Abstract
A biocomposite prepared from poly(vinyl alcohol) (PVA) and oil palm fiber (OPF) is proposed with clarification of intermolecular interactions, physical properties, and biodegradability behavior. OPF in this study was converted from oil palm frond, which can be counted as value-added agricultural waste. The OPF content in the biocomposite is varied from 5 to 25 phr and glycerol is used as plasticizer. Scanning electron micrograph presents the good dispersion of OPF in hydrophilic PVA matrix, even at high OPF content of 25 phr. This result provides the improved compressive strength and modulus for 2 and 2.5 times, respectively, as compared to plasticized PVA. The successfully induced rigidity structure of OPF to biocomposite system is also confirmed by increasing of glass transition and melting temperatures, but decreased degree of crystallinity at high contents of OPF (20 and 25 phr). Moreover, the biocomposite with 25 phr OPF exhibits the increase of water resistance at 60% relative humidity obviously. In this study, biodegradability behavior is also traced by Fourier transform infrared spectra which suggests the fast degradation of highly moisture-sensitive PVA as compared with the highly crystalline fibrous structure of OPF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(vinyl alcohol) (PVA) is a synthetic hydrophilic polymer used in various applications such as medical devices, food-contact materials, textiles, etc. In general, the physical properties of PVA are dependent on degree of hydrolyzation of polyvinyl acetate and molecular weight [1]. The unique characteristics of PVA are odorless, non-toxic, gas barrier, and highly ductile [2]. In the molecular level, PVA contains an abundance of hydroxyl groups on main chain, resulting in good water solubility, and consequently, biodegradability through hydrolysis and microorganisms activities [3, 4]. However, PVA has some drawbacks of poor mechanical strength and low-dimensional stability at high relative humidity.
Polymer blending and composites are alternative approaches to combine the different physical and chemical properties of various polymers together. Up to the present, several studies have been reported the use of natural fiber as reinforcing agent in polymer matrix, including PVA/fiber biocomposites [5]. Some fibers can be obtained from agricultural waste which is not only environmental friendliness but also value addition for these wastes. For instance, Lu et al. used microfibrillated cellulose (MFC) with web-like structure, separated from kraft pulp, to reinforce the mechanical properties of PVA film. The composite film containing with 10 wt% MFC increased the storage modulus for 53%, as compared to pure PVA at 25 °C, and the web-like structure could provide the much easier preparation method and lower processing costs, as compared to isolated cellulose fibrils or nanowhiskers [6]. In addition, Mohanty et al. also studied the effect of date palm leaf fiber (DPL) on the mechanical properties of PVA/DPL biocomposite. They applied the chemical surface modification to improve interfacial interaction between fiber and PVA matrix. It was found that DPL treated with acrylic acid could slightly increase the mechanical strength and stiffness in comparison with the case of without chemical treatment. The PVA/treated DPL biocomposite as obtained could also increase water resistance [7].
Oil palm (Elaeis guineensis Jacq.) is an economically valuable plant cultivated in more than 40 countries around the world [8]. The major oil palm cultivating regions include West Africa, Latin America, and Southeast Asia [9], where countries such as Malaysia, Indonesia, India, and Thailand produce large quantities of crude palm oil. Thus, vast amounts of oil palm biomass, such as empty fruit bunches, fronds, and shells, are generated, and finally, discarded [10]. Typically, oil palm fronds are abandoned in the cultivating field after the oil palms are harvested which can be converted to fiber structure using a sulfite pulping process. Not only oil palm fronds have very high fibrous biomass (about 55 tons per ha of total dry matter) [11], but they also consist of abundant cellulosic matter. The oil palm fiber (OPF) as obtained is becoming an attractive reinforcing phase for biocomposites due to its fully biodegradation and value-added agricultural waste. It comes to our viewpoint that OPF has a potential in biocomposites to produce the superior mechanical properties of highly moisture-sensitive PVA with remained biodegradability. In addition, up to the present, the reports on the utilization of fiber from agricultural waste in biocomposite are still limited.
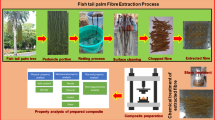
In this work, the biocomposite of PVA and OPF was prepared using glycerol (G) as plasticizer and formed tubular-shaped PVA/G/OPF containers by an injection-molding process. The structural interaction of biocomposite was characterized by Fourier transform infrared (FTIR) spectra. Moreover, the product obtained was also investigated the mechanical properties, thermal stability, and water resistance of PVA/G/OPF container, including the biodegradation behavior.
