Abstract
A two-step enrichment procedure led to the isolation of a strain of Rhodococcus ruber (C208) that utilized polyethylene films as sole carbon source. In liquid culture, C208 formed a biofilm on the polyethylene surface and degraded up to 8% (gravimetrically) of the polyolefin within 30 days of incubation. The bacterial adhesion to hydrocarbon assay and the salt aggregation test both showed that the cell-surface hydrophobicity of C208 was higher than that of three other isolates which were obtained from the same consortium but were less efficient than C208 in the degradation of polyethylene. Mineral oil, but not nonionic surfactants, enhanced the colonization of polyethylene and increased biodegradation by about 50%. Fluorescein diacetate (FDA) hydrolysis and protein content analysis were used to test the viability and biomass density of the C208 biofilm on the polyethylene, respectively. Both FDA activity and protein content of the biofilm in a medium containing mineral oil peaked 48–72 h after inoculation and then decreased sharply. This finding apparently reflected rapid utilization of the mineral oil adhering to the polyethylene. The remaining biofilm population continued to proliferate moderately and presumably played a major role in biodegradation of the polyethylene. Fourier transform infrared spectra of UV-photooxidized polyethylene incubated with C208 indicated that biodegradation was initiated by utilization of the carbonyl residues formed in the photooxidized polyethylene
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past three decades, non-biodegradable plastic materials have replaced biodegradable products in a variety of applications. The drastic rise in the use of plastic materials has not been accompanied by a corresponding development of procedures for the safe disposal or degradation of these materials. As a consequence, plastic wastes accumulating in the environment pose an ever increasing ecological threat to terrestrial and marine wildlife.
The most problematic plastic, in this regard, is probably polyethylene, which—being resistant to microbial attack—is one of the most inert synthetic polymers. In a long-term study of the biodegradation of 14C-labeled polyethylene, Albertsson and Karlsson (1990) found that polyethylene subjected to 26 days of artificial UV irradiation before being buried in soil evolved less than 0.5% carbon (as CO2) by weight after 10 years. Without prior irradiation, less than 0.2% carbon dioxide was produced. Similarly, a polyethylene sheet that had been kept in contact with moist soil for a period of 12 years showed no evidence of biodeterioration (Potts 1978). Only partial degradation was observed in a film of polyethylene that had been buried in soil for as long 32 years (Otake et al. 1995). Some studies have, however, demonstrated partial biodegradation of polyethylene after shorter periods of time. These studies showed that UV photooxidation (Cornell et al. 1984), thermal oxidation (Albertsson et al. 1998; Volke-Sepulveda et al. 2002) or chemical oxidation with nitric acid (Brown et al. 1974) of polyethylene prior to its exposure to a biotic environment did enhance biodegradation. Furthermore, Albertsson et al. (1987) reported a synergistic effect between photooxidation and the biodegradation of polyethylene. It has been suggested that the biodegradation of polyethylene is enhanced by oxidation pretreatment, which increases surface hydrophilicity by the formation of carbonyl groups that can be utilized by microorganisms (Albertsson 1978, 1980; Cornell et al. 1984).
Most studies on the biodegradation of polyethylene are based on natural soil as the biotic environment, but some studies have used axenic bacterial or fungal cultures amended with polyethylene. Albertsson et al. (1998), for example, studied the biodegradation of thermooxidized polyethylene incubated with Arthrobacter paraffineus for 3.5 years. They showed that oxidation of the polyethylene resulted in the formation of organic acids, most of which could not be detected at the end of the study, presumably due to utilization by the bacteria. The incubation with A. paraffineus resulted in a small increase in the molecular weight of the polyethylene, apparently due to the consumption of lower-molecular-weight fragments by the bacteria (Albertsson et al. 1998). In contrast, Yamada-Onodera et al. (2001) reported a small decrease in the molecular weight of oxidized (a combination of thermooxidation and chemical oxidation) polyethylene compared with that of unoxidized polyethylene, both of which had been incubated with Penicillium simplicissimum for 3 months. It may well be that some microorganisms are indeed capable of degrading the high-molecular-weight polymer (Yamada-Onodera et al. 2001), as was evident from a recent report on the biodegradation of thermooxidized polyethylene by P. pinophilum (Volke-Sepulveda et al. 2002). Although the rate of mineralization of the polymer by the latter fungus was very low (0.37% after 31 months), it improved when the cultures containing polyethylene were amended with ethanol, presumably due to co-metabolism of polyethylene and ethanol, which facilitated the biodegradation of polyethylene (Volke-Sepulveda et al. 2002).
