckmann et al. [48] conducted exposure experiments on polyethylene terephthalate (PET). They found that PET harbors more diverse microbial communities in summer than in winter. The Shannon index (microbial-community diversity index) for the PET biofilm was the highest in summer (2.38 ± 0.34) and the lowest in winter (1.79 ± 0.43). One plausible explanation is the higher temperatures in summer than in other seasons, which increase the reaction rates of microbial enzymes and hasten the metabolic development of cells [44, 47].
MPs and microbial factors
The colonization of MP surfaces by microorganisms profoundly depends on the nature of the MPs and the unique structural characteristics of the colonizing microorganisms [49, 50]. First, the type of MPs affects the abundance of the biofilm community. Hossain et al. [51] found that bacteria in freshwater environments colonize different MPs differently. For instance, bacterial abundance is highest on low-density polyethylene (LDPE) and lowest on polypropylene (PP) [51]. Meanwhile, microbes are tightly bound to PP surfaces and dispersed on polyethylene (PE) surfaces [52]. However, some researchers have found that the MP type is not a major affecter of biofilm formation. For example, Dudek et al. [53] found that the formation of bacterial community in biofilms on MPs is more strongly related to the time of exposure to the environment than to the MP type. Visualization of bacterial rRNA gene sequences via Principal co-ordinates analysis (PCoA) revealed that the prokaryotes deviated from the community with time in the environment, rather than because of the type of polymer. In addition, Deng et al. [54] performed exposure experiments and found that the number of operational taxonomic units (OTUs) of PS was not noticeably different from that of PE and polylactic acid (PLA) at the same exposure time. Therefore, we speculate that the type of MPs is not the main factor influencing biofilm formation.
In general, the different particle sizes of MPs may also affect the biofilm on the surface of MPs. Li et al. [55] performed high-throughput sequencing of biofilms on PE surfaces with three particle sizes (10, 40 and 120 μm). After 28 days of experimentation, it was found that the Chao 1 index (the Chao1 index was used to represent community richness) of biofilms on the surface of microplastics with three particle sizes differed. Compared to 10 μm (Chao 1 index of about 2700), microplastic surface biofilms with a particle size of 120 μm (Chao 1 index of about 2500) have a lower community richness of biofilms. This reduction is attributed to the fact that the larger particle sizes of MPs cause more effective shading, resulting in a decrease in community abundance. Gong et al. [56] found that MPs with different particle sizes had surface biofilms with different microbial-community compositions. For example, the proportion of phylum cyanobacteria in the surface biofilm of MPs was 69.54% for MPs with a particle size of 0.065 μm and 52.18% for those with a particle size of 5 μm, while the proportions of Proteobacteria in these MPs were 15.13% and 23.11%, respectively. Yao et al. [57] suggested that larger MPs lead to a more incompact biofilm on the surface, which may be detrimental to the maintenance of biomass in the biofilm.
Additives are added to MPs to ensure their properties [58]. However, the presence of additives may affect the microbial growth on the surfaces of MPs. Additives can be better utilized by microorganisms to promote the microbial colonization of MP surfaces [59]. For example, plasticizers, an additive used with MPs, can be metabolized by microorganisms during biofilm production and may play an important role in microbial colonization [58, 60]—this result is consistent with the findings of Chen et al. [61]. Meanwhile, the addition of antioxidants and UV stabilizers may play an important role in altering the physicochemical properties of MPs during aging, which indirectly affects microbial colonization in biofilms [61].
Microbial colonization is also related to the properties of MPs [62]. In a monitoring study of biofilms on four different MPs [63], polyolefins yielded the highest total suspended solids and organic matter content owing to their low surface energy. Xie et al. [64] performed exposure experiments on nine MPs. They reported that the dominant bacteria on the surfaces of four MPs were associated with specific groups on the MP molecules [64]. For example, carbonyl-containing MPs are dominated by Erythrobacter, which uses carbonyl compounds as the sole carbon source [64]. Sooriyakumar et al. [65] concluded that surface roughness affects the type of microorganisms colonizing the plastic surface. Second, bacterial adhesion and growth may be related to the electrical charge carried on the MP surface [66]. Bacteria are negatively charged and adhere fastest to positively charged surfaces [66]. Because PE and PS are negatively charged, they are less favorable for bacterial adhesion than other MPs [30]. Gottenbos et al. [66] found that the bacteria that were cultured in their study adhered to positively charged poly (methacrylate) surfaces the fastest. The original PP was neutral [67]. Hossain et al. [51] performed an 8-week MP biofilm culture experiment and demonstrated that the bacterial richness in PP was low, which may be related to the neutral surface of PP.
Whether biofilms will form also depends on the properties of the microorganisms. The rate and extent of adhesion depend on the cell hydrophobicity and on cell surface structures such as flagella, mycorrhizal hairs, and EPS [68]. Strains without flagella are weakly adhered and their biofilm formation is slow [30]. Some bacteria co-aggregate in the aquatic environment. Such co-aggregation is an important physiological feature of bacteria in biofilms, as it inhibits the successful integration of noncoaggregating bacteria into the biofilm [69]. Some autotrophic microorganisms, such as cyanobacteria and phototrophic microorganisms, adapt by releasing organic substance that enhance their metabolic activity and thereby promote biofilm development [70]. In addition, communities in biofilms may compete for similar nutrients [70]. As described by Rendueles and Ghigo [71] and others, a particular strain that adheres, colonizes, and develops into a biofilm can inhibit similar behavior in other strains.
Other factors affecting microbial colonization and difference discussion
Biofilms can occur on various substrates but the composition of microbial communities may vary on different substrates. Wu et al. [72] performed incubation experiments to compare the biofilms grown on the surface of MPs with those on natural substrates (e.g., rocks and leaves) and showed that the biofilms on MPs have a unique microbial-community structure compared to those on rocks and leaves. Compared to the proportions of Chlorobi, Acidobacteria, Gemmatimonadetes, Actinobacteria, Planctomycetes, and Hydrogenidentes in rocks (2.48%, 0.2%, 0.33%, 0.23%, 0%, and 0%, respectively) and leaves (0.2%, 0%, 0%, 0.03%, 0%, and 0%, respectively), the proportions were higher in MPs (3.3%, 1.3%, 0.93%, 0.58%, 0.1%, and 0.1%, respectively). McCormic et al. [21] found that the community of the biofilms on MP surfaces in rivers differs from that of the biofilms in water columns or suspended organic matter, that there are clear differences in the taxonomic composition of these biofilms, and that pathogens and other groups are more abundant on MPs. Oberbeckmann et al. [48] compared the microbial communities of biofilms on different substrates and found a difference of at least 57% between those growing on PET and those on glass. A comparison of biofilms on plastic and other artificial substrates found that the surfaces of hydrophilic stainless steel and hydrophobic PVC had almost similar bacterial richness [73]. In conclusion, MPs, as a new artificial substrate, can be easily colonized by microorganisms to form biofilms on the surface and have a unique community structure different from that of the biofilms formed on the surface of other materials [14].
Biofilms occur on various natural and artificial substrates. Although biofilm formation differs on different substrates, the determinants of growth and development are similar on all substrates (Fig. 3). However, as pointed out in some studies, colonization by microorganisms depends less on the MP surface than on the nutrients required for biofilm development, the salinity and temperature of the environment, and other factors [37]. For example, Bellou et al. [74] found differences among deep-sea biofilm communities on different MP types at the same depth. This difference appears to widen when the exposure depths differ. In addition, the MP age influences biofilm formation and may be more important than MPs. For example, Hong et al. found no significant difference in the settlement of Hidradenia larvae on MPs of different types aged to a similar degree, also demonstrating that MPs with different degrees of aging exhibited a greater effect on the formation of bacterial community structures in biofilms [75]. The aging of MPs is related to the environment, emphasizing that environmental factors can more likely explain the formation of biofilms under different growth conditions than many other influencing factors.
In addition, we found that the results of studies on the influence of environmental factors on biofilm formation are not always consistent. For example, regarding the flow rate, Katsikogianni et al. [31] proposed that a high rate would reduce the number of bacteria on the plastic surface. In contrast, Lehtola et al. [76] found that the total bacterial count in the same PE tube increased to 0.8 L/min, which was on average 15 times higher than the total bacterial count detected at 0.2 L/min. This is because the increased flow provides more nutrients to the microorganisms in the tube, leading to increased nutrient consumption and a greater number of bacteria. Moreover, pH has a regulatory effect on the growth of biofilms [41]. However, Miao et al. [77] reported that the biofilm biomass on the PP was significantly correlated with the physicochemical properties of the sampling point, particularly the levels of TN, nitrate nitrogen (NO3− − N), ammonia nitrogen (NH4+ − N), TP, and suspended solids (SS) (r > 0.9). In contrast, pH exhibited negligible (r = 0.356) or no correlation with the biofilm biomass. Therefore, future studies of the factors affecting microbial colonization should focus on environmental factors.
MPs are a new type of pollutant. Microorganisms form a biofilm on the surface of MPs through a series of adhesion and reproduction processes. The environment, MPs (i.e., type, particle size, presence of additives, and surface groups on MPs), and microorganisms have varying degrees of influence on the surface biofilms of MPs. Importantly, environmental factors play a more important role in biofilm formation than MPs and microorganisms. Because of the complexity of natural environmental conditions, the influence of environmental conditions on biofilm formation is inconsistent and large owing to a variety of uncontrollable factors. Future research must be devoted to more in-depth studies analyzing the effects of environmental changes on biofilm formation.
