Abstract
The feasibility of the selective surface hydrophilization of poly vinyl chloride (PVC) using microwave treatment to facilitate the separation of PVC via froth flotation from automobile shredder residue (ASR) and electronic waste shredder residue (ESR) was evaluated. In the presence of powder-activated carbon (PAC), 60-s microwave treatment selectively enhanced the hydrophilicity of the PVC surface (i.e., the PVC contact angle decreased from 86.8° to 69.9°). The scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS) results are consistent with increased hydrophilic functional groups (i.e., ether, hydroxyl, and carboxyl), amounting to significant changes in the morphology and roughness of the PVC surface after treatment. After only 60 s of microwave treatment, 20 % of the PVC was separated in virgin and ASR/ESR plastics with 33 and 29 % purity, respectively, as settled fractions by froth flotation at a 150 rpm mixing speed. The microwave treatment with the addition of PAC had a synergetic effect with the froth flotation, which brought about 100 and 90 % selective separation of PVC from the other virgin and ASR/ESR plastics, with 91 and 82 % purity. The use of the combined froth flotation and microwave treatments is an effective technology for separating PVC from hazardous waste plastics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
End of Life Vehicles (ELVs) and Waste Electric & Electronic Equipment (WEEE/E-waste) are increasingly important secondary sources of ferrous metals, nonferrous metals, and plastics. About 1 million ELVs are generated every year in Korea [1, 2] and the generation of WEEE has rapidly increased in recent years [3]. The most problematic fractions of WEEE and ELV recycling treatments that have been mainly landfilled/incinerated in the past are shredding residues (SRs), including automobile shredder residue (ASR) from ELVs and electronic waste shredder residue (ESR) from WEEE, composed of plastics, small metals, wires, rubber, textiles, etc. [4, 5]. The disposition and management of ASR and ESR is an emerging environmental issue of concern to the solid waste community in Korea and around the world [6]. A Korean directive regarding the resource recycling of electrical and electronic equipment and vehicles has set a recycling target, including thermal recycling, at 85 % by 2006 and 95 % by 2015 [7]. Plastics are the largest portion in ASR and ESR; plastic comprises about 30 wt% of the whole and includes up to 15 different types of engineering plastics, rendering separating and recovery difficult [8].
The main plastics found in ASR/ESR, and their specific densities, are: polypropylene (PP 0.93 g/cm3), polyethylene (PE 0.90 g/cm3), polycarbonate (PC 1.20 g/cm3), polyethylene terephthalate (PET 1.41 g/cm3), high-impact polystyrene (HIPS), polyvinyl chloride (PVC 1.31 g/cm3), polymethyl methacrylate (PMMA 1.13 g/cm3), polyamide (PA 1.20 g/cm3), polystyrene (PS 1.1 g/cm3), acrylonitrile butadiene styrene (ABS 1.03 g/cm3), polyoxymethylene (POM 1.37 g/cm3), and styrene-acrylonitrile (SAN 1.10 g/cm3), etc. [8, 9]. Chlorinated plastics (i.e., PVC) comprise around 6–8 wt% of the total mixed plastics in ASR/ESR. When such plastics are subjected to incineration or uncontrolled burning processes for disposal, recycling, or metals recovery, their high chlorine content can contribute to the formation of highly toxic and persistent chlorinated dioxins [10]. Furthermore, when PVC is landfilled, some of its chemical additives can leach and contribute to the overall contaminant burden of landfill leachate [11]. Therefore, any end-of-life product or waste stream involving products containing chlorinated plastics must be managed in a way that minimizes the potential harmful impact on human health and the environment [12].
Wet gravity separation can be used to separate heavy plastics of specific density >1.0 g/cm3 (including PVC, ABS, PET, PS, HIPS, SAN, PA, PMMA, and POM of about 70–80 wt%) from light plastics of specific density <1.0 g/cm3 (including PP and PE, about 20–30 wt%) in ASR/ESR [13, 14]. However, further separation of chlorinated plastics such as PVC from other heavy plastics is difficult due to their similar densities. Heavy plastic fraction separation, therefore, cannot be applied for material and chemical recycling, even if the fraction is mainly composed of non-chlorinated plastics. Numerous techniques have been developed for plastics separation, such as electrostatic separation [15, 16], gravity separation [17], and selective dissolution [18]. In addition, in order to obtain the desired purity at a reasonable throughput, automated sorters have been developed. These typically have expensive plastics identification systems based on laser scanning and infrared or X-ray spectroscopy [19]. Selective wetting of hydrophobic plastic surfaces by chemical reagents [20], flame treatments [21], boiling treatments [22], and ozonation [23] has also been developed to facilitate plastics flotation separation. However, plastic surface modification using chemical reagents is relatively time intensive and requires additional facilities and chemicals to treat the wastewater. Plasma/flame treatment systems have high operating and maintenance costs and are difficult to handle [24]. Conversely, with ozone treatment, air pollution control devices need to be installed at the outlets of ozonation systems to prevent air pollution caused by the emission of the remaining ozone after the reaction with plastic surfaces.