In most habitats—both natural and artificial—the majority of microbial populations form biofilms on solid surfaces (Atkinson and Fowler 1974). In many cases, the metabolic activity of the microbial populations that form the biofilm is higher than that of suspended bacteria (Kirchman and Mitchell 1982). When the solid surface also serves as the substrate (as in the case of polyethylene challenged by microbial populations), it is clear that carbon availability is greater in a biofilm. However, the hydrophobicity of polyethylene constitutes an obstacle that interferes with colonization and biofilm formation. In an attempt to overcome this obstacle to biodegradation, Albertsson et al. (1993) added a nonionic surfactant (Tween 80) to the culture medium of Pseudomonas aeruginosa. The surfactant apparently increased the hydrophilicity of the polyethylene surface and thus facilitated the adhesion of bacteria to the polymer.
In the present study, we used a two-step enrichment technique to isolate polyethylene-degrading bacteria: the first enrichment was performed in soil amended with polyethylene and the second in a synthetic medium (SM) containing polyethylene as the sole carbon source. This protocol yielded a Rhodococcus strain (C208) capable of forming a biofilm on the polyethylene. In the course of the study, we characterized the cell-surface hydrophobicity of Rhodococcus and tested the biofilm formation and biodegradation of polyethylene by this bacterium.
Materials and methods
Polyethylene
Films of branched low-density (0.92 g cm−3) polyethylene (Ipiten 111) with an average molecular weight of 191,000 (Carmel Olefins, Haifa, Israel), containing a UV photosensitizer (undisclosed compound) and designated L0235 (Plastopil, Hazorea, Israel), were kindly provided by Mr. R. Harpaz of Plastopil.
UV irradiation of polyethylene
To induce partial photolysis of the polyethylene film and to simulate natural weathering of polyethylene exposed to the sun (e.g., polyethylene used for soil mulching or greenhouse cover), polyethylene samples were treated in a QUV accelerated weathering tester (Q-Panel, Cleveland, Ohio). The polyethylene was subjected to a program of alternating exposure to UV and humidity: five cycles per day of UV exposure (four of 4 h each, one of 3 h at 70 °C) separated by 1-h intervals (50 °C) during which time water condensed on the polyethylene surface. The overall cumulative UV irradiation time to which the polyethylene samples were exposed was 60 h. Prior to transfer to liquid media, the polyethylene films were cut into pieces (about 3×3 cm each), weighed, disinfected in 95% ethanol and air-dried for 15 min in a laminar-flow hood. Unless otherwise specified, all experiments were carried out with UV-irradiated polyethylene.
Bacterial culture media
Bacterial strains assayed for their ability to utilize polyethylene as the sole source of carbon and energy were grown in a minimal SM containing (per liter of distilled water): 1.0 g NH4NO3, 0.2 g MgSO4·7H2O, 1.0 g K2HPO4, 0.1 g CaCl2·2H2O, 0.15 g KCl, 0.1 g yeast extract (Difco) and 1.0 mg of each of the following microelements: FeSO4·6H2O, ZnSO4·7H2O and MnSO4. In some experiments, mineral oil (light white oil, d=0.84 g l−1; Difco) was added to the medium. In other experiments, nonionic surfactants (Tween 60 or Tween 80) were added to SM medium at a concentration of 0.01–0.50% to test the effect of a surfactant on the colonization of polyethylene by bacteria.
Bacterial cultures were maintained on nutrient broth or nutrient agar (Difco). Unless otherwise specified, liquid cultures (100 ml) were incubated in flasks (250 ml) on a rotary shaker (150 rpm) at 30 °C.