Biofilms affect the properties of MPs
MPs in water environments have become new habitats for microbial life [78]. The biofilm generated by microorganisms colonising the surface of microplastics can change some of the physicochemical properties of MPs, including crystallinity, surface hydrophobicity, surface functional groups, etc. [27, 36].
Physical changes in MPs
Biofilm formation is influenced by the nature of MPs, but biofilms themselves can alter the properties of MPs. When microorganisms attach to PE, they roughen its surface compared to that of the original MPs [67]. As the involved biofilm accumulates, MPs undergo several changes: decrease in tensile strength [79] and reduction in surface hydrophobicity of the surface of MPs with concomitant increase in surface hydrophilicity [36]. McGivney et al. [79] experimentally found that the stiffness of PP was reduced by the involved biofilm to an average of 35 N/mm owing to bacterial exposure. Lobelle et al. [36] found that the drop depth of the PE with a biofilm attached increased from 25 to ~ 40 mm and that the plastic was initially very hydrophobic and remains at the air-sea interface, but begins to sink below the surface after the third week. For example, Kaiser et al. [85] found that PS to which biofilms are attached exhibited varying increases in sinking velocity. This was demonstrated by increases of 16% and 81% in the sinking rate of PS in estuarine and seawater conditions, respectively, after 6 weeks of biofilm incubation. The particle size and density of MPs are considered as the main controllers of the sinking rate [86]. Biofilm formation increases the size and density of plastic particles, causing the settling of MPs [35, 87]. Chen et al. [47] experimentally found that biofilm development is a possible major cause of the sinking of floating MPs during the warm summer months. The development of biofilms led to an increase in the density of MPs from 910 to ~ 1000 mg/cm3 in 30 days. Morét-Ferguson et al. [27] found that the presence of biofilms led to an increase in the density of MPs to 0.97–1.04 g/mL, a range of densities not normally found in virgin plastics. Through a 44-d microbial colonization experiment in three freshwater systems, Miao et al. [77] found that the density of biofilm-attached PET and PVC increased up to 1.81 and 1.62 g/cm3, respectively, and the sinking rate increased by 47.6% and 5.04%, respectively, compared to that of pristine PET (1.38 g/cm3) and PVC (1.4 g/cm3). The results suggest that biofilm attachment affects MP density and thus its sinking behavior. Rozman et al. [78] conducted a 12-week biofilm incubation experiment under controlled laboratory conditions and found that the average particle size of the biofilm-covered PE increased from 149 ± 75 to 165 ± 106 μm, and the density also increased by 8% compared with the original PE. However, this density was still lesser than that of water; therefore, most of the MPs biofilm continued to float on the water surface. These biofilm effects can alter the horizontal and vertical transport of MPs [28, 29, 87, 155]. This behavior of aggregated microorganisms on MP surfaces might explain why MPs are removed from the surface of water columns and are sometimes found in sediment [28, 88] (Fig. 4).
Chemical changes in MPs
In addition to altering the physical properties (i.e., density and surface roughness) biofilms change certain chemical properties of MPs. Changes and increases in the functional groups of MPs are closely linked to biofilm formation [54]. One study found that MPs with attached biofilms display more peaks in their Fourier transform infrared spectra than the original MPs, suggesting that biofilm formation introduces new functional groups [18]. When covered with surface biofilms, some MPs acquire nitrogen-containing and oxygen-containing functional groups, which play important roles in the adsorption of metal ions [89]. Functional group changes can also affect the adsorption of pollutants to MPs [90].
According to a study, bacteria readily colonize MP surfaces, but no microorganisms have been found to be present that can degrade MPs [36]. In contrast, it has been found that the catalytic activities of exogenous enzymes secreted by microorganisms on MPs weaken the carbon skeleton structure of the MP polymer, promoting cleavage and consequent degradation of the MPs [58, 59]. However, this degradation was mainly the biodegradation of single plastics. For example, it has been shown that a strain that uses PE as its sole carbon source forms a biofilm on the surface and reduces the PE weight by 8% after 30 d [83]. Experiments conducted by Santo et al. [82] showed that when Cu-induced laccase secreted by actinomycete Rhodococcus ruber was incubated with polyethylene, the average molecular weight and average molecular number of polyethylene decreased by 20% and 15%, respectively. In addition, Hadad et al. [84] found that after incubating a thermophilic bacterium, Brevibacillus borstelensis, with PE for 30 d, the weight and molecular weight of the PE were degraded by 11% and 30%, respectively. Meanwhile, signaling molecules (called community sensors) control many metabolic processes in microbial communities. Such signaling molecules are speculated to facilitate the formation of hydrocarbon-degrading communities that decompose and mineralize MPs [91] (Fig. 5). However, in some cases, microbial colonization enhances the stability of MPs and protects them from degradation; for example, such colonization protects the MPs from ultraviolet radiation at the surface of the aqueous environment [58]. Degradation of MPs is highly uncertain under complex environmental conditions in the real world. Degradation depends on the size of the compound (larger molecules are difficult to degrade), the concentration of the compound (degradation is difficult if the concentration is very low), or the cleavage site of the compound (degradation is difficult if the cleavage site cannot be easily accessed) [92]. Experiments conducted by Brandon et al. [158] on PE and PP under natural weathering conditions demonstrated that, following a period of three years, the surface of the microplastic exhibited only slight changes. Auta et al. [159] isolated eight bacterial strains from mangrove sediments in Peninsular Malaysia and investigated their ability to degrade PE, PET, PS, and PP. It was found that only two strains were able to grow predominantly under conditions where the four MPs were used as the sole source of carbon for the 40-day experiment. Moreover, Bacillus cereus caused only 1.6%, 6.6% and 7.4% mass loss for PE, PET and PS, respectively. Bacillus gottheilii caused only 6.2%, 3.0%, 5.8% and 3.6% loss in PE, PET, PS and PP, respectively. Therefore, the degradation of MPs may depend on key factors such as bioavailability and stability of the MP compounds.
The properties of MPs change with the formation of biofilms. According to our review it, the basic properties of MPs, such as surface roughness, density, and surface hydrophilicity, vary due to the presence of biofilms. Microorganisms in the biofilm affect the functional groups of MPs, and the MPs are degraded under certain conditions. However, the degradation phenomenon is affected by the size and concentration of MPs. The study of MP degradation may need more attention and thinking.
Biofilm affects contaminant adsorption by MPs
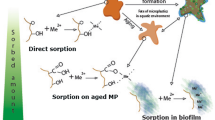
The environment is replete with pollutants such as heavy metals, POPs, and pathogenic microorganisms, which inevitably react with MPs. With their hydrophobicity of the surface of MPs and large specific surface area, MPs can adsorb and carry various types of pollutants [24, 90]. After entering the water environment, MPs provide a new ecological niche and are quickly colonized by microorganisms [12, 93]. Biofilm formation influences the performance and pollutant-adsorption capability of MPs [35] (Fig. 6). The physicochemical properties of MPs are altered by the presence of biofilms, which in turn affects the adsorption of pollutants by MPs. Below, we summarize the effects of biofilm on the adsorption of different pollutants onto MPs.
Heavy metals
Heavy metals are commonly used in industrial, domestic, agricultural, and medical applications. Accordingly, they have become widely distributed in the environment, raising concerns on their potential impacts [94]. High concentrations of heavy metals have been found on MPs [95] (Table 2). When MPs are ingested by aquatic organisms and transferred to higher nutrient levels, they are potentially hazardous [96].
In many cases, the ability of MPs to adsorb heavy metals depends on the functional groups on the polymer surface, π–π interactions, electrostatic interactions, and other chemical properties [97, 98]. According to Rochman et al. [99], the type of MPs exerts no significant effect on heavy-metal accumulation. They hypothesized that the adsorption of heavy metals on MPs is mediated by biofilms [99]. MPs in the natural environment can be colonized by several microorganisms to form biofilms, which can affect both the oxygenated groups and surface hydrophobicity of MPs [79, 81]. The changes in these properties of MPs affect the adsorption of heavy metals to MPs [18, 97].
Some studies have reported that biofilm formation facilitates the adsorption of heavy-metal ions on MPs. For instance, Johansen et al. [100] found that under estuarine conditions, the microorganisms in rapidly formed biofilms on MP surfaces reduced the amount of Al in the region from 21 to 13% [100]. Biofilm-induced changes in the functional groups of MPs can feasibly explain (at least partly) the enhanced sorption of heavy metals on MPs. For example, Guan et al. [18] experimentally found that biofilms can alter the kinetics of metal adsorption on MPs, enhancing the adsorption of metals. As a main cause of adsorption enhancement, they suggested that biofilms lead to complexation of functional groups such as carboxyl and amino groups in MPs [18]. Enhanced adsorption has also been attributed to increased numbers of adsorption sites. As heavy-metal ions are usually charged, they will be adsorbed at the positively and negatively charged sites in biofilms through attractive electrostatic interactions and ion-exchange mechanisms [98]. Wang et al. [89] found that the adsorption rate and capacity of metal-ion adsorption was highest on biofilm-coated MPs than on bare MPs, probably because biofilms accelerate the availability of surface-adsorption sites. The maximum adsorption capacity of biofilm-attached MPs reached 31.4048 µmol/g for Cu and 43.8846 µmol/g for Pb [89]. Microorganisms in the biofilms on MPs also affect the adsorption of some heavy metals during the growth process. For example, Cu can coexist with bacterial cells in biofilms and promotes the enrichment of Cu-metabolizing microorganisms, thus enhancing the adsorption of Cu on the MP surface [81].