Recently, investigations of microwave technologies have been conducted for various applications, including pyrolysis [25] and the dehydrochlorination of PVC [26, 27]. Although microwave techniques are developing, little work is currently available in the published literature on the surface hydrophilicity of PVC [26, 27]. PVC is known to have a higher dielectric loss coefficient than other plastics [28]. Exposed to microwave frequencies, PVC may be selectively hydrophilized/decomposed thermally [29, 30]. This is due to the scission of the C–C main chain of PVC followed by the vaporization of fragment molecules (probably small hydrocarbons) [31–33]. The resultant variations influence surface reactions, especially for PVC, which increases the presence of hydrophilic functional groups such as ether, hydroxyl, and carboxyl, i.e., C–Cl (ether carbon) [34, 35]. Further, carbon materials are in general very good adsorbents of microwaves and are easily heated by microwave radiation [36]. This characteristic allows them to be transformed by microwave heating, giving rise to new carbons with tailored properties, to be used as microwave receptors in order to heat other materials indirectly, or to act as a catalyst and microwave receptor in different heterogeneous reactions. The objective of the present work is to clarify the possibility of surface hydrophilization on PVC by microwave irradiation and subsequent separation from ASR/ESR plastic waste by froth floatation. Optimum treatment conditions, namely via the influence of powder-activated carbon (PAC) acting as a heat absorbent and the detailed mechanisms of PVC separation efficiency in froth floatation were also examined.
Materials and methods
ASR and ESR plastic samples collection
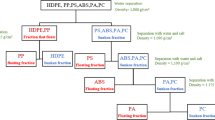
The ASR plastic samples were collected at automobile recycling/shredding plants in Pohang, Korea. ESR plastic samples were also collected at a WEEE recycling plant in Ulsan, Korea. The plastic compositions of both the ASR and ESR were identified by recording the IR spectra using a Fourier transform infrared (FT-IR) spectrometer (Perkin-Elmer, Spectrum One). The individual plastic compositions of the ASR/ESR samples were then quantified. Furthermore, in order to optimize the microwave treatment and froth flotation conditions, virgin plastic, PMMA, PC, and PVC were also obtained from the Kasai Sangyo Co., Ltd, Japan. Each virgin plastic shape was rectangular with a length of 1500 mm, a width of 1200 mm, and a thickness of 2 mm. Usually, ASR/ESR plastics are of non-uniform size and shape, but each plastic shape was cut to a small and uniform size (10 mm × 10 mm × 1 mm) using a saw and nippers and the four edges of the cut plastics were smoothed using sandpaper to remove attached particles generated by sawing. Further, the samples used for the tests were of different colors, rendering it easier to analyze the concentrated samples by manual sorting before and after each experiment (Fig. 1). The samples used for each test for the microwave treatments and froth flotation experiments consisted of ten pieces of each virgin or ASR/ESR plastic fraction.
Effect of microwave treatment on the hydrophobicity of virgin plastics
Microwave radiation was produced with the help of a microwave oven (Dongbu Daewoo Electronics Corp., KR-G20EW), producing a frequency of 2450 MHz and 1120 W as the rated microwave input. For the powder-activated carbon (PAC) pretreatment as a heat absorbent, we used a commercial PAC product (Activated Charcoal Norit®, Sigma-Aldrich, Co.). Mixtures of virgin or ASR/ESR plastic samples and the PAC (10 g) were manually shaken in 50 ml centrifuge tubes for 10 min to completely coat the plastic samples with PAC. Ten pieces of each plastic sample, both untreated (control) and pretreated with PAC, were heated in the microwave oven on a glass plate for specified time intervals (i.e., 30, 60, and 90 s). Appropriate alkaline washing was performed as a pretreatment for the removal of the pollutants from the ASR/ESR plastics surface according to our previous study [37].
