Abstract
We have developed the hybrid jig which combines the principles of jig separation and flotation. However, the selectivity of bubble attachment in water was poor because most plastics have inherently hydrophobic surfaces; so, development of surface modification techniques for plastic particles would expand the application of hybrid jig to the material recycling of plastics. In this study, hybrid jig separation of polypropylene using glass fiber and high impact polystyrene having similar specific gravities and surface wettability were investigated with three wetting agents [Di-2-ethylhexyl sodium sulfosuccinate (Aerosol OT, AOT), sodium lignin sulfonate, and tannic acid]. The results showed that the probability of bubble attachment was influenced by wetting agents because of their strong effects on the surface tension of solution and surface wettability of plastics. The results also suggest that wetting agents could be utilized to control the selectivity of bubble attachment and improve the hybrid jig separation efficiency. In addition, since the hybrid jig separation of polyvinyl chloride and polyamide (nylon-66) using AOT was imperfect, a two-step approach, composed of a pre-wetting step (first step) in a solution containing the wetting agent (AOT) and hybrid jig separation in water (second step), is proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The total global production of plastics has grown from around 1.5 million tons in 1950 to 348 million tons in 2017 and worldwide consumption has been increasing annually at a rate of 5% with the largest increase reported in Asia [1, 2]. There are two common ways to recycle plastics: (1) material recycling whereby plastic wastes are recovered for the production of new plastics, and (2) thermal recycling through which plastic wastes are used as fuel for power generation. Between the two, material recycling is more sustainable and profitable but requires exceptionally efficient separation of different types of plastics from mixed-plastic wastes to obtain products with very high purities (> 99.9%). Achieving this very high plastic purity in recycling is very difficult; so, mixed-plastic wastes are more commonly used in thermal recycling. In recent years, however, several studies have utilized mineral processing techniques to separate mixed-plastic wastes into their individual components to facilitate material recycling (e.g., gravity separation [3,4,5,6,7,8,9,10], dense medium separation [11,12,13], electrical separation [14,15,16], and flotation [12, 17,18,19,20,21,22,23,24,25,26,27,28]).
Flotation is a common and very efficient mineral processing technique for fine fraction (minerals: − 75 μm; coal: − 150 μm) because fine grinding is required (µm) to achieve sufficient liberation of target minerals [9, 29]. Most minerals have hydrophilic surfaces so a collector (e.g., xanthate and aerofloats [30]) is usually added to selectively change their surface wettability and enhance the separation efficiency. In contrast, plastic flotation is usually carried out with wetting agents (e.g., AOT [22], NaLS [22], CaLS [12, 22,23,24], TA [22, 25, 26], PVA [22, 26,27,28]) since most plastics have inherently hydrophobic surfaces. Plastic flotation is also very challenging because in resources recycling, especially plastics, sufficient liberation is already achieved at relatively coarse particle sizes (mm–cm) [30,31,32]. Moreover, additional size reduction (i.e., crushing and grinding) is required for flotation that requires more energy and incurs higher costs and energy [30].
One potentially effective technique for the separation of coarse plastics from mixed-plastic wastes is through the use of jigs. Jigs can separate coarse size fractions (+ 0.5 mm) based on differences in particle densities or specific gravities (SG) [7, 30, 33]. Among the various types of jigs, the TACUB (Takakuwa air chamber under bed) jig, commercially marketed as BATAC jig, is a popular and commonly used type of jig especially in coal cleaning because of its more uniform pressure distribution inside the separation chamber that provides higher separation efficiency. This uniform pressure distribution is facilitated by a series of pneumatically operated multiple air chambers, usually two cells under the separation chamber, that extend to its full width. Pressurized air is injected into the air chambers, causing water within the separation chamber to pulsate and induce stratification [3, 6, 30].