However, we found that the biofilm effects on heavy-metal adsorption differ among heavy metals. In general, MPs frequently adsorb more Pb(II) than Cu(II). Similarly, Wang et al. [89] found that PS adsorbs more Pb(II) than Cu(II), whereas Zou et al. [101] found that Pb2+ most strongly adsorbed to their MP adsorbents, followed by Cu2+. Ashton et al. [102] analyzed the heavy metals in PE particles collected from the beach and found that the concentrations of Pb and Cu were 0.15 ± 0.04 and 0.06 ± 0.03 μg/g, respectively. In general, the adsorption capacities of biofilm-attached MPs for heavy-metal ions are pH-dependent. At lower pH, H + competes with cationic metal ions for the adsorption sites. However, one study reported that when the pH did not significantly change, the Cu content was higher on biofilm-covered MPs (3004.0 ± 260.0 ng per 20 pieces), than on the original MPs (2508.0 ± 28.0 ng per 20 pieces) [81].
MPs hosting biofilms can also adsorb certain amounts of radioactive elements. For example, Johansen et al. [100] found measurable amounts of the radioactive elements 137Cs and 90Sr on different types of plastic biofilms. This finding suggests that MPs act as sinks for 137Cs and 90Sr radionuclides, which are associated with nuclear activity. Ashton et al. [102] found uranium at concentrations below 5% in PE suspended in a harbor for eight weeks. However, to understand the adsorption properties of biofilms for radionuclides, the fate of radionuclides must be investigated in future studies.
POPs
POPs are persistent organic compounds that resist physical, chemical, and biological degradation and tend to accumulate in organisms, with adverse effects on their growth [103, 104]. Consequently, POPs are difficult to remove from the environment and can be detected in many animals. Notably, perfluoroalkyl and polyfluoroalkyl substances (PFASs) are known as “forever chemicals” because of their extremely high chemical and thermal stability; moreover, they are detected in most aquatic environments worldwide [105]. MP biofilms can adsorb and accumulate PFASs in the water environment. Munoz et al. [106] analyzed the PFASs content in a river in northern France. They found that the total concentration of 14 PFASs in LDPE biofilms was 4.3–32 ng g−1 (dry weight), which was much higher than that detected in sediments (0.18–5.1 ng g−1, dry weight). In addition, the main groups in PFASs are carboxyl and sulfonic acid groups, which usually behave negatively in aqueous environments and are repulsed by negatively charged substances [105]. However, biofilms can act as mediators to mitigate this electrostatic repulsion [105]. For example, Fu et al. [107], when studying the effects of biofilm on perfluorooctanoic acid (PFOA) transport in sand columns, found that a PA biofilm had a significant effect on PFOA retention. These results indicate that the presence of a biofilm reduces the zeta potential in the sand column, thus reducing the electrostatic repulsion between the sand column and PFOA. The specific adsorption mechanism of PFASs in the natural environment is highly complex and influenced by numerous factors, which warrants considerable attention.
Despite the complex adsorption kinetics in contaminant–biofilm–microplastic systems, the effects of biofilms on POPs adsorption to MPs are extensively reported (Table 3). For example, Guasch et al. [108] concluded that the adsorption of POPs (such as antibiotics) to MPs is enhanced in the presence of biofilms. Another study similarly found that biofilms increase the adsorption of several POPs—polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and α-hexabromocyclododecane (α-HBCDD)—to HDPE [146]. In particular, it was suggested that the metabolic activity of microorganisms increases the adsorption capacity of MPs for POPs [14]. Meng et al. [109] found that pollutant-degrading microorganisms increase their adsorption and degradation capacities for polycyclic aromatic hydrocarbons (PAHs) during the summer months when microbial activity is high. The authors obtained a maximum adsorption of 1092.5 ± 93.0 ng g−1 for PAHs in summer, versus 826 ± 50.3 ng g−1 in winter.
Other researchers have suggested additional explanations for the adsorption-altering effects of biofilms. For example, Bhagwat et al. [110] found that the adsorption of perfluorooctane sulfonates (PFOS) was 20%–85% higher on biofilm-attached MPs than on the original MPs, possibly because the biofilm increases the specific surface area of the MPs and changes its surface hydrophobicity. Rosato et al. [80] concluded that some biofilm microorganisms promote the reduction dechlorination process of PCBs, initiating dechlorination after 2 weeks. During this process, the average number of chlorines per biphenyl molecule decreased from 5.2 to 4.8 to 4.3. This finding indicates that biofilms can change the toxicity of PCBs by changing its composition, thus affecting its adsorption to MPs.
Biofilms do not always increase the adsorption capacity of MPs for POPs. The EPS secreted by microorganisms in biofilms contain humic acids that can compete with PCBs for adsorption sites, attenuating the adsorption of PCBs to MPs [14]. Zhang et al. [17], who conducted exposure experiments of nine pollutants, also concluded that biofilms reduce the adsorption of compounds to MPs. For example, the estrone concentration on biofilm-coated MPs was only 12.7 ng g−1, versus 48.4 ng g−1 on bare MPs.
Adsorption to MPs is additionally affected by the nature of the pollutant being adsorbed. For example, when the temperature is high in summer, strong microbial activity decreases the concentration of pollutants in the surrounding environment. This decline is especially noticeable for PAHs with low (2–3 rings) or mid-range (4 rings) molecular weights, such as phenanthrene, chrysene, fluoranthene, and benz[a]anthracene [109]. Therefore, when studying the effect of biofilms on adsorption, we should also consider the effects of the pollutant properties.
Pathogenic microorganisms
The potential hazards of plastic-associated microbial communities have roused growing concern [111]. Pathogens can take advantage of the transmissibility of MPs in effluent discharged from wastewater treatment plants and thus spread to pathogen-free ecosystems. [112]. In particular, they can enter animal guts when ingested with MPs, causing health hazards [112]. Numerous studies have shown that MP surfaces can harbor many microorganisms, including various harmful algae along with Bacillus and Vibrio species [39, 53, 54, 72, 85].
Algal species abound on MP surfaces. Among the harmful algae are diatoms, which are believed to attach to MPs in coastal waters [113]. MPs can also be colonized by Salmonella bacteria, a major cause of fish diseases. [114]. Meanwhile, pathogenic Vibrio bacteria are early colonizers of MPs in marine environments [115]. Vibrio can strongly colonize and establish biofilms on PS surfaces [116]. Yang et al. [115] reported that members of the Flavobacteriaceae, Redobacteriaceae, and Foliobacteriaceae families become more abundant in the later stages of microbial colonization of MPs. Moreover, some biofilm microorganisms mutate after colonization. For example, certain pathogens in MP biofilms acquire antibiotic resistance genes from environmental bacteria. Such antibiotic-resistant pathogens are difficult to kill and can be transported along with MPs to remote environments, posing a threat to ecosystems and human health [72].
Some harmful microorganisms exploit the compositions of MPs. As is well known, carbon is an indispensable source of microbial growth and development. The pathogenic microorganisms in biofilms can utilize MPs as a carbon source. Plastic production also introduces many additives that further promote the growth of bacterial pathogens colonizing their surfaces [117].
In summary, the surfaces of MPs provide pathogenic microorganisms with new substrates for colonization [30]. MPs are highly durable and can transport pathogenic microorganisms over large horizontal and vertical distances through the aquatic environment. MPs can carry disease-causing microorganisms into the food web, where they transfer to different nutrient levels, posing health risks to animals and humans [111, 118]. Therefore, the dual pollution effects of pathogenic microorganisms and MPs are important considerations.
Other factors affecting adsorption and difference discussion
The interactions between MPs and pollutants are complex. Different particle sizes of MPs may lead to differences in pollutant adsorption. Cui et al. [119] found experimentally that different particle sizes affected the adsorption of organic pollutants by HDPE. HDPE with particle sizes smaller than 53 µm took longer to reach equilibrium (~ 5 d) than HDPE with particle sizes of 53–300 and 300–1000 µm (~ 1 d). The reason for this may be that smaller particles have greater specific surface area. Zhao et al. [125] found that PVC with a particle size of 10 μm had the highest adsorption of 39.5 mg/g for gentamycin, while for particles smaller than 10 μm, it was 32.21–38.42 mg/g. In addition, the adsorption rate of PLA with small particles (0.06 g mg/min) was greater than that of PLA with large particles (0.01 g mg/min) under identical biochar addition conditions. This may be due to the fact that MPs with small particles have a larger specific surface area and more adsorption sites [125]. MPs in the aquatic environment carry a wide range of chemicals, including their own additives and organic and inorganic chemicals absorbed from the surrounding environment [99, 126, 127]. The additive contents in plastic products may be higher than 50% and may include organic forms of toxic metals such as cadmium, lead, antimony, and tin, which are commonly used to improve the durability and processability of plastic products. The additives may also include PAFSs and PFOA, commonly added as lubricants to plastics [128]. These chemicals may leach or migrate into the surrounding environment, including onto plastic surfaces [128]. For example, dimethyl phthalate, which is used as a plasticizer, is easily released from plastics [126]. MPs can act as a new carrier of pollutants to adsorb heavy metals and organic pollutants, and the presence of additives may cause variations in the pollutant adsorption by MPs. According to Chen’s experiments on two types of PVC, namely, PVC1 and PVC2 (PVC1 is a soft material used in table mats and PVC2 is a granule used in the construction industry and present in the electrical component (e.g., electrical insulation, wires, and cable coatings)). PVC1 showed surface cracks and new functional groups and resulted in a BPA adsorption capacity higher than that of PVC2 by 0.57 μg/L. The aging characteristics of PVC2 are not obvious, resulting in no significant change in adsorption capacity, possibly because of the presence of light stabilizers and antioxidants [61].