Materials and methods
Materials
Oil palm frond was collected from Krabi province, located in the southern part of Thailand. Poly(vinyl alcohol) (PVA-124) and glycerol (G) were obtained from Japan Vam & Poval Co., Ltd., Japan, and QRëC Quality Reagent Chemical, New Zealand, respectively.
Preparation of poly(vinyl alcohol)/oil palm fiber biocomposites
First, oil palm frond was converted to oil palm frond fiber (OPF) by a sulfite pulping process. The OPF obtained was further mixed with PVA resin and glycerol using a co-rotating twin screw extruder (COLLIN model T-20, Germany) at processing temperature range of 140–220 °C and a screw speed of 60 rpm. After that, the extrudate was cut into pellet-form with 2.5 mm in length and dried in a hot-air oven at 60 °C for 24 h. The contents of OPF in the biocomposite were varied at 0, 5, 10, 15, 20, and 25 phr of PVA resin, whereas 35 phr glycerol (G) based on OPF weight was used in all samples.
Furthermore, the PVA/G/OPF biocomposites obtained were formed tubular-shaped container by an injection-molding machine with screw L/D ratio of 30:1 (Battenfeld BA 250 CDC, USA). The processing temperature of injection molding was in the range of 150–245 °C and a screw speed of 100 rpm.
Characterizations
Structural analysis
The intermolecular interactions of PVA/G/OPF biocomposites and the biodegradation of PVA in the biocomposites were traced using a Brüker Tensor 27 Fourier transform infrared spectrophotometry (FTIR) spectrometer in attenuated total reflectance (ATR) mode. The ATR-FTIR spectra were recorded over a wavenumber range of 500–4000 cm−1 with 32 scans and a resolution of 4 cm−1.
Thermal properties
Thermal behavior of the biocomposite was investigated by an METTLER TOLEDO differential scanning calorimeter (DSC) STARe system. The samples in aluminium pan were heated from 25 to 220 °C and down to − 20 °C with a rate of 10 °C/min under nitrogen gas flow of 50 mL/min.
The DSC thermogram from the first heating scan was used to determine degree of crystallinity (χc), as shown in the following equation:
where ΔHm and \(\Delta H_{{\text{m}}}^{ \circ }\) are the heat fusion of PVA in DSC thermogram and 100% crystalline PVA (138.6 J/g), respectively [12] and w is the weight fraction of PVA in the biocomposite.
In addition, thermal stability of PVA/G/OPF biocomposite was also studied by an STA PT1000 thermal gravimetric analyzer (TGA). The 20 mg sample was weighed, and consequently, heated from 30 to 800 °C at a heating rate of 10 °C/min under nitrogen atmosphere (40 mL/min).
Biocomposite morphology
Cross-sectional surfaces of PVA/G/OPF biocomposites coated with a thin layer of gold/platinum mixture were analyzed by a Philips XL-30 FEI scanning electron microscopy (SEM) at an accelerating voltage of 1.5 kV.
Mechanical properties and moisture absorption
Compression test was carried out by an Instron model 5965 universal testing machine. The samples were studied in tubular-shaped container with a crosshead speed of 10 mm/min.
Moisture absorption of the PVA/G/OPF biocomposite was determined from percentage of weight gain. All tubular-shaped biocomposites obtained were placed under 60 and 100% relative humidity (RH), representing the ambient atmosphere in Thailand and the worst-case scenario, respectively.
Biodegradation behavior
Commercial loamy sand from Ratchaburi, a western province of Thailand, was used for soil burial test to determine the biodegradation of the PVA/G/OPF biocomposites. The commercial loamy sand with pH 6.72 and 4.5 dS/m electrical conductivity was composed of 8.7% soil organic matter, 652.4 mg phosphorus/kg soil, and 480.5 mg potassium/kg soil, respectively. In this test, it was carried out on a laboratory scale as followed by Azahari et al. [13]. The samples were cut into 2 cm × 5 cm rectangular pieces in triplicate, and then, weighed before placed at a depth of 5 cm from the soil surface in a composting chamber covered with a plastic net. After all samples were further exposed to environmental conditions at average soil temperature of 31 ± 3 °C for 60 days, they were removed from the soil, and cleaned with a brush and dried at 60 °C until a constant weight was achieved. The weight loss of the degraded samples was calculated.