Enrichment and isolation of polyethylene-degrading bacteria
A two-step enrichment procedure aimed at the isolation of polyethylene-degrading bacteria was used: the initial enrichment was carried out in soil and the second in liquid SM. Soil samples were obtained from 15 sites at which polyethylene waste from agricultural use (mainly films for soil mulching) had been buried. Soil samples, 10 g each, were placed in test tubes containing 4 ml of SM and about 300 mg of polyethylene film. The test tubes were incubated for 12 weeks at 30 °C, after which time the polyethylene samples were removed from the soil, washed, dried at 60 °C and weighed. In the second step, flasks containing 50 ml of SM were amended with polyethylene films and, as the inoculum source, 1 g of soil from the samples in which degradation of the polyethylene had been observed. The cultures were incubated at 30 °C for 30 days and the dry weight of the polyethylene samples was determined. Pure bacterial cultures were then isolated from mixed cultures in which weight loss of the polyethylene had been observed. The isolated strains were tested for their ability to degrade polyethylene, compared with the bacterial consortia from which the single isolates had originated.
Removal of bacterial biofilm from the polyethylene surface
To facilitate accurate measurement of the weight of the residual polyethylene, the bacterial biofilm was washed off the polyethylene surface with 2% sodium dodecyl sulfate (SDS) for 4 h, followed by washing with distilled water. Residual polyethylene from cultures containing mineral oil was treated with chloroform prior to the SDS washing to remove the oil.
Identification of strain C208
Total genomic DNA for 16S rDNA amplification was isolated from cells grown to the late log phase by means of a standard protocol (Ausubel et al. 1992). Amplification of the 5′ end of the gene 16S rDNA was performed with universal primers from Escherichia coli: forward primer 8-F (5′-AGAGTTTGATYMTGGCTCAG-3′) and reverse primer: 1942-R (5′-GGTTACCTTGTTACGACTT-3′). The homology of the obtained sequence with other 16S rDNA sequences from closely related bacteria was tested with BLASTN ver. 2.2.1 (Altschul et al. 1997).
Evaluation of bacterial hydrophobicity
Two methods were used to determine bacterial cell-surface hydrophobicity: the bacterial adhesion to hydrocarbon (BATH) test (Rosenberg et al. 1980) and the salt aggregation test (SAT; Lindahl et al. 1981). The BATH assay for bacterial hydrophobicity is based on the affinity of bacterial cells for an organic hydrocarbon such as hexadecane. The more hydrophobic the bacterial cells, the greater their affinity for the hydrocarbon, resulting in a transfer of cells from the aqueous suspension to the organic phase and a consequent reduction in the turbidity of the culture. For the BATH test, bacteria were cultured in NB medium until the mid-logarithmic phase, centrifuged, and washed (twice) with PUM buffer containing (per liter): 17 g K2HPO4, 7.26 g KH2PO4, 1.8 g urea and 0.2 g MgSO4·7H2O. The washed cells were resuspended in PUM buffer to an optical density at 400 nm (OD400) value of 1.0–1.2. Aliquots (1.2 ml each) of this suspension were transferred to a set of test tubes, to which were added increasing volumes (range 0–0.2 ml) of hexadecane. The test tubes were shaken for 10 min and allowed to stand for 2 min to facilitate phase separation. The OD400 of the aqueous suspension was measured. Cell-free buffer served as the blank.
The SAT method is based on the phenomenon of “salting out” of bacterial cells in a salt solution: the higher the cell-surface hydrophobicity, the lower the salt concentration required to induce cell aggregation and precipitation. Ammonium sulfate solutions (0–4.0 M) were prepared in 0.002 M sodium phosphate buffer, pH 6.8. Logarithmic-stage bacterial cells grown in NB (10 ml) were pelleted by centrifugation and resuspended in 10 ml of the sodium phosphate buffer. Droplets of the bacterial suspension were gently mixed on a glass slide with droplets of each salt solution. The level of aggregation was compared with that in 4.0 M ammonium sulfate (positive control) and that in ammonium sulfate-free buffer (negative control).
Quantitative estimation of bacterial biomass in biofilms colonizing the polyethylene surface
Since the bacterial biofilm was strongly attached to the polyethylene surface, it was impossible to estimate the population density by standard techniques, such as direct cell-counting or plating. Therefore, the population density of the biofilm on the polyethylene surface was estimated by determination of protein concentration. Pieces taken from polyethylene film colonized in SM or SM plus 0.05% mineral oil were washed briefly in water and then boiled for 30 min in 5 ml of 0.5 N NaOH. The suspension was centrifuged, the supernatant was saved and the pellet was subjected to the same procedure once again. The two supernatants were combined, the protein concentration was determined in each supernatant according to Sedmak and Grossberg (1977) and the values were then combined.