In addition to the particle size of MPs and the ability of additives to influence the adsorption of contaminants by MPs, the presence of biofilms makes the mechanism of MP–contaminant interaction more difficult to explore [35]. Studying the effects of MPs on pollutant adsorption is difficult because of the presence of biofilms. First, the effect of biofilms on the adsorption of organic pollutants is inconsistent. For example, it has been suggested that the large amounts of EPS secreted by microorganisms in biofilms can reduce the adsorption of PCBs by MPs because of the competitive behavior of EPS in the presence of pollutants [14]. In contrast, Zhong et al. [129] found that higher levels of PFASs could be retained in the presence of a greater amount of EPS secreted by the membrane. This may be because the microorganisms were stimulated to secrete more EPS when the biofilm came into contact with PFASs, and the interaction of EPS with PFASs resulted in increased retention levels. In addition, the coexistence of multiple pollutants may affect the adsorption results. In general, biofilms tend to trap and degrade pollutants more easily compared with complex ones [105]. For example, Wu et al. [130] found that the addition of ammonium nitrogen significantly increased the biosorption of PFASs by microorganisms and regulated the PFASs accumulation in biofilms. The uptake of ammonium nitrogen by the biofilm triggered the microorganisms in the biofilm to release more EPS, resulting in a reduced retention of PAFS in soil particles and an increased tendency for retention in the biofilm. Overall, most studies on biofilms affecting heavy-metal adsorption by MPs were usually conducted using in situ experiments or under laboratory conditions [18, 89, 100, 102]. Biofilm-attached MPs adsorb more metals than pristine MPs, and the results of in situ experiments and laboratory studies are largely consistent [131]. In particular, biofilms that affect heavy-metal adsorption appear to be related to the degradability of MPs [131]. PE with a biofilm on its surface exhibited 3.46 times greater metal adsorption compared with pristine PE [132]. Wang et al. [89] found that the presence of a biofilm resulted in the adsorption of Cu to PS at 31.4 μg/g compared with 16.15 μg/g in pristine PS. The amount of Cu adsorbed by PS with a biofilm was about twice that of the original PS. However, it was found that degradable polybutylene succinate (PBS) MPs adsorbed Pb(II) approximately ten times more frequently than pristine PBS [133]. In conclusion, the effect of biofilms present on the surface of degradable MPs should be considered. The effects of biofilms on pollutant adsorption onto MPs are related to many factors. This also suggests that we need to subsequently focus on the effects of biofilm composition, the coexistence of multiple pollutants, and degradable MPs.
The ability of microplastics to accumulate pollutants has been widely proven [156, 157]. One of the factors affecting adsorption is the particle size of the MPs. Smaller particle sizes usually adsorb more pollutants because they have a larger specific surface area. In addition, the use of additives has an indirect effect on the adsorption results. The emergence of biofilms has made the adsorption of contaminants by microplastics relevant to a wider range of factors. Biofilms facilitate the adsorption of heavy metals by MPs through enhanced complexation and an increased number of adsorption sites. The effect of the presence of biofilms on the adsorption of POPs by MPs does not seem to be absolute, possibly because of the complex nature of POPs. The effect of the presence of biofilms attached to MP surfaces on adsorption is pronounced in the case of degradable MPs compared to nondegradable MPs. These findings suggest that when studying pollution adsorption by MPs, attention should be paid to the combined effects of the MP type, particle size, additives, degradability, biofilm composition, and coexistence of multiple pollutants.
Conclusions and future directions
This review first introduced MPs as a new ecological niche for biofilm establishment and development. Biofilm formation is facilitated by the strong hydrophobicity of MP surfaces and the self-release of MP components. Reciprocally, biofilms affect the properties of MPs. We then described how biofilm formation depends on the environment, MPs, and microorganism characteristics. Among these factors, environmental factors exerted more influence than MPs and microorganism-related factors. Finally, we summarized how biofilms on MPs influence the adsorption of environmental pollutants, heavy metals, POPs, and pathogenic microorganisms. The adsorption of pollutants by MPs will be affected by the biodegradability, particle size, and additives of MPs, and the adsorption mechanism becomes complicated owing to biofilm formation. In general, the adsorption kinetics on biofilm-coated MPs are inherently complex and the mechanism by which biofilms promote adsorption is only vaguely understood. More in-depth studies could be directed toward the following goals:
-
The mechanism by which biofilms promote the adsorption of contaminants on MPs must be elucidated. The internal and external factors that influence the promotional effect of biofilm on contaminant adsorption to MPs must also be identified and the adsorption pattern should be summarized.
-
In future work, adsorption experiments should be carried out in natural environments to avoid laboratory limitations. Under natural conditions, the colonisation of microorganisms on the surface of microplastics and the effect of biofilm on the modification of microplastic properties and the adsorption of pollutants by microplastics should be further investigated.
-
Biofilm formation on MP surfaces increases the risk of pathogenic microorganisms entering the food chain along with ingested MPs. Follow-up studies should focus on the interactions between pathogenic microorganisms and MPs and the health problems caused by their entry into organisms. The transmission and accumulation patterns of pathogenic microorganisms on MPs should be determined.
Data availability
Data, associated metadata, and calculation tools are available from the corresponding author (qinyan@cqjtu.edu.cn).
Abbreviations
- MP:
-
Microplastic
- POPs:
-
Persistent organic pollutants
- PS:
-
Polystyrene
- PVC:
-
Polyvinyl chloride
- PA:
-
Polyamide
- HDPE:
-
High-density polyethylene
- LDPE:
-
Low-density polyethylene
- PE:
-
Polyethylene
- PET:
-
Polyethylene terephthalate
- PP:
-
Polypropylene
- PLA:
-
Polylactic acid
- PBS:
-
Polybutylene succinate
- PCoA:
-
Principal co-ordinates analysis
- OTUs:
-
Operational taxonomic units
- NOR:
-
Norfloxacin
- DMP:
-
Dimethyl phthalate
- TN:
-
Total nitrogen
- NO3 − − N:
-
Nitrate nitrogen
- NH4 + − N:
-
Ammonia nitrogen
- TP:
-
Total phosphorus
- SS:
-
Suspended solids
- TN:
-
Total nitrogen
- PFASs:
-
Perfluoroalkyl and polyfluoroalkyl substances
- PFOA:
-
Perfluorooctanoic acid
- PCBs:
-
Polychlorinated biphenyls
- PBDEs:
-
Polybrominated diphenyl ethers
- α-HBCDD:
-
α-Hexabromocyclododecane
- PAHs:
-
Polycyclic aromatic hydrocarbons
- EPS:
-
Extracellular polymeric substances
- PFOS:
-
Perfluorooctane sulfonates
References
Hernández EG, Nowack B, Mitrano DM (2017) Polyester textiles as a source of microplastics from households: a mechanistic study to understand microfiber release during washing. Environ Sci Technol 51(12):7036–7046. https://doi.org/10.1021/acs.est.7b01750
Thompson RC, Olsen YS, Mitchell RP, Davis A, Rowland SJ, John A, McGonigle DF, Russell AE (2004) Lost at sea: where is all the plastic? Science 304(5672):838. https://doi.org/10.1126/science.1094559
Borrelle SB, Ringma J, Law KL, Monnahan CC, Lebreton L, McGivern A, Murphy EL, Jambeck J, Leonard GH, Hilleary MA, Eriksen M, Possingham HP, De Frond H, Gerber LR, Polidoro B, Tahir A, Bernard M, Mallos NJ, Barnes M, Rochman CM (2020) Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 369(6510):1515–1518. https://doi.org/10.1126/science.aba3656
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62(8):1596–1605. https://doi.org/10.1016/j.marpolbul.2011.05.030
Plastic Europe (2023) https://plasticseurope.org/knowledge-hub/. Accessed 23 Mar 2023
Cole M, Lindeque PK, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62(12):2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Hurley R, Woodward J, Rothwell J (2018) Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat Geosci 11(4):251–257. https://doi.org/10.1038/s41561-018-0080-1
Mamun AA, TaE P, Dewi IR, Ahmad M (2023) Microplastics in human food chains: food becoming a threat to health safety. Sci Total Environ 858:159834. https://doi.org/10.1016/j.scitotenv.2022.159834