Plastics surface characterization tests
To observe the changes on the surface of the untreated (control) and microwave-treated samples, the following characterization techniques were employed. We measured the contact angle formed between the water drops and the surface of the plastic sample to evaluate the wettability (hydrophilic or hydrophobic characteristics) of the plastic samples. This measurement was performed using a Contact Angle Analyzer (Smartdrop, Femtofab, Korea). A needle with a diameter of 0.55 mm (Dispovan) was used to place a drop of water onto the surface of the sample. The sample was photographed for 30 s after placing the water droplet on it, and the contact angle was then measured. The changes in the morphology and roughness of the plastic surfaces after microwave treatments were analyzed using a Field Emission Scanning Electron Microscope (FE-SEM, JSM-6500F, JEOL, Japan). To identify the elemental states of the carbons on the plastic surfaces before and after each treatment, X-ray Photoelectron Spectroscopy (XPS) analyses were performed using an XPS Spectrometer (K-Alpha, Thermo Scientific, USA).
Froth flotation experiments for the separation of PVC
To selectively separate PVC from the plastic samples, froth flotation experiments were conducted under different conditions. The main glass reactor, with a height of 14 cm, an inner diameter of 7 cm, and a volume of 0.54 dm3, consisted of a ceramic bubble diffuser at the bottom that was connected to a mini air pump (MP-Σ300, Sibata, Japan) which generated small air bubbles that enhanced the flotation efficiency of the plastic samples. An auto overhead stirrer (WiseStir, Daihan scientific Co., Ltd.) was installed to mix the floated plastic samples after bubbling, at a steady mixing speed and for a specific time. For the froth flotation experiment, 400 ml of tap water was used and a small amount (0.5 ml/L) of methyl isobutyl carbinol (MIBC) was added as a frother to facilitate the ease of flotation of the plastic samples. Ten pieces of each plastic (PMMA, PC, and PVC) (before and after treatment) were placed into the glass reactor and air bubbles were formed through the ceramic bubble diffuser at the bottom of the reactor by operating the mini air pump at 0.5 l/min for 1 min, to float all the plastic samples. After floating all the plastic samples, the auto overhead stirrer was operated at various mixing speeds (0–300 rpm) for 1 min and the number of floated and settled plastic samples of each type at each mixing speed was recorded to determine the optimum PVC separation condition. The recovery ratio and purity ratio for each experiment were calculated using Eqs. (1) and (2), respectively [20].
Here, PVC s and PVC f are the number of settled and floating PVC pieces, respectively, and “others” refer to the number of settled non-PVC plastic pieces.
Results and discussion
Quantification of ASR plastics composition
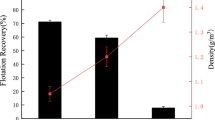
The compositions of main plastics in the ASR/ESR samples were identified with FT-IR and quantified. Figure 2 shows the results, giving the averages of the three replicates. The main plastics found in the ASR/ESR fractions were PP, PE, PC, PET, HIPS, ABS, PVC, PMMA, PA, POM, and SAN. In ASR, rubber and other plastics form the major portion of about 45.6 %; in ESR, the ABS and HIPS plastics were the dominant types, amounting to about 51 %. In the ASR and ESR plastic compositions, “others” are included in the heavy fraction. The FT-IR results further indicated that the PVC plastics in ASR and ESR were about 4.6 and 5.9 %, respectively. With wet gravity separation, heavy plastics of specific density >1.0 g/cm3 (including PVC, ABS, PET, PS, HIPS, SAN, PA, PMMA, and POM) were separated from light plastics of specific density <1.0 g/cm3 (including PP and PE) in ASR/ESR [13, 14]. The ASR and ESR consisted of about 74.2 and 80.8 % of heavy plastics, respectively.