Viability of the bacterial biofilm
The viability and activity of the bacterial biofilm was determined indirectly by measuring the hydrolysis of fluorescein diacetate (FDA) to fluorescein according to Schnurer and Rosswall (1982), as follows. A piece of polyethylene film was transferred from SM (with or without 0.05% mineral oil) cultures of C208 to a flask containing 60 ml of sodium phosphate buffer, 60 mM, pH 7.6. A solution of FDA in acetone, 0.3 ml, was added to give a final concentration of 10 µg ml−1. The flasks were shaken at 140 rpm at 30 °C and 1-ml aliquots were withdrawn at various times during the incubation. The samples were centrifuged at 17,000 g and read in a spectrophotometer at 494 nm. Samples without FDA served as blanks and a sample of polyethylene from a sterile SM medium served as a control.
Scanning electron microscopy for biofilm analysis
Polyethylene samples (1×1 cm) colonized with a biofilm of C208 were removed from the medium and dried in a desiccator for 24 h under vacuum. The samples were vapor-fixed at room temperature for 3 days in a sealable glass container containing two beakers, one with 10 ml of 25% glutaraldehyde in H2O and the other with 5 ml of 5% OsO4 in 0.1 M phosphate buffer, pH 7.0. After fixation, the container was aerated for 20 h. The samples were gold-coated in deep vacuum and visualized by scanning electron microscopy (SEM), using a JEOL JSM-35CF (JEOL, Japan).
Fourier transform infrared analysis of polyethylene
Changes in the polyethylene structure following UV irradiation and subsequent incubation with bacteria were analyzed by attenuated total reflectance (ATR)–Fourier transform infrared (FTIR) (Impact 410; Nicolet, USA), using the ATR 350 method with a ZnSe crystal. Three types of polyethylene sample were analyzed: (1) untreated, (2) UV-irradiated (as described above), (3) UV-irradiated and incubated with bacteria.
Results
Isolation and identification of strain C208
The two-step enrichment cultures gave rise to a mixture of bacteria capable of growing in liquid SM containing polyethylene as the sole carbon source. Since the composition of mineral oil resembles that of short polyolefin oligomers, we screened the above-mentioned bacterial mixture on plates containing SM supplemented with 2% mineral oil. A number of isolates obtained from this bacterial mixture were capable of growing on the mineral-oil-supplemented medium. Among these, a gram-positive strain, designated C208, exhibited the fastest growth. When inoculated into liquid SM containing polyethylene as the sole carbon source, C208 colonized the polyethylene surface within a few days. After 30 days of incubation, the polyethylene lost about 8.0% of its initial dry weight. Based on 16S rRNA sequence homology, the bacterium was identified as R. ruber strain C208 (data not shown).
Bacterial hydrophobicity
The BATH assay clearly showed the higher hydrophobicity of strain C208 compared with that of C332, B334 or E478 (Fig. 1). The latter three isolates were obtained from the same consortium and were capable of growing in SM containing polyethylene as the sole carbon source, but they were less efficient than C208 in degrading the polyethylene (data not shown). For C208, the adhesion of bacterial cells to hexadecane was evident even at the lowest concentration of the hydrocarbon, resulting in a reduction of more than 20% in the turbidity of the culture. Logarithmic cells were more hydrophobic than those taken from the stationary phase. An evaluation of the cell hydrophobicity of these isolates with the SAT method gave similar results: Table 1 shows that strain C208 is very hydrophobic, since it aggregated in aqueous solution, even without the addition of salt. The other three strains were less hydrophobic, since aggregation started at higher salt concentrations (Table 1).
Hydrophobicity of bacterial isolates determined by the bacterial adhesion to hydrocarbon test. Aliquots of logarithmic (Log.) and stationary (Stat.) cell suspensions were supplemented with increasing concentrations of hexadecane. The transfer of hydrophobic cells from the aqueous phase to the hexadecane is reflected as a decrease in the turbidity (optical density at 600 nm, O.D. 600 ) of the bacterial suspension
Effect of mineral oil and nonionic surfactants on colonization of polyethylene by C208
Experiments to investigate whether polyethylene colonization and biofilm formation by strain C208 could be promoted by the addition to the culture medium of mineral oil or surfactants showed that mineral oil improved the colonization, but the nonionic surfactants Tween 60 and Tween 80 had a negative effect on biofilm formation (Table 2).