Results and discussion
FTIR spectra of PVA/G/OPF biocomposite
In this study, the structure and intermolecular interaction of pure PVA and PVA/G/OPF biocomposites were investigated using ATR-FTIR technique, as shown in Fig. 1. For pure PVA, the ATR-FTIR spectrum shows the broad peak in the region of 3000–3600 cm−1 and 2900–2950 cm−1, assigned to stretching vibration of hydroxyl group (–OH) and methylene unit (–CH2), respectively. Moreover, the peak at 1730 cm−1 representing C=O stretching is also observed which is due to residual acetate groups from the incomplete hydrolysis of polyvinyl acetate to form PVA, as reported by Shehap and Gohil et al. [14, 15].
When PVA was blended with glycerol (PVA/G), the peak at 1045 cm−1 of C–O stretching in G molecule becomes sharpen as also presented in the study of van Soest and coworkers [16]. At the same time, the characteristic vibrational peak at 1100 cm−1, assigned to C–O stretching of secondary alcohols of PVA, still presents obviously as also found by Rajendran et al. [17]. In addition, it was found that the broad peak of –OH stretching of pure PVA at 3332 cm−1 shifts to lower wavenumber at 3317 cm−1 after blending PVA with G. It indicates that the intra- and intermolecular hydrogen bonds of PVA in the system are decreased by penetration of plasticizer molecule (i.e., glycerol) in between PVA chains for promoting polymer chain movement and increasing free volume in the PVA/G blend.
In the case of ATR-FTIR spectrum of OPF, it shows the unique broad peaks in the region of 950–1180 cm−1 for C–O stretching and –CH2 rocking of anhydroglucose unit which is generally found in cellulose-based structure. For PVA/G/OPF-10 phr biocomposite, the ATR-FTIR spectrum is from combining of important peaks at 1045, 1100, 1147, 1265, 1730, and 3300 cm−1, belonging to PVA, G, and OPF structures which were the similar wavenumbers as reported by Sudhamani et al. and Priya et al. [18, 19]. However, there is no presence of significant shifting of –OH-stretching band at 3317–3313 cm−1 by adding highly crystalline fiber into the biocomposite system, even though the OPF content was increased to 25 phr, as shown in Fig. 2. It should be noted that the intermolecular hydrogen bonds in pure PVA and the PVA/G/OPF biocomposite have not been changed clearly by adding OPF, and varying the OPF content, respectively.
Thermal properties of PVA/G/OPF biocomposite
After the PVA/G blend and the PVA/G/OPF biocomposite with varied OPF contents were obtained by twin screw extruder, their thermal properties are traced by DSC technique and are presented in Table 1. At the first heating scan, pure PVA shows glass transition temperature (Tg) at 66.9 °C, and melting temperature (Tm) at 202.8 °C. Whereas the crystallization temperature (Tc) of PVA could be observed at 128.8 °C during cooling scan, indicating the fast crystallization rate of PVA with relatively high degree of crystallinity (χc) of 46.35%. As anticipated, Tg, Tc, and Tm of PVA plasticized with G shifts to lower temperature at 51.3, 101.7, and 178.7 °C, respectively, while χc of PVA significantly increases to 69.07%. This result is because of plasticization function of G which provided the flexibility and crystallization acceleration to PVA molecule.
For thermal properties of the PVA/G/OPF biocomposite, the values of Tg, Tc, and χc of PVA/G/OPF-5 phr are similar to those of PVA/G blend, excepting Tm increasing to 186.7 °C. Notably, when the content of OPF in the composite increases to 25 phr, Tg and Tm of the biocomposites gradually increase to around 55 °C and 220 °C, respectively, and χc drastically decreases to 29.70%. At the same time, there is no impact of OPF addition on Tc shifting in the biocomposite when compared with Tc of PVA/G blend. It could be summarized that OPF induces the rigid arrangement of fiber structure in PVA/G/OPF biocomposite which obstructs the free movement of PVA chains, resulting in increased Tg and Tm, but decreased χc. These consequences had an agreement with the study of Patel and coworkers which reported the increasing of Tg and Tm of PVA/palm leaf biocomposite as determined by DSC and X-ray diffraction techniques [20].