The effect of microwave treatment on the hydrophobicity of virgin and ASR/ESR plastics
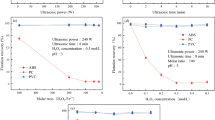
The contact angles were measured for virgin, ASR, and ESR (PC, PMMA, and PVC) plastics before and after microwave-only and microwave-with-PAC treatment, at specified time intervals (i.e., 30, 60, and 90 s), as shown in Fig. 3. All values shown in the figure are averages of three sample replications. For major plastics in ASR and ESR, having normally hydrophobic surface characteristics, the contact angles are usually greater than 85°. Even with 90 s microwave-only treatment, the contact angles of the virgin PC, PMMA, and PVC decreased by 0.8°, 5.1°, and 3.2°, respectively (Fig. 3a). On the other hand, as shown in Fig. 3a, the combination of PAC and the 60 s microwave treatment produced a sharp and significant decrease of 19° of the contact angle of PVC. Similarly, for the plastics in the ASR and ESR, as shown in Fig. 3b, the combination of 60 s microwave treatment and PAC decreased the contact angles of PVC in ASR and ESR significantly, by about 16.5° and 17.1°, respectively, whereas the contact angles of other plastics only slightly decreased (Fig. 3b). It was thus confirmed that the contact angle of PVC significantly decreased even in the ASR and ESR, compared to the decreases for the other plastics. Moreover, the decreased PVC contact angle values of the ASR and ESR samples are smaller than those in the virgin plastics. This might be because contaminants such as paint and oil were attached to the plastics collected from ASR and ESR, even though the plastics were washed by alkaline washing before the experiment [37]. These contaminants on the plastics would have changed the contact angles of the ASR/ESR waste plastics.
However, the microwave treatment alone did not affect any significant changes to the plastic surfaces. On the other hand, the combination of microwave treatment and PAC as a heat absorbent led to a synergetic effect that selectively decreased the contact angles of the PVCs, causing an increase in the hydrophilicity of the PVC surface [38, 39]. This could be because the chloride groups on the surface of PVC were replaced with hydrophilic functional groups such as hydroxyl groups and carboxyl groups [40, 41]. If a PVC surface has a high hydrophilicity, it would be the first material to settle on the bottom during the froth flotation experiment. Therefore, to selectively separate the PVC from the plastic samples using the froth flotation method, it is necessary to determine the lowest contact angle for the PVC surface among the other plastics.
Plastics surface characterization tests
Field emission scanning electron microscope (FE-SEM) images were taken to visualize the changes in the morphology and roughness induced in the surfaces of the microwave-treated virgin plastic samples. Similar to the contact angle measurements, untreated samples and samples treated with 60-s microwave with PAC were utilized for the SEM analysis. The SEM images provided a qualitative type of analysis by visualizing changes in the surfaces of the plastic samples (Fig. 4). The SEM images showed significant changes in the morphology and roughness of the plastic samples after treatment compared to the untreated samples (Fig. 4). In particular, after treatment, the PVC surfaces had larger pitted areas than the other treated plastic samples. This shows a substantial decrease in the contact angle of PVC surfaces after treatment, which can be subsequently used to facilitate the selective separation of PVC during froth flotation experiments.
XPS results were also used to determine the elemental state of carbon on the plastic sample surfaces. In the C 1 s spectra of the plastic sample surfaces, the peaks observed at 285, 286.5, and 289.1 eV can be assigned to neutral carbon, ether carbon, and carbonyl carbon, respectively [42, 43]. The ether carbon (C–O, C–Cl) and carbonyl carbon (O–(C=O)–O) are classified as hydrophilic groups, while neutral carbons (C–C, C=C, C–H) are classified as hydrophobic groups. The peak areas of the hydrophilic groups in the curved fits of the C 1 s peaks were calculated using a fit peak program (XPSPEAK41, version 4.1). The hydrophilicity levels of the plastic surfaces could be identified based on the relative peak area (RPA) of the hydrophilic groups. If the peak area of the hydrophilic groups was increased, the contact angle could be decreased [40–43]. Summarized values of the peak areas and contact angles of the hydrophilic groups for untreated, microwave-treated, and microwave treatment with PAC, are presented in Fig. 5. As shown in Fig. 5a, the percentage of PVC relative peak area (hydrophilic groups) increased significantly, even after 30 s of microwave treatment with PAC. On the contrary, the contact angle of the PVC decreased (Fig. 5b). On the other hand, in the case of PC and PMMA, the relative peak area percentage and contact angle difference were considered to be insignificant.