The effect of mineral oil on the colonization of polyethylene samples by C208 is shown in SEM photomicrographs (Fig. 2). In the absence of mineral oil, only sparse colonization was observed after 16 h of incubation (Fig. 2A), while after the same incubation period in a medium amended with 0.05% mineral oil, significant colonization of the polyethylene surface was obtained (Fig. 2B). Analysis of samples incubated for 1 week in the absence of mineral oil showed the formation of a uniform bacterial biofilm, whereas incubation in a mineral-oil-amended medium resulted in a multi-layer dense biofilm (Fig. 2C, D).
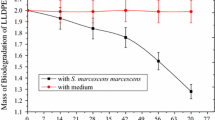
Similarly, the addition of mineral oil to the medium markedly enhanced the biodegradation of polyethylene by C208 (Fig. 3). A maximal increase (about 50% compared with mineral-oil-free medium) in weight loss of the polyethylene was obtained at a concentration of 0.05% mineral oil. Higher concentrations of mineral oil had a smaller effect on the biodegradability of the polymer, probably because the oil itself was readily utilized by C208 as a carbon source (Fig. 3).
When the FDA assay was applied to two biofilms of C208, one small (10 cm2) and one large (30 cm2) as an indirect measure of biofilm viability and metabolic activity, the difference in the rate of FDA hydrolysis by the biofilms was proportional to the difference in their areas (Fig. 4).
Hydrolysis of fluorescein diacetate (FDA) as an assay for the estimation of biofilm viability and metabolic activity. The production of fluorescein (as a result of FDA hydrolysis by extracellular esterases produced by biofilms of R. ruber C208 formed on the surface of polyethylene) is shown as a function of time for two bacterial biofilms of different sizes (30 cm 2, 10 cm 2)
Analysis of the protein content of the biofilm was performed to monitor the biomass density and rate of colonization of the polyethylene by C208 (Fig. 5). The results followed a pattern similar to that of the viability of the biofilm measured by the FDA assay. Both FDA hydrolysis activity and protein content increased rapidly after inoculation, peaking during days 2–3 of incubation and then decreasing sharply to minimal values (day 8 after inoculation). The residual low-level biofilm population was viable, as indicated by FDA-hydrolyzing activity. This biofilm population showed a moderate increase in protein content and FDA hydrolysis during the following 3 weeks of incubation (Fig. 5). The steep decline in protein content and FDA hydrolysis suggests that the fast-proliferating population of C208 (which adheres to the polyethylene surface during the early stage of incubation) utilized the mineral oil as a carbon source. Once the mineral oil had been consumed, a slow and constantly proliferating biofilm developed on the polyethylene.
Effect of UV irradiation and biodegradation on the structure of polyethylene
The FTIR spectrum of UV-photooxidized polyethylene showed the typical carbonyl peak at 1,712 cm−1. Incubation of the photooxidized polyethylene with C208 for 30 days resulted in a marked reduction in the amount of carbonyl residues (Fig. 6). To quantify this reduction, the carbonyl index (the ratio between the absorbance peak of the carbonyl groups and the average between the two CH2 peaks at 1,460 cm−1 and 1,470 cm−1) was calculated (Table 3). The data showed that incubation of UV-irradiated polyethylene with C208 reduced the carbonyl index by 66%. Similarly, the terminal double-bond index (the ratio between the absorbance peak at 910 cm−1 and the two CH2 peaks) showed a reduction of about 20% in the UV-irradiated polyethylene treated with C208, compared with the irradiated but untreated polyethylene (Table 3).
Discussion
A two-step culture-enrichment protocol was employed to isolate soil bacteria capable of growing on polyethylene as the sole carbon source. One such isolate, C208 (identified as a strain of R. ruber), from a polyethylene-waste burial site colonized the polyethylene surface and degraded up to 8% of the initial dry weight of the polyethylene in as little as 4 weeks. This biodegradation rate is higher than the rates reported previously for polyethylene incubated in soil, which ranged from 3.5% to 8.4% after 10 years (Albertsson and Karlsson 1990; Potts 1978; Yabannavar and Bartha 1994). These low rates are in agreement with the argument of Otake et al. (1995) that 10 years is a relatively short period for the biodegradation of synthetic polymers such as polyethylene.