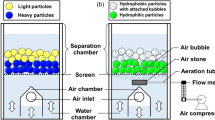
The TACUB jig was, however, ineffective for mixed-plastic wastes because SG differences of the various types of plastics in mixed-plastic wastes are very small. To address this problem, Tsunekawa et al. [3] developed the RETAC jig (R&E, Co., Ltd., Japan), an improved version of the TACUB jig, that facilitates plastic–plastic separation via precision control of the wave form during jig separation (Fig. 1a) [3, 4]. The RETAC jig, for example, successfully separated polystyrene (PS), acrylonitrile butadiene styrene (ABS), and polyethylene terephthalate (PET) from crushed copy machines [3]. Although effective, separation efficiency of the RETAC jig dramatically decreases when the density difference of plastics to be separated becomes small. Unfortunately, many types of plastics developed in recent years for identical applications have very similar SG. For example, two types of plastics used in the manufacture of large home appliances, polypropylene with glass fiber (PPGF) and high impact polystyrene (HIPS), have SG equal to 1.04. In theory, separation of materials with similar SG is impossible using gravity separation techniques like jigs.
A new type of jig called the hybrid jig was developed to address this limitation. The hybrid jig works by combining the principles of gravity separation and flotation, and could separate materials with similar SG as long as their surface wettability properties are different (Fig. 1b) [5]. Separation occurs due to the attachment of bubbles to more hydrophobic plastic particles that make them lighter than the less hydrophobic plastic particles, causing stratification during water pulsation.
In a previous study of the authors, hybrid jig separation of pure plastic mixtures [i.e., polyethylene (PE), polyethylene terephthalate (PET), polyvinyl chloride (PVC)] in water was carried out and good separation efficiency was reported [5]. However, the selectivity of bubble attachment in water for other plastics was poor because most plastics have inherently hydrophobic surfaces. This means that surface modification of plastics is required for the improvement of mixed-plastic waste separation using the hybrid jig; so in this study, the effects of wetting agents on bubble attachment probability and hybrid jig separation efficiency were investigated. In addition, a two-step approach to improve hybrid jig separation efficiency using wetting agents is proposed.
Materials
Samples
PPGF and HIPS obtained from a recycling facility of home appliances in Japan, and virgin plastic boards of polyvinyl chloride (PVC) and polyamide (nylon-66 or PA) were used in this study and the SG of these samples are listed in Table 1. The virgin plastic boards were crushed by an orient mill (VH16, Seishin Enterprise Co. Ltd., Japan) and screened to obtain suitable size fractions for the hybrid jig separation experiments.
Reagents
Methyl isobutyl carbinol (MIBC, Wako Pure Chemical Industries Ltd., Japan), a reagent widely utilized in flotation to stabilize bubble formation in solution, was used in the hybrid jig separation experiments. Three types of wetting agents were evaluated in this study: (1) Di-2-ethylhexyl sodium sulfosuccinate (Aerosol OT, AOT, Wako Pure Chemical Industries Ltd., Japan), (2) sodium lignin sulfonate (NaLS, Tokyo Chemical Industry Co. Ltd., Japan), and (3) tannic acid (TA, Wako Pure Chemical Industries, Ltd., Japan).
Experimental methods
Surface tension and contact angle measurements
Surface tension of solutions containing each of the wetting agents was measured using a temperature-controlled reaction vessel connected to a tensiometer (Krüss K100, Krüss GmbH, Germany). For the contact angle measurements, an air bubble generated from a syringe needle was introduced underneath the plastic sample submerged in solutions containing each of the wetting agents and the contact angle [angles between particle surface (solid–liquid interface) and bubble surface (gas–liquid interface)] (Fig. 2) was measured using a high-resolution digital microscope with image analysis capability (VHX-1000, Keyence Corporation, Japan).