Froth flotation separation of PVC
From the FE-SEM and XPS analysis and from the contact angle measurements, we were able to conclude that microwave treatment with PAC pretreatment can significantly alter the hydrophobicity of PVC, which is consequently drastically increasing the wettability/hydrophilicity of the PVC samples. Hence, the separation of PVC plastic from mixed virgin, ASR, and ESR plastics via froth flotation can be achieved. Therefore, the froth flotation experiments were conducted using ten pieces of each plastic of PC, PMMA, and PVC for the virgin, ASR, and ESR plastic samples, with the ideal treatment time selected (i.e., 60 s) based on the preceding contact angle and surface characterization study. Initially, all plastic samples were submerged in pure water; then, after the froth flotation, all plastics floated away with the air bubbles attached. The floating/settling behaviors of each plastic were evaluated before and after microwave-only treatment and microwave treatment with PAC, the results of which are presented in Fig. 6. With 60 s of microwave-only treatment, about 20 % of PVC was observed to have settled, along with 20 % each of PC and PMMA, at the 150 rpm mixing speed. However, when the plastic samples were treated for 60 s by microwave with PAC at a mixing speed of 150 rpm, 100 % of the PVC was separated in the settled fraction from the other plastic samples (0 % of PVC floated), with only 10 % PC contamination (Fig. 6). Further, the microwave treatment with PAC promoted the settlement of PVC at a lower mixing speed (150 rpm). At a high mixing speed of 300 rpm, all the PC, PMMA, and PVC samples had settled by removing all attached air bubbles.
Optimized ideal froth flotation separations of PVC conditions based on virgin plastics (i.e., 60 s microwave treatment with PAC and 150 rpm) were further considered for ASR and ESR samples. The obtained froth flotation results are presented in Table 1, which shows that a slightly lower separation efficiency was obtained for ASR and ESR compared to virgin plastics. In ASR plastics, with 60 s of microwave-only treatment, about 20 % of the PVC was observed to have settled, along with 20 and 30 % of PC and PMMA, respectively, at the 150 rpm mixing speed. However, when the plastic samples treated for 60 s by microwave with PAC were mixed at 150 rpm, 90 % of the PVC was separated in the settled fraction from the other plastic samples, with 10 % each of PC and PMMA contamination (Table 1). Similar results were obtained for ESR: when the plastic samples treated for 60 s by microwave with PAC were mixed at 150 rpm, 90 % of PVC settled with only 10 % of PMMA contamination (Table 1).
The recovery and purity ratios of the separated PVC versus the other plastic samples were also calculated using Eqs. (1) and (2) for each treatment. The purity ratios of the PVC that was recovered were, respectively, about 33.3 and 29 % for the virgin and ASR/ESR plastic samples, with the microwave-only treatment. However, the highest purity ratios of the recovered PVC, about 91 and 82 % for the virgin and ASR/ESR plastic samples, respectively, were obtained after microwave treatment with PAC. The use of the combined froth flotation and microwave treatments is, therefore, a simple and effective technology for separating PVC from plastic waste. Although chemical reactions occur on the PVC surfaces, microwave treatment has little influence on the bulk characteristics of the PVC, since only a few nanometers of the PVC surfaces were destroyed during the treatment process (Fig. 4). Therefore, microwave treatment does not affect the post-recycling process by froth flotation. As a result of this research, the selective surface modification of PVC with microwave treatment can be efficiently used to separate PVC from other mixed plastic waste of similar density, including ASR/ESR.
Conclusions
In this research, we evaluated the selective surface modification of PVC by microwave treatment, as a method for separating PVC from hazardous automobile shredder residue (ASR) and electronic waste shredder residue (ESR). This enabled the optimization of the microwave treatment/froth flotation conditions. With the combination of 60 s of microwave treatment with PAC, a sharp and significant decrease of about 19° in the contact angle of the PVC was observed. The SEM images of the PVC plastic samples after treatment displayed significant changes in surface morphology and roughness compared to the PC and PMMA samples. The XPS results of the plastic samples demonstrated that increased amounts of hydrophilic functional groups (ether, hydroxyl, and carboxyl) appeared on the PVC surface after microwave treatment using PAC. After only 60 s of microwave-only treatment, approximately 20 % of the PVC was separated in the virgin and ASR/ESR plastics, with 33 and 29 % purity, respectively, as settled fractions by froth flotation at a 150 rpm mixing speed. However, the microwave treatment with the addition of PAC resulted in a synergetic effect for the froth flotation, which brought about 100 and 90 % selective separation of PVC from the virgin and ASR/ESR plastics, with 91 and 82 % purity, respectively. Further studies are in progress, to optimize the process to improve the recovery and purity of PVC from ASR/ESR.