Fu L, Li J, Wang G, Luan Y, Dai W (2021) Adsorption behavior of organic pollutants on microplastics. Ecotoxicol Environ Saf 217:112207. https://doi.org/10.1016/j.ecoenv.2021.112207
Costigan E, Collins A, Hatinoglu MD, Bhagat K, Macrae J, Perreault F, Apul O (2022) Adsorption of organic pollutants by microplastics: overview of a dissonant literature. J Hazard Mater Adv 6:100091. https://doi.org/10.1016/j.hazadv.2022.100091
Tu C, Chen T, Zhou Q, Liu Y, Wei J, Waniek JJ, Luo Y (2020) Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Sci Total Environ 734:139237. https://doi.org/10.1016/j.scitotenv.2020.139237
Sun X, Xin H, Xiong H, Fang Y, Wang Y (2023) Bioremediation of microplastics in freshwater environments: a systematic review of biofilm culture, degradation mechanisms, and analytical methods. Sci Total Environ 863:160953. https://doi.org/10.1016/j.scitotenv.2022.160953
De Tender C, Devriese L, Haegeman A, Maes S, Ruttink T, Dawyndt P (2015) Bacterial community profiling of plastic litter in the Belgian part of the North Sea. Environ Sci Technol 49(16):9629–9638. https://doi.org/10.1021/acs.est.5b01093
Rummel C, Jahnke A, Gorokhova E, Kühnel D, Schmitt-Jansen M (2017) Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Tech Lett 4(7):258–267. https://doi.org/10.1021/acs.estlett.7b00164
Verdú I, Amariei G, Rueda-Varela C, González-Pleiter M, Leganés F, Rosal R, Fernández-Piñas F (2023) Biofilm formation strongly influences the vector transport of triclosan-loaded polyethylene microplastics. Sci Total Environ 859:160231. https://doi.org/10.1016/j.scitotenv.2022.160231
He S, Tong J, Xiong W, Xiang Y, Peng H, Wang W, Yang Y, Ye Y, Hu M, Yang Z, Zeng G (2023) Microplastics influence the fate of antibiotics in freshwater environments: biofilm formation and its effect on adsorption behavior. J Hazard Mater 442:130078. https://doi.org/10.1016/j.jhazmat.2022.130078
Zhang H, Zhang C, Rao WK, Zhang H, Liang G, Deng X, Zhao J, Guan Y, Ying G (2022) Influence of biofilms on the adsorption behavior of nine organic emerging contaminants on microplastics in field-laboratory exposure experiments. J Hazard Mater 434:128895. https://doi.org/10.1016/j.jhazmat.2022.128895
Guan J, Qi K, Wang J, Wang W, Wang Z, Lü N, Qu J (2020) Microplastics as an emerging anthropogenic vector of trace metals in freshwater: significance of biofilms and comparison with natural substrates. Water Res 184:116205. https://doi.org/10.1016/j.watres.2020.116205
Davey ME, O’Toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64(4):847–867. https://doi.org/10.1128/mmbr.64.4.847-867.2000
Stabnikova O, Stabnikov V, Marinin A, Kļaviņš M, Vaseashta A (2022) The role of microplastics biofilm in accumulation of trace metals in aquatic environments. World J Microbiol Biotechnol. https://doi.org/10.1007/s11274-022-03293-6
McCormick AR, Hoellein TJ, Mason SA, Schluep J, Kelly JJ (2014) Microplastic is an abundant and distinct microbial habitat in an urban river. Environ Sci Technol 48(20):11863–11871. https://doi.org/10.1021/es503610r
Nguyen HT, Choi W, Kim E, Cho K (2022) Microbial community niches on microplastics and prioritized environmental factors under various urban riverine conditions. Sci Total Environ 849:157781. https://doi.org/10.1016/j.scitotenv.2022.157781
Sathicq MB, Sabatino R, Corno G, Di Cesare A (2021) Are microplastic particles a hotspot for the spread and the persistence of antibiotic resistance in aquatic systems? Environ Pollut 279:116896. https://doi.org/10.1016/j.envpol.2021.116896
Bradney L, Wijesekara H, Palansooriya KN, Obadamudalige N, Bolan N, Ok YS, Rinklebe J, Kim K, Kirkham M (2019) Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ Int 131:104937. https://doi.org/10.1016/j.envint.2019.104937
Lorite GS, Rodrigues CM, De Souza AA, Kranz C, Mizaikoff B, Cotta MA (2011) The role of conditioning film formation and surface chemical changes on Xylella fastidiosa adhesion and biofilm evolution. J Colloid Interface Sci 359(1):289–295. https://doi.org/10.1016/j.jcis.2011.03.066
Ke C, Wigglesworth-Cooksey B (1995) Adhesion of bacteria and diatoms to surfaces in the sea: a review. Aquat Microb Ecol 9:87–96. https://doi.org/10.3354/ame009087
Morét-Ferguson S, Law KL, Proskurowski G, Murphy EK, Peacock EE, Reddy CM (2010) The size, mass, and composition of plastic debris in the western north Atlantic Ocean. Mar Pollut Bull 60(10):1873–1878. https://doi.org/10.1016/j.marpolbul.2010.07.020
Michels J, Stippkugel A, Lenz M, Wirtz K (1885) Rapid aggregation of biofilm-covered microplastics with marine biogenic particles. Proc R Soc Biol Sci 285:20181203. https://doi.org/10.1098/rspb.2018.1203
Woodall LC, Sanchez-Vidal A, Canals M, Paterson GL, Coppock R, Sleight V, Calafat A, Rogers AD, Narayanaswamy BE, Thompson RC (2014) The deep sea is a major sink for microplastic debris. R Soc Open Sci 1(4):140317. https://doi.org/10.1098/rsos.140317
He S, Jia M, Xiang Y, Song B, Xiong W, Cao J, Peng H, Yang Y, Wang W, Yang Z, Zeng G (2022) Biofilm on microplastics in aqueous environment: physicochemical properties and environmental implications. J Hazard Mater 424:127286. https://doi.org/10.1016/j.jhazmat.2021.127286
Katsikogianni MG, Missirlis YF (2004) Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater. https://doi.org/10.22203/ecm.v008a05
Renner LD, Weibel DB (2011) Physicochemical regulation of biofilm formation. MRS Bull 36(5):347–355. https://doi.org/10.1557/mrs.2011.65
Hori K, Matsumoto S (2010) Bacterial adhesion: from mechanism to control. Biochem Eng J 48(3):424–434. https://doi.org/10.1016/j.bej.2009.11.01
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2(2):95–108. https://doi.org/10.1038/nrmicro821
Kalčíková G, Skalar T, Marolt G, Kokalj AJ (2020) An environmental concentration of aged microplastics with adsorbed silver significantly affects aquatic organisms. Water Res 175:115644. https://doi.org/10.1016/j.watres.2020.115644
Lobelle D, Cunliffe M (2011) Early microbial biofilm formation on marine plastic debris. Mar Pollut Bull 62(1):197–200. https://doi.org/10.1016/j.marpolbul.2010.10.013
Wright RJ, Erni-Cassola G, Zadjelovic V, Latva M, Christie-Oleza JA (2020) Marine plastic debris: a new surface for microbial colonization. Environ Sci Technol 54(19):11657–11672. https://doi.org/10.1021/acs.est.0c02305
Kirstein IV, Kirmizi S, Wichels A, Garin-Fernandez A, Erler R, Löder MGJ, Gerdts G (2016) Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar Environ Res 120:1–8. https://doi.org/10.1016/j.marenvres.2016.07.004
Bao R, Cheng Z, Hou Y, Xie C, Pu J, Peng L, Liu G, Chen W, Su Y (2022) Secondary microplastics formation and colonized microorganisms on the surface of conventional and degradable plastic granules during long-term UV aging in various environmental media. J Hazard Mater 439:129686. https://doi.org/10.1016/j.jhazmat.2022.129686
Li W, Zhang Y, Wu N, Zhao Z, Wang X, Ma Y, Niu Z (2019) Colonization characteristics of bacterial communities on plastic debris influenced by environmental factors and polymer types in the Haihe estuary of Bohai Bay. China Environ Sci Technol 53(18):10763–10773. https://doi.org/10.1021/acs.est.9b03659
De Tender C, Devriese L, Haegeman A, Maes S, Vangeyte J, Cattrijsse A, Dawyndt P, Ruttink T (2017) Temporal dynamics of bacterial and fungal colonization on plastic debris in the North Sea. Environ Sci Technol 51(13):7350–7360. https://doi.org/10.1021/acs.est.7b00697
Caruso G (2020) Microbial Colonization in Marine Environments: Overview of current knowledge and emerging research topics. J Mar Sci Eng 8(2):78. https://doi.org/10.3390/jmse8020078
Xu X, Wang S, Gao F, Li J, Zheng L, Sun C, He C, Wang Z, Qu L (2019) Marine microplastic-associated bacterial community succession in response to geography, exposure time, and plastic type in China’s coastal seawaters. Mar Pollut Bull 145:278–286. https://doi.org/10.1016/j.marpolbul.2019.05.036
Garrett TR, Bhakoo M, Zhang Z (2008) Bacterial adhesion and biofilms on surfaces. Prog Nat Sci: Mater Int 18(9):1049–1056. https://doi.org/10.1016/j.pnsc.2008.04.001
Stoodley P, Dodds I, Boyle JD, Lappin-Scott HM (1998) Influence of hydrodynamics and nutrients on biofilm structure. J Appl Microbiol 85(S1):19S-28S. https://doi.org/10.1111/j.1365-2672.1998.tb05279.x
Xiao C, Lian X, Wang Y, Chen S, Sun Y, Tao G, Tan Q, Feng J (2023) Impacts of hydraulic conditions on microplastics biofilm development, shear stresses distribution, and microbial community structures in drinking water distribution pipes. J Environ Manag 325:116510. https://doi.org/10.1016/j.jenvman.2022.116510
Chen X, Xiong X, Jiang X, Shi H, Wu C (2019) Sinking of floating plastic debris caused by biofilm development in a freshwater lake. Chemosphere 222:856–864. https://doi.org/10.1016/j.chemosphere.2019.02.015