Adsorption and desorption experiments
To obtain the adsorption isotherm of AOT on PA [34], adsorption experiments of AOT on PA were carried out. The particles of PA (25 g) were stirred (400 rpm) in AOT solutions (30, 50, 80, 100, 150, 180, 200, and 250 ppm) for 10 min and then, allowed to equilibrate for 120 min. The dissolved sulfur concentration remaining in solution was measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES, ICPE-9820, Shimadzu Corporation, Japan) (margin of error = ± 2%).
For desorption experiments, PA particles were put in 10-ppm AOT solution for 3 min and then washed with water. The particles were then put in another beaker filled with water and the dissolved sulfur concentration of solution was measured by ICP-AES after 30, 60, 90, and 120 min to calculate the desorption amount of AOT from PA.
Hybrid jig separation experiments
Figure 1b shows a schematic diagram of the desktop hybrid jig separation apparatus used in this study. This device is a modified desktop RETAC jig that has additional aeration tubes fitted with air stones installed under the separation chamber (145-mm long, 155-mm wide and 320-mm high) to generate air bubbles. The hybrid jig separation experiments were done by putting plastic samples and a solution (20 L) containing water, MIBC, and one of the wetting agents into the separation chamber (Table 2). Air bubbles were introduced underneath the screen simultaneously as water pulsation begun (Fig. 1b). Table 2 summarizes the conditions of hybrid jig separation experiments conducted in this study. The water pulsation was decided based on the previous work of Hori et al. [4]. After each run, the products were divided from the top into six layers (Fig. 1c) and then collected using a vacuum sampling system. Different plastics in each layer were separated by hand-picking to measure the purity of products.
Results and discussion
Separation of PPGF and HIPS using bubble attachment probability
PPGF is typically used for drum-type washing machines, while HIPS is a common type of plastic used in the manufacture of many home appliances because of its durability. Although the SG of PP is less than 1.0, addition of glass fiber to create the stronger PPGF increased the SG to 1.04, which is equal to that of HIPS. Because both of these types of plastics are common components of large home appliances, they are mixed during recycling and should be separated to improve material recycling efficiency.
Preliminary experiments to separate PPGF and HIPS using the hybrid jig without any wetting agent showed that separation was impossible because of the indiscriminate attachment of bubbles on both types of plastics. Figure 3a shows the hybrid jig experimental results for the PPGF–HIPS mixture (3.0–8.0 mm) in solutions containing 350 ppm of AOT, NaLS or TA. Purity in the top (1st) and bottom (6th) layers (Fig. 1c) with AOT (Fig. 3a-1) was lower than those with NaLS (Fig. 3a-2) and TA (Fig. 3a-3). With NaLS, the PPGF purity in the top layer and the HIPS purity in the bottom layer were 97% and 99%, respectively, and with TA, the PPGF purity in the top layer and the HIPS purity in the bottom layer were 99% and 86%, respectively. These results suggest that separation of these plastics is possible, and the type of wetting agent and their concentrations are important parameters to improve the efficiency of hybrid jig separation.
The effects of a wetting agent on particle–bubble interactions depend on tensile forces acting on the particle that leads to the development of an angle between the particle surface and the bubble surface [30]. At equilibrium,
where γs⁄a, γs⁄w, and γw⁄a are the surface energies between solid and air, solid and water, and water and air, respectively, and θ is the contact angle [angles between particle surface (solid–liquid interface) and bubble surface (gas–liquid interface)] (Fig. 1). The attachment of bubbles on the surface of particles is strongly dependent on the work of adhesion (Ws/a), which is defined as the force required to break the particle–bubble interface and is equal to the work needed to separate the solid–air interface and produce separate solid–air, air–water and solid–water interfaces (Eq. 2) [30].
The work of adhesion is further influenced by the surface wettability of the particle, determined by the contact angle (θ), and the surface tension of solution. More hydrophobic (i.e., larger contact angle) particles have larger work of adhesion when in contact with bubbles. Similarly, solutions with higher surface tension (γw⁄a) increase the work of adhesion between particle and bubble. Both the surface tension and surface wettability of particles could be changed by adding surface acting agents called surfactants [30], so measurements of the contact angles of PPGF and HIPS as well as the surface tension of solutions containing each of the wetting agents were carried out.