Since we used photooxidized polyethylene, it was not surprising to find the typical carbonyl peak in the FTIR spectrum. The amount of carbonyl residues in the polyethylene decreased after incubation with C208, due to their utilization by the bacterium. This finding is in accordance with the biodegradation mechanism suggested by Albertsson et al. (1987) of a synergistic effect between photooxidation and the biodegradation of polyethylene. However, in contrast with the study of Albertsson et al. (1987), who reported on the formation of terminal double bonds only in samples exposed to a biotic environment, we found an increase in the amount of terminal double bonds after photooxidation of the polyethylene. This could have resulted from a Norish type I degradation of the carbonyl residues. The decrease in the amount of double bonds obtained after incubation with C208 may be explained by the degradation of short polyethylene oligomers produced during photooxidation.
Microbial degradation of a solid polymer, such as polyethylene, requires the formation of a biofilm on the surface of the polymer to enable the microorganism to efficiently utilize the non-soluble substrate. Indeed, C208 effectively colonized the polyethylene surface. This may explain the relatively rapid biodegradation of polyethylene, which was evident (as measured by weight loss and FTIR) as early as 2 weeks after inoculation. In contrast, three other isolates (C332, B334, E478) obtained from the same consortium as C208 were less hydrophobic, did not produce a significant biofilm and were less efficient in degrading polyethylene.
The hydrophobicity of polyethylene usually interferes with bacterial adhesion to the surface since most bacterial surfaces are hydrophilic. Surprisingly, the addition of mineral oil to the culture of C208 increased both colonization and biodegradation of polyethylene, while the addition of a nonionic surfactant (Tween 60, Tween 80) had no effect. This finding indicates that hydrophobic interactions between C208 and the polyethylene are more dominant than hydrophilic interactions mediated by nonionic surfactants. Indeed, hydrophobicity assays of C208 showed that its cell surface is very hydrophobic. In contrast, it was previously shown that the interaction of other microorganisms with polyethylene depends on the presence of nonionic surfactants in the medium. Albertsson et al. (1993) showed that Tween 80 increased the adhesion and biodegradation of polyethylene by Pseudomonas aeruginosa and Yamada-Onodera et al. (2001) reported that the nonionic surfactant Triton X-100 improved the growth of Penicllium simplicissimum in a medium containing polyethylene without being utilized by the fungus.
The addition of mineral oil to SM for C208 improved the degradation of the polyethylene film by about 50% after 4 weeks of incubation. In contrast, Albertsson and Karlsson (1993) reported that high-density polyethylene (HDPE), with or without paraffin, showed the same level of degradation after 4 years of incubation in a biotic environment. This could have resulted from a rapid consumption of the paraffin, which therefore had no effect on overall biodegradation during the 4-year period.
It has long been argued that polyethylene cannot be considered a biodegradable polymer as long as enzymes that catalyze the degradation of high-molecular-weight olefins to yield a measurable product have not been identified (Griffin 1983). At present, 20 years later, such enzymes have still not been identified. However, our study provides an indication of the presence of enzymatic activity that might affect polyethylene. Our SEM photomicrographs of the bacterial biofilm showed some localized degradation of the polyethylene around the bacterial cells in the biofilm, forming a cell-like molded pattern in the polyethylene. Such patterns have previously been observed for biodegradable polymers, e.g., poly-β-hydroxybutyrate (Otake et al. 1995).
Protein assays and FDA hydrolysis by extracellular esterases proved to be efficient tools for determining the state of polyethylene colonization and biofilm formation (Fig. 6). Both methods provided strong evidence of rapid colonization of the polyethylene during the first 2 days of incubation, followed by a sharp decrease in biomass density. It seems that the rapid early colonization of the polyethylene was due to utilization of the mineral oil adhering to the polyethylene surface as a carbon source. Presumably, once the mineral oil was completely consumed, most of the biomass in this high-density, multi-layered biofilm did not have access to the polyethylene surface and was washed off into the medium. Thus, it may be hypothesized that a low-density population, with a low growth rate—consisting of cells able to utilize polyethylene as a carbon source—is responsible for the biodegradation of the polyethylene. Similarly, Albertsson and Karlsson (1993) indicated that biodegradation of HDPE is limited in the presence of mineral oil, which is preferentially utilized as a carbon source.