Oberbeckmann S, Loeder MG, Gerdts G, Osborn AM (2014) Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters. FEMS Microbiol Ecol 90(2):478–492. https://doi.org/10.1111/1574-6941.12409
Feng L, He L, Jiang S, Chen J, Zhou C, Qian Z, Hong P, Sun S, Li C (2020) Investigating the composition and distribution of microplastics surface biofilms in coral areas. Chemosphere 252:126565. https://doi.org/10.1016/j.chemosphere.2020.126565
Pompilio A, Piccolomini R, Picciani C, D’Antonio D, Savini V, Di Bonaventura G (2008) Factors associated with adherence to and biofilm formation on polystyrene by Stenotrophomonas maltophilia: the role of cell surface hydrophobicity and motility. FEMS Microbiol Lett 287(1):41–47. https://doi.org/10.1111/j.1574-6968.2008.01292.x
Hossain MR, Jiang M, Wei Q, Leff LG (2018) Microplastic surface properties affect bacterial colonization in freshwater. J Basic Microbiol 59(1):54–61. https://doi.org/10.1002/jobm.201800174
Frère L, Maignien L, Chalopin M, Huvet A, Rinnert E, Morrison HG, Kerninon S, Cassone A, Lambert C, Réveillaud J, Paul-Pont I (2018) Microplastic bacterial communities in the Bay of Brest: Influence of polymer type and size. Environ Pollut 242:614–625. https://doi.org/10.1016/j.envpol.2018.07.023
Dudek KL, Cruz B, Polidoro B, Neuer S (2020) Microbial colonization of microplastics in the Caribbean Sea. Limnol Oceanogr Lett 5(1):5–17. https://doi.org/10.1002/lol2.10141
Deng H, Fu Q, Li D, Zhang Y, He J, Feng D, Zhao Y, Du G, Yu H, Ge C (2021) Microplastic-associated biofilm in an intensive mariculture pond: Temporal dynamics of microbial communities, extracellular polymeric substances and impacts on microplastics properties. J Clean Prod 319:128774. https://doi.org/10.1016/j.jclepro.2021.128774
Li W, Luo D, Yan N, Miao L, Adyel TM, Kong M, Hou J (2023) Effects of polyethylene microplastics with different particle sizes and concentrations on the community structure and function of periphytic biofilms. J Environ Chem Eng 11(6):111287. https://doi.org/10.1016/j.jece.2023.111287
Gong X, Ge Z, Ma Z, Li Y, Huang D, Zhang J (2023) Effect of different size microplastic particles on the construction of algal-bacterial biofilms and microbial communities. J Environ Manag 343:118246. https://doi.org/10.1016/j.jenvman.2023.118246
Yao S, Lyu S, An Y, Lu J, Gjermansen C, Schramm A (2018) Microalgae-bacteria symbiosis in microalgal growth and biofuel production: a review. J Appl Microbiol 126(2):359–368. https://doi.org/10.1111/jam.14095
Debroy A, George N, Mukherjee G (2021) Role of biofilms in the degradation of microplastics in aquatic environments. J Chem Technol Biotechnol 97(12):3271–3282. https://doi.org/10.1002/jctb.6978
Ru J, Huo Y, Yang Y (2020) Microbial degradation and valorization of plastic wastes. Front Microbiol. https://doi.org/10.3389/fmicb.2020.00442
Wang H, Yu P, Schwarz C, Zhang B, Huo L, Shi B, Alvarez PJJ (2022) Phthalate esters released from plastics promote biofilm formation and chlorine resistance. Environ Sci Technol 56(2):1081–1090. https://doi.org/10.1021/acs.est.1c04857
Chen X, Chen X, Chen X, Chen X (2024) Bisphenol A sorption on commercial polyvinyl chloride microplastics: effects of UV-aging, biofilm colonization and additives on plastic behavior in the environment. Environ Pollut 356:124218. https://doi.org/10.1016/j.envpol.2024.124218
Dang H, Lovell CR (2000) Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified RRNA gene restriction analysis and sequence analysis of 16S RRNA genes. Appl Environ Microbiol 66(2):467–475. https://doi.org/10.1128/aem.66.2.467-475.2000
Artham T, Sudhakar M, Venkatesan R, Nair CM, Murty KVGK, Doble M (2009) Biofouling and stability of synthetic polymers in sea water. Int Biodeterior Biodegrad 63(7):884–890. https://doi.org/10.1016/j.ibiod.2009.03.003
Xie H, Chen J, Feng L, He L, Zhou C, Hong P, Sun S, Zhao H, Liang Y, Ren L, Zhang Y (2021) Chemotaxis-selective colonization of mangrove rhizosphere microbes on nine different microplastics. Sci Total Environ 752:142223. https://doi.org/10.1016/j.scitotenv.2020.142223
Sooriyakumar P, Bolan N, Kumar M, Singh L, Yu Y, Li Y, Weralupitiya C, Vithanage M, Ramanayaka S, Sarkar B, Wang F, Gleeson D, Zhang D, Kirkham M, Rinklebe J, Siddique KH (2022) Biofilm formation and its implications on the properties and fate of microplastics in aquatic environments: a review. J Hazard Mater Adv 6:100077. https://doi.org/10.1016/j.hazadv.2022.100077
Gottenbos B, Grijpma DW, Van Der Mei HC, Feijén J, Busscher HJ (2001) Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J Antimicrob Chemother 48(1):7–13. https://doi.org/10.1093/jac/48.1.7
Fotopoulou KN, Karapanagioti HK (2012) Surface properties of beached plastic pellets. Mar Environ Res 81:70–77. https://doi.org/10.1016/j.marenvres.2012.08.010
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8(9):881–890. https://doi.org/10.3201/eid0809.020063
Rickard AH, McBain AJ, Ledder RG, Handley PS, Gilbert P (2003) Coaggregation between freshwater bacteria within biofilm and planktonic communities. FEMS Microbiol Lett 220(1):133–140. https://doi.org/10.1016/s0378-1097(03)00094-6
Flemming H, Wingender J, Szewzyk U, Steinberg PD, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9):563–575. https://doi.org/10.1038/nrmicro.2016.94
Rendueles O, Ghigo J (2015) Mechanisms of competition in biofilm communities. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.mb-0009-2014
Xin-Rong W, Pan J, Li M, Yao L, Bartlam M, Wang Y (2019) Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res 165:114979. https://doi.org/10.1016/j.watres.2019.114979
Pedersen K (1990) Biofilm development on stainless steel and pvc surfaces in drinking water. Water Res 24(2):239–243. https://doi.org/10.1016/0043-1354(90)90109-j
Bellou N, Papathanassiou E, Dobretsov S, Lykousis V, Colijn F (2012) The effect of substratum type, orientation and depth on the development of bacterial deep-sea biofilm communities grown on artificial substrata deployed in the Eastern Mediterranean. Biofouling 28(2):199–213. https://doi.org/10.1080/08927014.2012.662675
Chung GHC, Lee OO, Huang Y, Mok SYF, Kolter R, Qian P (2010) Bacterial community succession and chemical profiles of subtidal biofilms in relation to larval settlement of the polychaete Hydroides elegans. ISME J 4(6):817–828. https://doi.org/10.1038/ismej.2009.157
Lehtola MJ, Laxander M, Miettinen IT, Hirvonen A, Vartiainen T, Martikainen PJ (2006) The effects of changing water flow velocity on the formation of biofilms and water quality in pilot distribution system consisting of copper or polyethylene pipes. Water Res 40(11):2151–2160. https://doi.org/10.1016/j.watres.2006.04.010
Miao L, Gao Y, Adyel TM, Huo Z, Li Z, Wu J, Hou J (2021) Effects of biofilm colonization on the sinking of microplastics in three freshwater environments. J Hazard Mater 413:125370. https://doi.org/10.1016/j.jhazmat.2021.125370
Rozman U, Filker S, Kalčíková G (2023) Monitoring of biofilm development and physico-chemical changes of floating microplastics at the air-water interface. Environ Pollut 322:121157. https://doi.org/10.1016/j.envpol.2023.121157
McGivney E, Cederholm L, Barth A, Hakkarainen M, Hamacher-Barth E, Ogonowski M, Gorokhova E (2020) Rapid physicochemical changes in microplastic induced by biofilm formation. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2020.00205
Rosato A, Barone M, Negroni A, Brigidi P, Fava F, Xu P, Candela M, Zanaroli G (2020) Microbial colonization of different microplastic types and biotransformation of sorbed PCBs by a marine anaerobic bacterial community. Sci Total Environ 705:135790. https://doi.org/10.1016/j.scitotenv.2019.135790
Zhou Q, Tu C, Liu Y, Li Y, Zhang H, Vogts A, Plewe S, Pan X, Luo Y, Waniek JJ (2022) Biofilm enhances the copper (II) adsorption on microplastic surfaces in coastal seawater: simultaneous evidence from visualization and quantification. Sci Total Environ 853:158217. https://doi.org/10.1016/j.scitotenv.2022.158217
Santo M, Weitsman R, Sivan A (2013) The role of the copper-binding enzyme – laccase – in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int Biodeterior Biodegr 84:204–210. https://doi.org/10.1016/j.ibiod.2012.03.001
Gilan I, Hadar Y, Sivan A (2004) Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-004-1584-8
Hadad D, Geresh S, Sivan A (2005) Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J Appl Microbiol 98(5):1093–1100. https://doi.org/10.1111/j.1365-2672.2005.02553.x
Kaiser D, Kowalski N, Waniek JJ (2017) Effects of biofouling on the sinking behavior of microplastics. Environ Res Lett 12(12):124003. https://doi.org/10.1088/1748-9326/aa8e8b
Elagami H, Ahmadi P, Fleckenstein JH, Frei S, Obst M, Agarwal S, Gilfedder B (2022) Measurement of microplastic settling velocities and implications for residence times in thermally stratified lakes. Limnol Oceanogr 67(4):934–945. https://doi.org/10.1002/lno.12046