Figures 3b and 4 show the contact angles of PPGF and HIPS as well as the surface tension of solutions containing the wetting agents (i.e., AOT, NaLS or TA). The results showed that PPGF is more hydrophobic than HIPS regardless of the type and concentration of wetting agent (Fig. 3b).
Among the three wetting agents, only AOT lowered the surface tension of solution while the effects of NaLS or TA on it were negligible (Fig. 4). The lower surface tension of solution in the presence of AOT decreased the work of adhesion, resulting in fewer bubble attachments on the particle surface. In comparison, NaLS or TA had negligible effects on the surface tension but decreased the contact angle at higher concentrations (Fig. 4). These results suggest that the work of adhesion and bubble attachment probability decreased in the presence of either NaLS or TA likely because of the adsorption of these two wetting agents onto the surface of plastic particles that made them more hydrophilic. Similarly, the contact angles on both plastics decreased in the presence of AOT (Fig. 3b-1) but because this wetting agent also lowered the surface tension of solution (Fig. 4), the net effect was the limited bubble attachment that resulted in low separation efficiency even though both PPGF and HDPS became more hydrophilic (Fig. 3a-1). In the case of NaLS, the change in contact angle of HIPS was more dramatic than that of PPGF, which caused selective bubble attachment onto PPGF making it lighter than HIPS that facilitated better stratification during hybrid jig separation (Fig. 3a-2, b-2). In the case of TA, the contact angle of PPGF decreased with increasing TA concentration (Fig. 3b-3). However, the contact angle with TA was slightly higher than that with AOT, which likely caused selective bubble attachment on the PPGF surface. These results showed that the control of surface characteristics by wetting agents could improve the separation efficiency of the hybrid jig. In the TA solution, similar results were obtained to that in NaLS even though the contact angles of HIPS and PPGF were similar. This phenomenon remains unclear because of the difficulty in quantifying the amounts of bubbles attached on plastic particles. Many previous papers reported the interaction between air bubbles and plastic particles during flotation [35, 36]; however, the particle and water movement during hybrid jig separation and flotation are different. This means that a reliable measurement technique to quantify bubble attachment volume during hybrid jig separation (with water pulsation) should be developed.
Separation of PVC and PA using a two-step approach
PVC is a common type of plastic used for a variety of applications such as in building and construction, packaging, electrical and electronics, and automobile; while PA (or nylon) is an engineering plastic used in clothing, electrical and electronic, and automobile [1]. These types of plastics can be found together in waste electrical and electronic equipment (WEEE) and automotive shredder residue (ASR) from end of life vehicle (ELV). These plastics also have similar SG (1.38 and 1.37), so in this study, hybrid jig was applied to separate them into individual components to improve material recycling efficiency.
The results from the previous section showed that wettability control by selective bubble attachment aided by wetting agents could improve the hybrid jig separation efficiency. In this section, separation of PVC and PA using a two-step approach was investigated. Hybrid jig tests of PVC (SG = 1.38) and PA (SG = 1.37) were carried out in water containing 10 ppm of AOT. The results showed that these two plastics could not be separated without AOT (i.e., separation efficiency was almost 0%) because of the indiscriminate attachment of bubbles to both types of plastics. Figure 5a-1 shows the result of hybrid jig separation with 10 ppm of AOT. The PVC purity in the top layer and the PA purity in the bottom layer were 84% and 98%, respectively. Separation of PVC and PA occurred because AOT lowered the contact angle of PA but not that of PVC as shown in Fig. 5b. However, bubble attachment probability on PVC slightly decreased with AOT addition (Fig. 5b) due to the decrease of surface tension as discussed earlier (Fig. 4), which likely caused imperfect separation (Fig. 5a-1).