References
Sakai SI, Yoshida H, Hiratsuka J, Vandecasteele C, Kohlmeyer R, Rotter VS, Passarini F, Santini A, Peeler M, Li J, Oh GJ, Chi NK, Bastian L, Moore S, Kajiwara N, Takigami H, Itai T, Takahashi S, Tanabe S, Tomoda K, Hirakawa T, Hirai Y, Asari M, Yano J (2014) An international comparative study of end-of-life vehicle (ELV) recycling systems. J Mater Cycles Waste Manag 16:1–20
KAMA (2003) http://www.kama.or.kr/eng/K_eng_main.jsp. Seoul, Korea
KASA (2003) http://www.kasa.or.kr/bbs_board_file/statistics/statistics_list.asp. Seoul, Korea
Kim IS (2004) E-waste issues and measures in Korea. In: Proceedings of the third workshop on material cycles and waste management in Asia (NIES E-waste Workshop), 14–15 December, NIES, Tsukuba, Japan
Zevenhoven R, Sayeed L (2003) Automotive shredder residue (ASR) and compact disc (CD): waste: options for recovery of materials and energy. TKK Eny 14, Espoo
Jang YC (2010) Waste electrical and electronic equipment (WEEE) management in Korea: generation, collection, recycling systems. J Mater Cycles Waste Manag 12:283–294
Korea Ministry of Environment (Korea MOE) (2007) The act on the resource recycling of waste electrical electronic equipment (WEEE) and end-of-life vehicles (ELVs). The Ministry, Gwacheon
Martinho G, Pires A, Saraiva L, Ribeiro R (2012) Composition of plastics from waste electrical and electronic equipment (WEEE) by direct sampling. Waste Manag 32:1213–1217
Schlummer M, Gruber L, Maurer A, Wolz G, Eldik RV (2007) Characterisation of polymer fractions from waste electrical and electronic equipment (WEEE) and implications for waste management. Chemosphere 67:1866–1876
Dimitrakakis E, Janz A, Bilitewski B, Gidarakos E (2009) Small WEEE: determining recyclables and hazardous substances in plastics. J Hazard Mater 161:913–919
Wagenaar H, Langeland K, Hardman R, Sergeant Y, Brenner K, Sandra P, Rappe C, Fernandes A, Tiernan T (1998) Analysis of PCDDs and PCDFs in virgin suspension PVC resin. Chemosphere 36:1–12
Greenpeace International (2010) Why BFRs and PVC should be phased out of electronic devices
Reddy MS, Okuda T, Kurose K, Tsai T-Y, Nakai S, Nishijima W, Okada M (2010) Surface ozonation of polyvinyl chloride for its separation from waste plastic mixture by froth flotation. J Mater Cycles Waste 12:326–331
Kangal MO (2010) Selective flotation technique for separation of PET and HDPE used in drinking water bottles. Min Proc Ext Met Rev 31:214–223
Inculet II, Castle GSP, Brown JD (1998) Electrostatic separation of plastics for recycling. Particul Sci Technol 16:91–100
Park CH, Jeon HS, Yu HS, Han OH, Park JK (2008) Application of electrostatic separation to the recycling of plastic wastes: separation of PVC, PET, and ABS. Environ Sci Technol 42:249–255
Pongstabodee S, Kunachitpimol N, Damronglerd S (2008) Combination of three-stage sink–float method and selective flotation technique for separation of mixed post-consumer plastic waste. Waste Manag 28:475–483
Pascoe RD (2006) Investigation of hydrocyclones for the separation of shredded fridge plastics. Waste Manag 26:1126–1132
Krummenacher B, Peuch P, Fisher M, Biddle M (1998) Automatic identification and sorting of plastics from different waste streams—a status report, APME
Wang C, Wang H, Liu Q, Fu J, Liu Y (2014) Separation of polycarbonate and acrylonitrile–butadiene–styrene waste plastics by froth flotation combined with ammonia pretreatment. Waste Manag 34:2656–2661
Pascoe RD, O’Connell B (2003) Flame treatment for the selective wetting and separation of PVC and PET. Waste Manag 23:845–850
Wang CQ, Wang H, Wu BX, Liu Q (2014) Boiling treatment of ABS and PS plastics for flotation separation. Waste Manag 34:1206–1210
Reddy MS, Kurose K, Okuda T, Nishijima W, Okada M (2007) Separation of polyvinyl chloride (PVC) from automobile shredder residue (ASR) by froth flotation with ozonation. J Hazard Mater 147:1051–1055