The biodegradation of polyethylene by a pure culture obtained via enrichment and selection, as opposed to that obtained by non-selected microbial populations (in soil microcosms or in axenic cultures in the laboratory), enables one to obtain an organism better adapted to the biodegradation of the target polymer.
References
Albertsson AC (1978) Biodegradation of synthetic polymers. 2. Limited microbial conversion of C-14 in polyethylene to (CO-2)-C-14 by some soil fungi. J Appl Polym Sci 22:3419–3433
Albertsson AC (1980) The shape of the biodegradation curve for low and high density polyethylenes in prolonged series of experiments. Eur Polym J 16:623–630
Albertsson AC, Karlsson S (1990) The influence of biotic and abiotic environments on the degradation of polyethylene. Prog Polym Sci 15:177–192
Albertsson AC, Karlsson S (1993) Aspects of biodeterioration of inert and degradable polymers. Int Biodeterior Biodegrad 31:161–170
Albertsson AC, Andersson SO, Karlsson S (1987) The mechanism of biodegradation of polyethylene. Polym Degrad Stabil 18:73–87
Albertsson AC, Sares C, Karlsson S (1993) Increased biodegradation of LDPE with nonionic surfactants. Acta Polym 44:243–246
Albertsson AC, Erlandsson B, Hakkarainen M, Karlsson S (1998) Molecular weight changes and polymeric matrix changes correlated with the formation of degradation products in biodegraded polyethylene. J Environ Polym Degrad 6:187–195
Altschul SF, Madden TL, Shaffer AA, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Atkinson B, Fowler HW (1974) The significance of microbial films in fermenters. Adv Biochem Eng 3:224–277
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1992) Short protocols in molecular biology, 2nd edn. Wiley, New York
Brown BS, Mills J, Hulse JM (1974) Chemical and biological degradation of plastics. Nature 250:161–163
Cornell JH, Kaplan AM, Rogers MR (1984) Biodegradation of photooxidized polyalkylenes. J Appl Polym Sci 29:2581–2597
Griffin GJL (1983) Conference on Polyethylenes 1933–1983. Plastic and Rubber Institute, London
Kirchman D, Mitchell R (1982) Contribution of particle-bound bacteria to total microheterotrophic activity in five ponds and two marshes. Appl Environ Microbiol 43:200–209
Lindahl M, Faris A, Wadstrom T, Hjerten SA (1981) A new test based on “salting out” to measure relative hydrophobicity of bacterial cells. Biochem Biophys Acta 677:471–476
Otake Y, Kobayashi T, Ashabe H, Murakami N, Ono K (1995) Biodegradation of low-density polyethylene, polystyrene, polyvinyl-chloride, and urea-formaldehyde resin buried under soil for over 32 years. J Appl Polym Sci 56:1789–1796
Potts JE (1978) Biodegradation. In: Jelinek HHG (ed) Aspects of degradation and stabilization of polymers. Elsevier, New York, pp 617–658
Rosenberg M, Gutnik D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell surface hydrophobicity. FEMS Microbiol Lett 9:29–33
Schnurer E, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 43:1256–1261
Sedmak JJ, Grossberg SE (1977) A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem 79:544–552
Volke-Sepulveda T, Saucedo-Castaneda G, Gutierrez-Rojas M, Manzur A, Favela-Torres E (2002) Thermally treated low density polyethylene biodegradation by Penicillium pinophilum and Aspergillus niger. J Appl Polym Sci 83:305–314
Yabannavar AV, Bartha R (1994) Methods for assessment of biodegradability of plastic films in soil. Appl Environ Microbiol 60:3608–3614
Yamada-Onodera K, Mukumoto H, Katsuyaya Y, Saiganji A, Tani Y (2001) Degradation of polyethylene by a fungus, Penicillium simplicissimum YK. Polym Degrad Stabil 72:323–327
Acknowledgements
The authors thank Mr. R. Harpaz of Plastopil, Hazorea, Israel, for providing the polyethylene for this study and V. Pavlov for technical assistance. This work was partially funded by the Ministry for the Environment. The experiments described in this article comply with the current laws of Israel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
(Orr), I.G., Hadar, Y. & Sivan, A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber . Appl Microbiol Biotechnol 65, 97–104 (2004). https://doi.org/10.1007/s00253-004-1584-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1584-8