Syberg K, Khan FR, Selck H, Palmqvist A, Banta GT, Daley JM, Sano LL, Duhaime MB (2015) Microplastics: addressing ecological risk through lessons learned. Environ Toxicol Chem 34(5):945–953. https://doi.org/10.1002/etc.2914
Long M, Moriceau B, Gallinari M, Lambert C, Huvet A, Raffray J, Soudant P (2015) Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar Chem 175:39–46. https://doi.org/10.1016/j.marchem.2015.04.003
Wang Q, Zhang Y, Zhang Y, Zhouqi L, Wang J, Chen H (2022) Effects of biofilm on metal adsorption behavior and microbial community of microplastics. J Hazard Mater 424:127340. https://doi.org/10.1016/j.jhazmat.2021.127340
Luo H, Liu C, He D, Xu J, Sun J, Li J, Pan X (2022) Environmental behaviors of microplastics in aquatic systems: a systematic review on degradation, adsorption, toxicity and biofilm under aging conditions. J Hazard Mater 423:126915. https://doi.org/10.1016/j.jhazmat.2021.126915
Galloway TS, Cole M, Lewis C (2017) Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol. https://doi.org/10.1038/s41559-017-0116
Jahnke A, Arp HPH, Escher BI, Gewert B, Gorokhova E, Kühnel D, Ogonowski M, Potthoff A, Rummel C, Schmitt-Jansen M, Toorman E, MacLeod M (2017) Reducing uncertainty and confronting ignorance about the possible impacts of weathering plastic in the marine environment. Environ Sci Technol Lett 4(3):85–90. https://doi.org/10.1021/acs.estlett.7b00008
Zettler ER, Mincer TJ, Amaral-Zettler L (2013) Life in the “Plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol 47(13):7137–7146. https://doi.org/10.1021/es401288x
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. EXS, pp 133–164
Massos A, Turner A (2017) Cadmium, lead and bromine in beached microplastics. Environ Pollut 227:139–145. https://doi.org/10.1016/j.envpol.2017.04.034
Anderson JC, Park BJ, Palace V (2016) Microplastics in aquatic environments: implications for Canadian ecosystems. Environ Pollut 218:269–280. https://doi.org/10.1016/j.envpol.2016.06.074
Gao X, Hassan I, Peng Y, Huo S, Ling L (2021) Behaviors and influencing factors of the heavy metals adsorption onto microplastics: a review. J Clean Prod 319:128777. https://doi.org/10.1016/j.jclepro.2021.128777
Kurniawan A, Yamamoto T, Tsuchiya Y, Morisaki H (2012) Analysis of the ion adsorption-desorption characteristics of biofilm matrices. Microbes Environ 27(4):399–406. https://doi.org/10.1264/jsme2.me11339
Rochman CM, Hentschel BT, Teh SJ (2014) Long-term sorption of metals is similar among plastic types: implications for plastic debris in aquatic environments. PLoS One 9(1):e85433. https://doi.org/10.1371/journal.pone.0085433
Johansen MP, Cresswell T, Davis J, Howard DL, Howell NR, Prentice E (2019) Biofilm-enhanced adsorption of strong and weak cations onto different microplastic sample types: use of spectroscopy, microscopy and radiotracer methods. Water Res 158:392–400. https://doi.org/10.1016/j.watres.2019.04.029
Zou J, Liu X, Zhang D, Yuan X (2020) Adsorption of three bivalent metals by four chemical distinct microplastics. Chemosphere 248:126064. https://doi.org/10.1016/j.chemosphere.2020.126064
Ashton K, Holmes L, Turner A (2010) Association of metals with plastic production pellets in the marine environment. Mar Pollut Bull 60(11):2050–2055. https://doi.org/10.1016/j.marpolbul.2010.07.014
Tufail MA, Iltaf J, Zaheer T, Tariq L, Amir MB, Fatima R, Asbat A, Kabeer T, Fahad M, Naeem H, Shoukat U, Noor H, Awais M, Umar W, Ayyub M (2022) Recent advances in bioremediation of heavy metals and persistent organic pollutants: a review. Sci Total Environ 850:157961. https://doi.org/10.1016/j.scitotenv.2022.157961
Groffen T, Rijnders J, Van Doorn L, Jorissen C, De Borger SM, Luttikhuis DO, De Deyn L, Covaci A, Bervoets L (2021) Preliminary study on the distribution of metals and persistent organic pollutants (POPs), including perfluoroalkylated acids (PFAS), in the aquatic environment near Morogoro, Tanzania, and the potential health risks for humans. Environ Res 192:110299. https://doi.org/10.1016/j.envres.2020.110299
Ji B, Zhao Y (2024) Interactions between biofilms and PFASs in aquatic ecosystems: literature exploration. Sci Total Environ 906:167469. https://doi.org/10.1016/j.scitotenv.2023.167469
Munoz G, Fechner LC, Geneste E, Pardon P, Budzinski H, Labadie P (2016) Spatio-temporal dynamics of per and polyfluoroalkyl substances (PFASs) and transfer to periphytic biofilm in an urban river: case-study on the River Seine. Environ Sci Pollut Res 25(24):23574–23582. https://doi.org/10.1007/s11356-016-8051-9
Fu J, Gao B, Xu H, Shi H, Ren J, Wu J, Sun Y (2023) Effects of biofilms on the retention and transport of PFOA in saturated porous media. J Hazard Mater 443:130392. https://doi.org/10.1016/j.jhazmat.2022.130392
Guasch H, Bernal S, Bruno D, Almroth BC, Cochero J, Corcoll N, Cornejo D, Gacia E, Kröll A, Lavoie I, Ledesma JLJ, Lupon A, Margenat H, Morin S, Navarro E, Ribot M, Riis T, Schmitt-Jansen M, Tlili A, Martı E (2022) Interactions between microplastics and benthic biofilms in fluvial ecosystems: knowledge gaps and future trends. Freshwater Science 41(3):442–458. https://doi.org/10.1086/721472
Meng J, Yu X, Yao Z, Tao P, Li G, Yu X, Zhao J, Peng J (2020) How biofilms affect the uptake and fate of hydrophobic organic compounds (HOCs) in microplastic: insights from an In situ study of Xiangshan Bay. China Water Research 184:116118. https://doi.org/10.1016/j.watres.2020.116118
Bhagwat G, Tran TKA, Lamb D, Senathirajah K, Grainge I, O’Connor W, Juhasz AL, Thavamani P (2021) Biofilms enhance the adsorption of toxic contaminants on plastic microfibers under environmentally relevant conditions. Environ Sci Technol 55(13):8877–8887. https://doi.org/10.1021/acs.est.1c02012
Oberbeckmann S, Labrenz M (2020) Marine microbial assemblages on microplastics: diversity, adaptation, and role in degradation. Ann Rev Mar Sci 12(1):209–232. https://doi.org/10.1146/annurev-marine-010419-010633
Oberbeckmann S, Löder MGJ, Labrenz M (2015) Marine microplastic-associated biofilms—a review. Environ Chem 12(5):551. https://doi.org/10.1071/en15069
Masó M, Fortuño J, De Juan S, Demestre M (2016) Microfouling communities from pelagic and benthic marine plastic debris sampled across Mediterranean coastal waters. Sci Mar 80(S1):117–127. https://doi.org/10.3989/scimar.04281.10a
Viršek MK, Lovšin MN, Koren Š, Kržan A, Peterlin M (2017) Microplastics as a vector for the transport of the bacterial fish pathogen species Aeromonas salmonicida. Mar Pollut Bull 125(1–2):301–309. https://doi.org/10.1016/j.marpolbul.2017.08.024
Yang Y, Liu W, Zhang Z, Grossart H, Gadd GM (2020) Microplastics provide new microbial niches in aquatic environments. Appl Microbiol Biotechnol 104(15):6501–6511. https://doi.org/10.1007/s00253-020-10704-x
Foulon V, Roux FL, Lambert C, Huvet A, Soudant P, Paul-Pont I (2016) Colonization of polystyrene microparticles by Vibrio crassostreae: light and electron microscopic investigation. Environ Sci Technol 50(20):10988–10996. https://doi.org/10.1021/acs.est.6b02720
Hirt N, Body-Malapel M (2020) Immunotoxicity and intestinal effects of nano- and microplastics: a review of the literature. Part Fibre Toxicol. https://doi.org/10.1186/s12989-020-00387-7
Shapiro K, Krusor C, Mazzillo FFM, Conrad PA, Largier JL, JaK M, Silver MW (2014) Aquatic polymers can drive pathogen transmission in coastal ecosystems. Proc R Soc B: Biol Sci 281(1795):20141287. https://doi.org/10.1098/rspb.2014.1287
Cui W, Hale RC, Huang Y, Zhou F, Wu Y, Liang X, Liu Y, Tan H, Chen D (2023) Sorption of representative organic contaminants on microplastics: effects of chemical physicochemical properties, particle size, and biofilm presence. Ecotoxicol Environ Saf 251:114533. https://doi.org/10.1016/j.ecoenv.2023.114533
Singh S, Chakma S, Alawa B, Kalyanasundaram M, Diwan V (2023) Assessment of microplastic pollution in agricultural soil of Bhopal, Central India. J Mater Cycles Waste Manag 26(2):708–722. https://doi.org/10.1007/s10163-023-01805-6
Lee A, Liew MS (2019) Ecologically derived waste management of conventional plastics. J Mater Cycles Waste Manag 22(1):1–10. https://doi.org/10.1007/s10163-019-00931-4
Chen Y, Niu S, Yu J, Wu J, Wang T (2023) Microplastics and microorganisms in sediments from stormwater drain system. Sci Total Environ 889:164284. https://doi.org/10.1016/j.scitotenv.2023.164284
Yu Y, Miao L, Adyel TM, Kryss W, Wu J, Hou J (2023) Aquatic plastisphere: Interactions between plastics and biofilms. Environ Pollut 322:121196. https://doi.org/10.1016/j.envpol.2023.121196
Miao L, Wang P, Hou J, Yao Y, Liu Z, Liu S, Li T (2019) Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci Total Environ 650:2395–2402. https://doi.org/10.1016/j.scitotenv.2018.09.378