Wang C, Wang H, Liu Y (2015) Separation of polyethylene terephthalate from municipal waste plastics by froth flotation for recycling industry. Waste Manag 35:42–47
Jones DA, Lelyveld TP, Mavrofidis SD, Kingman SW, Miles NJ (2002) Microwave heating applications in environmental engineering—a review. Resour Conserv Recycl 34:75–90
Ito M, Ushida K, Nakao N, Kikuchi N, Nozaki R, Asai K, Washio M (2006) Dechlorination of poly (vinyl chloride) by microwave irradiation I: a simple examination using a commercial microwave oven. Polym Degrad Stab 91:1694–1700
Moriwaki S, Machida M, Tatsumoto H, Otsubo Y, Aikawa M, Ogura T (2006) Dehydrochlorination of poly(vinyl chloride) by microwave irradiation. Appl Therm Eng 26:745–750
Haviriliak S, Haviriliak SJ, Mark JE (eds) (1999) Physical properties of polymers handbook. Chapter 36. American Institute of Physics, New York, p 489
Steeman PAM, Turnhout JJ, Salamone JC (eds) (1996) Polymeric materials encyclopedia. CRC Press, Boca Raton, p 7026
Bacalogulu R, Fish MH, Kaufhold J, Sander HJ, Zweifel H (eds) (2001) Plastics additives handbook. Chapter 3. Hanser Gardner Publications, Cincinnati, p 427
Achilias C, Roupakias P, Megalokonomos AA, Lappas EVA (2007) Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J Hazard Mater 149:536–542
Gaboury SR, Urban MW (1991) Spectroscopic evidence for Si–H formation during microwave plasma modification of poly(dimethylsiloxane) elastomer surfaces. Polym Commun 32:390–392
Gaboury SR, Urban MW (1992) Quantitative analysis of the Si–H groups formed on poly(dimethylsiloxane) surfaces: an ATR FTi.r. approach. Polymer 33:5085–5089
Abbasi M, Salarirad MM, Ghasemi I (2010) Selective separation of PVC from PET/PVC mixture using floatation by tannic acid depressant. Iran Polym J 19:483–489
Kunkel JP, Urban MW (1993) Surface and interfacial FT–IR spectroscopic studies of latexes. VIII. The effect of particle and copolymer composition on surfactant exudation in styrene-n-butyl acrylate copolymer latex films. J Appl Polym Sci 50:1217–1223
Menéndez JA, Arenillas A, Fidalgo B, Fernández Y, Zubizarreta L, Calvo EG, Bermúdez JM (2010) Microwave heating processes involving carbon materials. Fuel Process Technol 91:1–8
Reddy MS, Kurose K, Okuda T, Nishijima W, Okada M (2008) Selective recovery of PVC-free polymers from ASR polymers by ozonation and froth flotation. Resour Conserv Recycl 52:941–946
Gaboury SR, Urban MW (1994) Quantitative attenuated total reflectance Fourier transform infrared analysis of microwave plasma reacted silicone elastomer surfaces. Langmuir 10:2289–2293
Marques GA, Tenorio JAS (2000) Use of froth flotation to separate PVC/PET mixtures. Waste Manag 20:265–269
Okuda T, Kurose K, Nishijima W, Okada M (2007) Separation of polyvinyl chloride from plastic mixture by froth flotation after surface modification with ozone. Ozone Sci Eng 29:373–377
Kurose K, Okuda T, Nakai S, Tsai T-Y, Nishijima W, Okada M (2008) Hydrophilization of polyvinyl chloride surface by ozonation. Surf Rev Lett 15:711–715
Bigot S, Louarn G, Kébir N, Burel F (2013) Facile grafting of bioactive cellulose derivativesonto PVC surfaces. Appl Surf Sci 283:411–416
Kojio K, Kugumia S, Uchiba Y, Nishino Y, Furukaw M (2009) The microphase-separated structure of polyurethane bulk and thin films. Polym J 41:118–124
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2014R1A1A2055487).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mallampati, S.R., Lee, CH., Park, M.H. et al. Processing plastics from ASR/ESR waste: separation of poly vinyl chloride (PVC) by froth flotation after microwave-assisted surface modification. J Mater Cycles Waste Manag 20, 91–99 (2018). https://doi.org/10.1007/s10163-016-0546-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-016-0546-4