Furthermore, the thermogravimetric (TGA) curves with differential thermal analysis (DTA) graphs for thermal stability of PVA/G/OPF biocomposite are also illustrated in Fig. 3, and all degradation temperatures (Tds) are listed in Table 1. Since the boiling point of glycerol is above 100 °C, the weight loss about 100 °C is merely related to moisture loss. The percentage of weight loss at temperatures between 100 and 300 °C was, therefore, not only caused by the volatilization of organic compounds, but also by glycerol (at 290 °C) as reported by Majdzadeh-Ardakania and Nazarib [21]. As a result, the weight loss curve of the plasticized PVA/G was observed to consist of two phases of decomposition. The first transition curve is related to the degradation of PVA by dehydration of polymer side chains (at 300–350 °C), whereas the second one is related to the degradation of the main chains of the polymer (at 440 °C) as also reported by Moshin et al. [22]. Normally, OPF is composed of lignocellulosic matter consisting of hemicellulose, cellulose, and lignin. The degradation of cellulose and hemicellulose occurs at 300–350 °C, whereas lignin is degraded at around 420 °C. When the system comprised of OPF to form biocomposite, the degradation of the first transition for moisture and glycerol presents between 100 and 200 °C. In addition, the endothermic peak at 255–260 °C, as assigned to OPF, can be detected before both PVA and OPF residue were further degraded at 310 °C. Finally, there is the sharp peak at 400–420 °C representing for disintegrating the remained PVA. It points out that Td of PVA in PVA/G/OPF biocomposite with varied OPF contents from Fig. 3 are significantly declined which could be caused by the loose tight packing structure of crystalline phase in PVA molecule due to plasticizer and fiber composition, resulting in lower Td of biocomposite. Although the content of OPF in the biocomposite in this study was varied, Td of all PVA/G/OPF biocomposites are similar. It should be noted that the shifting of Td to either lower or higher position is independent on the amount of OPF in the biocomposite. This could insist the major effect of plasticizer that played more important role than that of the reinforced fiber. This result was also found by Cinelli et al. [23], and they investigated the hybrid PVA/starch/lignocellulosics composite films with the presence of G as plasticizer, leading to the decreased Td of the composite.
Morphology of PVA/G/OPF biocomposite
Figure 4 shows the fiber structure of OPF with an average diameter of 13.74 µm and length of 760.80 µm. When OPF was added into the PVA/G/OPF biocomposite (Fig. 5b–f), the surface becomes rougher as compared to the smooth surface of PVA/G blend. In addition, there is the formation of void in PVA matrix depending on different OPF loadings (i.e., 5, 10, 15, 20, and 25 phr). Although PVA is hydrophilic polymer, the phase separation of OPF in PVA matrix still occurred. This result was from the strongly inter- and intramolecular hydrogen bonds in OPF molecule which obstructs the melting of OPF fibrous structure to form homogeneous surface. However, the OPF phase in PVA matrix still shows the good dispersion, even though the content of OPF reached 25 phr, as illustrated in Fig. 5. It can be the effect of similar hydrophilicity of several hydroxyl groups on PVA and cellulose (i.e., OPF) chains. These appearances were similar to the morphologies of PVA/microfibrillated cellulose biocomposite as presented by Nakagaito and Yano [24]. Moreover, this well dispersion of microfibrillated cellulose fiber was reported to inhibit the free polymer chain movement and restrict to chain fold ability for crystallization, leading to decreased χc which had an agreement with our study as discussed in thermal properties of biocomposite section.
Mechanical properties and moisture absorption of PVA/G/OPF biocomposite
After the tubular-shaped PVA/G/OPF biocomposites were produced, the mechanical properties were studied in terms of compressive strength and modulus, as reported in Table 2.
In general, compressive strength and modulus of pure PVA are approximately in the range of 40–70 and 200–250 MPa, respectively. These values are slightly decreased to 63.8 MPa strength and 170 MPa modulus after plasticized PVA with G. The tubular-shaped PVA/G container is more flexible than that of pure PVA. It reflects the plasticizer function of G to promote or assist PVA chain movement when the external force (i.e., compression) was applied on the tubular-shaped container.
Notably, the addition of the reinforcing OPF to form the PVA/G/OPF biocomposite, and the compressive strength was improved from 66.0 to 117.6 MPa according to increasing of OPF contents in the system (Table 2). Whereas the compressive modulus of PVA/G/OPF biocomposite was drastically increased for 1.5–2.5 folded, especially at OPF loading higher than 15 phr. This can be explained by the good dispersion of OPF in PVA matrix which also confirmed the reinforcing function of OPF for rigidity improvement of PVA. This increase of PVA mechanical properties by OPF loading was also revealed by Mohanty et al., Ching et al., and Yong et al. [7, 25, 26].