Zhao S, Zhang C, Zhang Q, Huang Q (2024) Small microplastic particles promote tetracycline and aureomycin adsorption by biochar in an aqueous solution. J Environ Manage 349:119332. https://doi.org/10.1016/j.jenvman.2023.119332
Teuten EL, Saquing JM, Knappe DRU, Barlaz MA, Jonsson S, Björn A, Rowland SJ, Thompson RC, Galloway TS, Yamashita R, Ochi D, Watanuki Y, Moore C, Viet PH, Tana TS, Prudente M, Boonyatumanond R, Zakaria MP, Akkhavong K, Takada H (2009) Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc Biol Sci 364(1526):2027–2045. https://doi.org/10.1098/rstb.2008.0284
Hoogenboom L (2016) Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. https://doi.org/10.2903/j.efsa.2016.4501
Yu Y, Kumar M, Bolan S, Padhye LP, Bolan N, Li S, Wang L, Hou D, Li Y (2024) Various additive release from microplastics and their toxicity in aquatic environments. Environ Pollut 343:123219. https://doi.org/10.1016/j.envpol.2023.123219
Zhong T, Lin T, Zhang X, Jiang F, Chen H (2023) Impact of biological activated carbon filtration and backwashing on the behaviour of PFASs in drinking water treatment plants. J Hazard Mater 446:130641. https://doi.org/10.1016/j.jhazmat.2022.130641
Wu J, Shen Z, Hua Z, Gu L (2023) Nitrogen addition enhanced Per-fluoroalkyl substances’ microbial availability in a wheat soil ecosystem. Chemosphere 320:138110. https://doi.org/10.1016/j.chemosphere.2023.138110
Pan H, Zhao X, Zhou X, Yan H, Han X, Wu M, Chen F (2023) Research progress on the role of biofilm in heavy metals adsorption-desorption characteristics of microplastics: a review. Environ Pollut 336:122448. https://doi.org/10.1016/j.envpol.2023.122448
Wang Y, Wang X, Li Y, Li J, Wang F, Xia S, Zhao J (2020) Biofilm alters tetracycline and copper adsorption behaviors onto polyethylene microplastics. Chem Eng J 392:123808. https://doi.org/10.1016/j.cej.2019.123808
Li Y, Wang X, Wang Y, Sun Y, Xia S, Zhao J (2022) Effect of biofilm colonization on Pb(II) adsorption onto poly(butylene succinate) microplastic during its biodegradation. Sci Total Environ 833:155251. https://doi.org/10.1016/j.scitotenv.2022.155251
Hoellein TJ, Rojas MG, Adam P, Gasior J, Kelly JJ (2014) Anthropogenic litter in urban freshwater ecosystems: distribution and microbial interactions. PLoS ONE 9(6):e98485. https://doi.org/10.1371/journal.pone.0098485
Weig A, Löder MGJ, Ramsperger A, Laforsch C (2021) In situ prokaryotic and eukaryotic communities on microplastic particles in a small headwater stream in Germany. Front Microbiol. https://doi.org/10.3389/fmicb.2021.660
Ayush PT, Ko J, Oh H (2022) Characteristics of initial attachment and biofilm formation of Pseudomonas aeruginosa on microplastic surfaces. Appl Sci 12(10):5245. https://doi.org/10.3390/app12105245
Dharmaraj I, Appavoo MS (2022) Occurrence of coliforms in microplastic associated biofilm in estuarine ecosystem. Pol J Environ Stud 32(1):547–557. https://doi.org/10.15244/pjoes/153970
Richard H, Carpenter E, Komada T, Palmer PT, Rochman CM (2019) Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci Total Environ 683:600–608. https://doi.org/10.1016/j.scitotenv.2019.04.331
Niu L, Hu J, Li Y, Wang C, Zhang W, Hu Q, Wang L, Zhang H (2022) Effects of long-term exposure to silver nanoparticles on the structure and function of microplastic biofilms in eutrophic water. Environ Res 207:112182. https://doi.org/10.1016/j.envres.2021.112182
Vedolin MC, Teophilo C, Turra A, Figueira RCL (2018) Spatial variability in the concentrations of metals in beached microplastics. Mar Pollut Bull 129(2):487–493. https://doi.org/10.1016/j.marpolbul.2017.10.019
Hu S, Zhou Y, Zhou L, Huang Y, Zeng Q (2018) Study on the adsorption behavior of cadmium, copper, and lead ions on the crosslinked polyethylenimine dithiocarbamate material. Environ Sci Pollut Res 27(3):2444–2454. https://doi.org/10.1007/s11356-018-3536-3
Johansen MP, Prentice E, Cresswell T, Howell NR (2018) Initial data on adsorption of Cs and Sr to the surfaces of microplastics with biofilm. J Environ Radioact 190–191:130–133. https://doi.org/10.1016/j.jenvrad.2018.05.001
Zhang W, Zhang L, Tian H, Yong-Gan L, Zhou X, Wang W, You Z, Wang S, Li M (2020) The mechanism for adsorption of Cr(VI) ions by PE microplastics in ternary system of natural water environment. Environ Pollut 257:113440. https://doi.org/10.1016/j.envpol.2019.113440
Liu Z, Adyel TM, Miao L, You G, Liu S, Hou J (2021) Biofilm influenced metal accumulation onto plastic debris in different freshwaters. Environ Pollut 285:117646. https://doi.org/10.1016/j.envpol.2021.117646
Zhao H, Li P, Su F, He X, Elumalai V (2022) Adsorption behavior of aged polybutylece terephthalate microplastics coexisting with Cd(II)-tetracycline. Chemosphere 301:134789. https://doi.org/10.1016/j.chemosphere.2022.134789
Velzeboer I, Kwadijk C, Koelmans AA (2014) Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ Sci Technol 48(9):4869–4876. https://doi.org/10.1021/es405721v
Wang F, Shih K, Li XY (2015) The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 119:841–847. https://doi.org/10.1016/j.chemosphere.2014.08.047
Guo X, Wang X, Zhou X, Kong X, Tao S, Xing B (2012) Sorption of four hydrophobic organic compounds by three chemically distinct polymers: role of chemical and physical composition. Environ Sci Technol 46(13):7252–7259. https://doi.org/10.1021/es301386z
Hüffer T, Hofmann T (2016) Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ Pollut 214:194–201. https://doi.org/10.1016/j.envpol.2016.04.018
Zhang H, Wang J, Zhou B, Yang Z, Dai Z, Zhou Q, Chriestie P, Luo Y (2018) Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: kinetics, isotherms and influencing factors. Environ Pollut 243:1550–1557. https://doi.org/10.1016/j.envpol.2018.09.122
Zhao Y, Gao J, Wang Z, Cui Y, Zhang Y, Dai H, Li D (2022) Distinct bacterial communities and resistance genes enriched by triclocarban-contaminated polyethylene microplastics in antibiotics and heavy metals polluted sewage environment. Sci Total Environ 839:156330. https://doi.org/10.1016/j.scitotenv.2022.156330
Li H, Wang F, Li J, Deng S, Zhang S (2021) Adsorption of three pesticides on polyethylene microplastics in aqueous solutions: kinetics, isotherms, thermodynamics, and molecular dynamics simulation. Chemosphere 264:128556. https://doi.org/10.1016/j.chemosphere.2020.128556
Liu G, Zhu Z, Yang Y, Sun Y, Yu F, Ma J (2019) Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ Pollut 246:26–33. https://doi.org/10.1016/j.envpol.2018.11.100
Steinman AD, Scott JW, Green LA, Partridge C, Oudsema M, Hassett M, Kindervater E, Rediske RR (2020) Persistent organic pollutants, metals, and the bacterial community composition associated with microplastics in Muskegon Lake (MI). J Great Lakes Res 46(5):1444–1458. https://doi.org/10.1016/j.jglr.2020.07.012
Porter A, Lyons BP, Galloway TS, Lewis C (2018) Role of marine snows in microplastic fate and bioavailability. Environ Sci Technol 52(12):7111–7119. https://doi.org/10.1021/acs.est.8b01000
Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J (2016) Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci 178:189–195. https://doi.org/10.1016/j.ecss.2015.12.003
Wang Z, Gao J, Zhao Y, Dai H, Jia J, Zhang D (2021) Plastisphere enrich antibiotic resistance genes and potential pathogenic bacteria in sewage with pharmaceuticals. Sci Total Environ 768:144663. https://doi.org/10.1016/j.scitotenv.2020.144663
Brandon J, Goldstein M, Ohman MD (2016) Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar Pollut Bull 110(1):299–308. https://doi.org/10.1016/j.marpolbul.2016.06.048
Auta H, Emenike C, Fauziah S (2017) Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ Pollut 231:1552–1559. https://doi.org/10.1016/j.envpol.2017.09.043
Funding
This research was funded by the Science and Technology Research Project of Chongqing Municipal Education Commission (Project No. KJQN202200704), National Engineering Research Center for Inland Waterway Regulation (Project No. SLK2021B08), Science and Technology Research Project of Chongqing Municipal Education Commission (Project No. KJQN202000743), Construction Project of Chongqing Joint Training Base (JDLHPYJD2022005).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, and supervision, Y.Q.; writing—original draft and reviewing, Y.T.; investigation and methodology, F.W.; investigation and visualization, C.C.; visualization, Y.Y.; investigation, Y.H. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, Y., Tu, Y., Chen, C. et al. Biofilms on microplastic surfaces and their effect on pollutant adsorption in the aquatic environment. J Mater Cycles Waste Manag (2024). https://doi.org/10.1007/s10163-024-02066-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10163-024-02066-7