To improve the separation efficiency, a two-step hybrid jig method (pre-wetting of PA) is proposed. In the first step, plastic mixture of PVC and PA is put in 10 -ppm AOT solution for 3 min. The AOT solution is then replaced with water is filled instead (second step) and hybrid jig separation in water is carried out. Figure 5a-2 shows that higher purities of PVC (94%) in the top layer and PA (99%) in the bottom layers were obtained compared with the single-step approach because higher surface tension was obtained in the two-step method than one-step method caused better bubble attachment on PVC (Fig. 5a-1). However, as the replacement of water in second step may cause some effects, the effect of time after water replacement was checked. The results showed that hybrid jig separation just after water replacement was effective (Fig. 5a-2); however, after several tens of minutes after water replacement, hybrid jig separation efficiency dropped because bubble attachment to PVC became difficult again. This lower bubble attachment probability to PVC could be caused by the decrease in surface tension due to the detachment of AOT from PA. To understand the attachment and detachment behaviors of AOT on PA, adsorption and desorption experiments were carried out. The adsorption of AOT on PA was confirmed (Fig. 6a) and desorption results of AOT from PA with time (Fig. 6b) showed that AOT was readily desorbed causing the surface tension of solution to decrease with time (Fig. 6b). In other words, the separation results worsed with time because the wettability of PVC was enhanced due to the decrease in surface tension of solution caused by the desorption AOT adsorbed on PA during the pre-wetting stage. This means that although pre-wetting was effective, the adsorption–desorption behavior of wetting agents could strongly affect the separation efficiency so, precise control of the wettability of samples is required.
Conclusions
This paper evaluated the improvement of hybrid jig separation of mixed-plastic wastes using wetting agents and the findings of this study are summarized as follows:
-
Wetting agents could change the surface tension of solutions by changing the water–air surface properties and the surface wettability (contact angle) of plastics by surface adsorption, both of which lowered the bubble attachment probability.
-
For the separation of PPGF and HIPS, surface modification using wetting agents (i.e., AOT, NaLS or TA) could improve the separation efficiency.
-
In the case of PPGF/HIPS with AOT, the changes in surface tension and decrease of contact angles of both plastics caused lower bubble attachment probability so, selective attachment of bubbles was limited and caused lower separation efficiency.
-
In the case of PPGF/HIPS with NaLS, the changes in surface tension were negligible and the contact angle on HIPS decreased while that on PPGF was unchanged; so, selective attachment of bubbles on PPGF occurred and higher separation efficiency was obtained.
-
In the case of PVC/PA with AOT, the surface tension decreased and the contact angle on PA decreased while that of PVC did not change, which caused selective attachment of bubbles on PVC.
-
A two-step approach could increase the surface tension, increase the bubble attachment probability on PVC, and dramatically improve the hybrid jig separation efficiency of PVC/PA.