Moreover, the tubular-shaped container of PVA/G/OPF biocomposite with varied OPF contents was also investigated the moisture absorption by determining the percentage of weight gain (%weight gain), as shown in Fig. 6. The samples were placed under the ambient atmosphere of 60% RH and the worst-case scenario of 100% RH.
Figure 6a shows that the low content of 5–10 phr OPF in the biocomposite under 60% RH presents slightly higher %weight gain than those of the high contents of OPF, in particular the PVA/G/OPF-25 phr biocomposite with 14% weight gain after 8 weeks under 60% RH atmosphere.
Under the 100% RH condition, the %weight gain of PVA/G/OPF biocomposite with varied OPF contents still exhibits the similar trend, as found in the case of 60% RH. However, the difference between the various OPF contents in the biocomposites at 100% RH which affects on %weight gain is not significant. It could be due to the high moisture sensitivity of PVA as compared to OPF with the intrinsic highly intermolecular hydrogen bond in the molecule. This moisture absorption characteristic of PVA/G/OPF biocomposite was similar to that of PVA/sago pith waste biocomposites by Yee et al. [27] which cellulose fibers could restrict the free mobility of water molecule in the biocomposite system.
Biodegradation of PVA/G/OPF biocomposite
Biodegradability of PVA/G/OPF biocomposite was carried out through soil burial test for 60 days, before using FTIR spectra to simply trace the degradation of the samples. Figure 7 exhibits the spectra of all tubular-shaped PVA/G blend and PVA/G/OPF biocomposite before and after the burial test. The hydroxyl group (–OH) stretching at 3296 cm−1, C–H stretching at 2914 cm−1, and the carbonyl group at 1714 cm−1 were noticeably changed. The peak intensity at 845 cm−1 (C–C stretching) vanished completely after 30 days of burial test, and subsequently, the spectra of the PVA/G/OPF biocomposites became similar to that of OPF at the 60th day of burial test. This suggests the easy degradation of PVA due to high moisture sensitivity of PVA molecule, leading to water absorption and being swollen before degraded. In the case of the remained OPF in the soil, OPF is hydrophilic molecule, but it has highly ordered structure from strong inter- and intramolecular hydrogen bond which limits OPF molecule to form the hydrogen bond with water in the environment.
Conclusions
Oil palm frond was converted before utilized as reinforcing fiber in PVA-based biocomposite. First, the intermolecular hydrogen bond in PVA molecule, as observed from ATR-FTIR spectra, was slightly depressed by plasticizer addition, whereas there was no significant effect of varied OPF contents on the interaction in the biocomposite system. The OPF can be well-dispersed in PVA matrix, resulting in the improved compressive strength with increasing of OPF content in the PVA matrix. The 25 phr of OPF content yielded the highest values of 117.6 MPa strength and 420 MPa modulus (around twofold of pure PVA). Moreover, the rigid fibrous OPF structure in the biocomposite showed the limitation of PVA chains movement with increased Tg and Tm, but decreased χc, allowing the enhanced strength of PVA/G/OPF biocomposite structure. Furthermore, the soil burial tests indicated the fast degradation of PVA within 30 days with remained OPF. The presence of OPF in the biocomposite clearly showed the reinforcement for PVA mechanical and thermal properties, but it did not disturb the water absorption of PVA as a key mechanism for degradation.
References
DeMerlis CC, Schoneker DR (2003) Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem Toxicol 41(3):319–326
Jang J, Lee DK (2003) Plasticizer effect on the melting and crystallization behavior of polyvinyl alcohol. Polymer 44(26):8139–8146
Jayasekara R, Harding I, Bowater I, Lonergan G (2005) Biodegradability of a selected range of polymers and polymer blends and standard methods for assessment of biodegradation. J Polym Environ 13(3):231–251
Briassoulis D, Dejean C, Picuno P (2010) Critical review of norms and standards for biodegradable agricultural plastics, part II: composting. J Polym Environ 18(3):364–383
Peijs T, Vught RJM, Govaert LE (1995) Mechanical properties of poly(vinyl alcohol) fibres and composites. Composites 26(2):83–90
Lu J, Wang T, Drzal LT (2008) Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Compos A 39(5):738–746
Mohanty JR, Das SN, Das HC, Swain SK (2013) Effective mechanical properties of polyvinylalcohol biocomposites with reinforcement of date palm leaf fibers. Polym Compos 34(6):959–966
Shinoj S, Visvanathan R, Panigrahi R (2010) Towards industrial utilization of oil palm fibre: physical and dielectric characterization of linear low density polyethylene composites and comparison with other fiber sources. Biosyst Eng 106:378–288
Joseph S, Joseph K, Thomas S (2006) Green composites from natural rubber and oil palm fiber: physical and mechanical properties. Int J Polym Mater 55(11):925–945
Al-Dulaimi AA, Wanrosli WD (2016) Isolation and characterization of nanocrystalline cellulose from totally chlorine free oil palm empty fruit bunch pulp. J Polym Environ 25(2):192–202
Hasamudin W, Soom RM (2002) Road-making using oil palm fibre (BIT5). Malaysian Palm Oil Board Information Series, Kuala Lumpur, Malaysia. http://palmoilis.mpob.gov.my/publications/TOT/tt171.pdf. Accessed 8 Oct 2017
Peppas NA, Merrill EW (1976) Differential scanning calorimetry of crystalline PVA hydrogels. J Appl Polym Sci 20(6):1457–1465
Azahari NA, Othman N, Ismail H (2011) Biodegradation studies of polyvinyl alcohol/corn starch blend films in solid and solution media. J Phys Sci 22(2):15–31
Shehap AM (2008) Thermal and spectroscopic studies of polyvinyl alcohol/sodium carboxy methyl cellulose blends. Egypt J Solids 31(1):75–91
Gohil JM, Bhattacharya A, Ray P (2006) Studies on the crosslinking of poly(vinyl alcohol). J Polym Res 13(2):161–169
van Soest JJG, de Wit D, Tournois H, Vliegenthart JFG (1994) The influence of glycerol on structural changes in waxy maize starch as studied by Fourier transform infra-red spectroscopy. Polymer 35(22):4722–4727
Rajendran S, Sivakumar M, Subadevi R (2004) Investigations on the effect of various plasticizers in PVA-PMMA solid polymer blend electrolytes. Mater Lett 58(5):641–649
Sudhamani SR, Prasad MS, Sankar KU (2003) DSC and FTIR studies on gellan and polyvinyl alcohol (PVA) blend films. Food Hydrocoll 17(3):245–250
Priya B, Gupta VK, Pathania D, Singha AS (2014) Synthesis, characterization and antibacterial activity of biodegradable starch/PVA composite films reinforced with cellulosic fibre. Carbohydr Polym 109:171–179
Patel AK, Bajpai R, Keller JM (2014) On the crystallinity of PVA/palm leaf biocomposite using DSC and XRD techniques. Microsyst Technol 20(1):41–49
Majdzadeh-Ardakani K, Nazari B (2010) Improving the mechanical properties of thermoplastic starch/poly(vinyl alcohol)/clay nanocomposites. Compos Sci Technol 70(10):1557–1563
Moshin M, Hossin A, Haik Y (2011) Thermal and mechanical properties of poly(vinyl alcohol) plasticized with glycerol. J Appl Polym Sci 122(5):3102–3109
Cinelli P, Chiellini E, Gordon SH, Imam SH (2003) Characteristics and degradation of hybrid composite films prepared from PVA, starch and lignocellulosics. Macromol Symp 197:143–155
Nakagaito AN, Yano H (2005) Novel high-strength biocomposites based on microfibrillated cellulose having nano-order-unit web-like network structure. Appl Phys A 80:155–159
Ching KS, Ealid M, Ching YC, Haniff M, Khalid M, Beg MTH (2014) Preparation and characterisation of polyvinyl alcohol/oil palm empty fruit bunch fibre composite. Mater Res Innov 18(6):364–367
Yong KC, Ching YC, Mohamad A, Lim ZK, Chong KE (2015) Mechanical and thermal properties of chemical treated oil palm empty fruit bunches fiber reinforced polyvinyl alcohol composite. J Biobased Mater Bioenergy 9:231–235
Yee TW, Choy LJ, Rahman WA (2011) Mechanical and water absorption properties of poly(vinyl alcohol)/sago pith waste biocomposites. J Compos Mater 45(11):1201–1207
Acknowledgements
The authors would like to thank the Graduate School of Kasetsart University, Kasetsart University Research and Development Institute (KURDI), and the Department of Packaging and Materials Technology for their financial support throughout the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sukudom, N., Jariyasakoolroj, P., Jarupan, L. et al. Mechanical, thermal, and biodegradation behaviors of poly(vinyl alcohol) biocomposite with reinforcement of oil palm frond fiber. J Mater Cycles Waste Manag 21, 125–133 (2019). https://doi.org/10.1007/s10163-018-0773-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-018-0773-y