References
Statista (2019) Global plastic production from 1950 to 2017 (in million metric tons). https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950. Accessed 15 Apr 2018
PlasticsEurope (2018) Plastics—the facts 2018. An analysis of European plastics production, demand and waste data. https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf. Accessed 22 Apr 2019
Tsunekawa M, Naoi B, Ogawa S, Hori K, Hiroyoshi N, Ito M, Hirajima T (2005) Jig separation of plastics from scrapped copy machines. Int J Miner Process 76:67–74. https://doi.org/10.1016/j.minpro.2004.12.001
Hori K, Tsunekawa M, Hiroyoshi N, Ito M (2009) Optimum water pulsation of jig separation for crushed plastic particles. Int J Miner Process 92:103–108. https://doi.org/10.1016/j.minpro.2009.01.001
Hori K, Tsunekawa M, Ueda M, Hiroyoshi N, Ito M, Okada H (2009) Development of a new gravity separator for plastics—a Hybrid-jig. Mater Trans 50:2844–2847. https://doi.org/10.2320/matertrans.M-M2009825
Ito M, Tsunekawa M, Ishida E, Kawai K, Takahashi T, Abe N, Hiroyoshi N (2010) Reverse jig separation of shredded floating plastic-separation of polypropylene and high density polyethylene. Int J Miner Process 97:96–99. https://doi.org/10.1016/j.minpro.2010.08.007
Kuwayama Y, Ito M, Hiroyoshi N, Tsunekawa M (2011) Jig separation of crushed automobile shredded residue and its evaluation by float and sink analysis. J Mater Cycles Waste Manag 13:240–246. https://doi.org/10.1007/s10163-011-0008-y
Tsunekawa M, Kobayashi R, Hori K, Okada H, Abe N, Hiroyoshi N, Ito M (2012) Newly developed discharge device for jig separation of plastics to recover higher grade bottom layer product. Int J Miner Process 114–117:27–29. https://doi.org/10.1016/j.minpro.2012.09.003
Phengsaart T, Ito M, Hamaya N, Tabelin CB, Hiroyoshi N (2018) Improvement of jig efficiency by shape separation, and a novel method to estimate the separation efficiency of metal wires in crushed electronic wastes using bending behavior and “entanglement factor”. Miner Eng 129:54–62. https://doi.org/10.1016/j.mineng.2018.09.015
Pita F, Castilho A (2016) Influence of shape and size of the particles on jigging separation of plastics mixture. Waste Manag 48:89–94. https://doi.org/10.1016/j.wasman.2015.10.034
Ferrara G, Bevilacqua P, Lorenzi L, Zanin M (2000) The influence of particle shape on the dynamic dense medium separation of plastics. Int J Miner Process 59:225–235. https://doi.org/10.1016/S0301-7516(99)00078-2
Pongstabodee S, Kunachitpimol B, Damronglerd S (2008) Combination of three-stage sink-float method and selective flotation technique for separation of mixed post-consumer plastic waste. Waste Manag 28:475–483. https://doi.org/10.1016/j.wasman.2007.03.005
Richard GM, Mario M, Javier T, Susana T (2011) Optimization of the recovery of plastics for recycling by density media separation cyclones. Resour Conserv Recycl 55:472–482. https://doi.org/10.1016/j.resconrec.2010.12.010
Dodbiba G, Shibayama A, Sadaki J, Fujita T (2003) Combination of triboelectrostatic separation and air tabling for sorting plastics from a multicomponent plastic mixture. Mater Trans 44:2427–2435. https://doi.org/10.2320/matertrans.44.2427
Dodbiba G, Sadaki J, Okaya K, Shibayama A, Fujita T (2005) The use of air tabling and triboelectric separation for separating a mixture of three plastics. Miner Eng 18:1350–1360. https://doi.org/10.1016/j.mineng.2005.02.015
Gente V, Marca FL, Lucci F, Massacci P (2003) Electrical separation of plastics coming from special waste. Waste Manag 23:951–958. https://doi.org/10.1016/S0956-053X(03)00088-6
Shent H, Pugh RJ, Forssbergc E (1999) A review of plastics waste recycling and the flotation of plastics. Resour Conserv Recycl 25:85–109. https://doi.org/10.1016/S0921-3449(98)00017-2
Fraunholcz N (2004) Separation of waste plastics by froth flotation—a review, part I. Miner Eng 17:261–268. https://doi.org/10.1016/j.mineng.2003.10.028
Wang C, Wang H, Fu J, Liu Y (2015) Flotation separation of waste plastics for recycling—a review. Waste Manag 41:28–38. https://doi.org/10.1016/j.wasman.2015.03.027
Güney A, Özdilek C, Kangal MO, Burat F (2015) Flotation characterization of PET and PVC in the presence of different plasticizers. Sep Purif Technol 151:47–56. https://doi.org/10.1016/j.seppur.2015.07.027
Burat F, Güney A, Kangal MO (2009) Selective separation of virgin and post-consumer polymers (PET and PVC) by flotation method. Waste Manag 29:1807–1813. https://doi.org/10.1016/j.wasman.2008.12.018
Shibata J, Matsumoto S, Yamamoto H, Lusaka E, Pradip (1996) Flotation separation of plastics using selective depressants. Int J Miner Process 48:127–134. https://doi.org/10.1016/S0301-7516(96)00021-X
Marques GA, Tenório JAS (2000) Use of froth flotation to separate PVC/PET mixtures. Waste Manag 20:265–269. https://doi.org/10.1016/S0956-053X(99)00333-5
Saisinchai S (2013) Separation of PVC from PET/PVC mixtures using flotation by calcium lignosulfonate depressant. Eng J 18:45–54. https://doi.org/10.4186/ej.2014.18.1.45
Wang H, Wang C, Fu J, Gu G (2014) Flotability and flotation separation of polymer materials modulated by wetting agents. Waste Manag 34:309–315. https://doi.org/10.1016/j.wasman.2013.11.007
Pita F, Castilho A (2017) Separation of plastics by froth flotation. The role of size, shape and density of the particles. Waste Manag 60:91–99. https://doi.org/10.1016/j.wasman.2016.07.041
Takoungsakdakun T, Pongstabodee S (2007) Separation of mixed post-consumer PET–POM–PVC plastic waste using selective flotation. Sep Purif Technol 54:248–252. https://doi.org/10.1016/j.seppur.2006.09.011
Dodbiba G, Haruki N, Shibayama A, Miyazaki T, Fujita T (2002) Combination of sink–float separation and flotation technique for purification of shredded PET-bottle from PE or PP flakes. Int J Miner Process 65:11–29. https://doi.org/10.1016/S0301-7516(01)00056-4
Jeon S, Ito M, Tabelin CB, Pongsumrankul R, Kitajima N, Hiroyoshi N (2018) Gold recovery from shredder light fraction of E-waste recycling plant by flotation-ammonium thiosulfate leaching. Waste Manag 77:195–202. https://doi.org/10.1016/j.wasman.2018.04.039
Wills BA, Napier-Munn TJ (2006) Mineral processing technology, 7th edn. Pergamon Press, Oxford
Brozek M, Surowiak A (2007) Effect of particle shape on jig separation efficiency. Physicochem Probl Miner Process 41:397–413
Beunder EM (2000) Influence of shape on particle behaviour in recycling techniques. Dissertation, Delft University of Technology
Boylu F, Cinku K, Cetinel T, Karakas F, Guven O, Karaagaclioglu IE, Celik MS (2015) Effect of coal moisture on the treatment of a lignitic coal through a semi-pilot-scale pneumatic stratification jig. Int J Coal Prep Util 35:143–153. https://doi.org/10.1080/19392699.2015.1005743
Butt HJ, Graf K, Kappl M (2003) Physics and chemistry of interfaces, 1st edn. Wiley, Weinheim
Firouzi M, Nguyen AV, Hashemabadi SH (2011) The effect of microhydrodynamics on bubble-particle collision interaction. Miner Eng 24:973–986. https://doi.org/10.1016/j.mineng.2011.04.005
Zhao Y, Li YP, Huang J, Liu J, Wang WK (2015) Rebound and attachment involving single bubble and particle in the separation of plastics by froth flotation. Sep Purif Technol 144:123–132. https://doi.org/10.1016/j.seppur.2015.02.016
Acknowledgements
The authors gratefully acknowledge the JSPS KAKENHI 23560990 for financial support and wish to thank Prof. Toshiaki Yoshioka, Prof. Shinichi Sakai, and the anonymous reviewers for their valuable inputs to this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ito, M., Takeuchi, M., Saito, A. et al. Improvement of hybrid jig separation efficiency using wetting agents for the recycling of mixed-plastic wastes. J Mater Cycles Waste Manag 21, 1376–1383 (2019). https://doi.org/10.1007/s10163-019-00890-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-019-00890-w