Abstract
Background
Tolvaptan (TLV) promotes aquaresis; however, little is known about its effect on solute excretion in chronic kidney disease (CKD).
Methods
We retrospectively studied CKD patients with decompensated heart failure (HF) or those with autosomal dominant polycystic kidney disease (ADPKD) receiving TLV. Patients with an increased urine volume of more than twice of daily variance were defined as “responders” in HF. We compared the ability of the urinary osmolality (U-OSM) change and urinary creatinine concentration ([U-Cr]) change to discriminate “responders”. The fractional excretion of sodium (FeNa) and urea nitrogen (FeUN), and blood urea nitrogen (BUN) were monitored.
Results
In 30 responders among 53 HF patients, TLV increased FeUN significantly from 36.1 to 44.2% after starting TLV, but not FeNa. Since U-OSM is determined partially by urinary UN concentration, the decrease of [U-Cr] after treatment outperformed the U-OSM decrement to discriminate responders, as shown in receiver operating characteristic curve analysis and significantly higher net reclassification index. In 13 ADPKD patients, TLV increased FeUN (34.8, 47.3%, p = 0.02), and significant decrease of BUN by 2.3 (95% confidence interval 0.4–4.2) mg/dL was observed even 3 months after the intervention. Systolic blood pressure decreased significantly by 14.2 (95% confidence interval 4.0–24.4) mmHg along with the increase in FeNa, leading to reduced dosage of antihypertensives in 6 patients.
Conclusion
TLV promotes the excretion of sodium and urea. The change in [U-Cr] is useful for early discrimination of responders. Hypotension should be carefully monitored during high-dose TLV therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vasopressin V2 receptor antagonist tolvaptan (TLV) promotes water excretion acting on the kidney collecting ducts. While TLV is approved for dilutional hyponatremia in various countries, TLV is approved and widely used for decompensated heart failure (HF) in Japan. Moreover, TLV was recently revealed to suppress the deterioration of renal function in patients with autosomal dominant polycystic kidney disease (ADPKD) [1,2,3]. TLV was first approved for ADPKD in Japan in 2014.
Vasopressin increases sodium reabsorption via increased expression of the epithelial sodium channels (ENaC) and activation of the Na–K–2Cl cotransporter (NKCC2), and also increases urea reabsorption via regulation of urea transporters [4,5,6,7]; therefore, TLV is expected to promote sodium and urea excretion. Recent studies showed that high-dose TLV therapy increased urinary sodium excretion in a healthy population [8], and TLV lowered blood urea nitrogen (BUN) in acute HF patients [9]. However, these effects remain to be explored in chronic kidney disease (CKD) patients, who have impaired natriuresis and high BUN levels.
Although TLV is useful for ameliorating congestion in HF patients [10,11,12], TLV non-responders, with no increase in urine volume (UV), certainly exist, especially among CKD patients. Early discrimination of TLV responders is clinically relevant in terms of avoiding continuation of unnecessary treatment. A recent study reported that % decrease in urinary osmolality (U-OSM) after TLV administration is useful in discriminating TLV responders [13]. However, if TLV has any effect on sodium and urea excretion, the discrimination of TLV responders using % decrease in U-OSM would be underestimated, because % decrease in U-OSM due to increased free water excretion by TLV can be partially canceled by its solute excretion effect. Because TLV does not affect the excretion of urinary creatinine, we hypothesized that % decrease in urinary creatinine concentration ([U-Cr]) would reflect the increase of free water excretion by TLV and, hence, discriminate TLV responders more accurately.
The present study examined how TLV influences urinary sodium and urea excretion in two distinct clinical situations: (1) in HF patients with CKD who received relatively low-dose TLV in addition to sodium-losing diuretics or (2) in ADPKD patients who started to take high-dose TLV with no concomitant sodium-losing diuretics. We investigated acute effect of TLV in these situations and relatively long-term effect regarding BUN (up to 3 months) and blood pressure (up to 12 months) in ADPKD patients. Moreover, we compared the superiority of % decrease in [U-Cr] to % decrease in U-OSM in discriminating TLV responders among HF patients.
Materials and methods
Study design
Study in HF patients
We retrospectively analyzed 53 HF patients with CKD stage G3–G5D who started TLV between December 2012 and June 2015 at the Nephrology Department of Osaka University Hospital. The daily variance of UV was calculated from UV for 1 week before TLV started, and the minimum significant change (MSC) was defined as twice the UV daily variance (coefficient of variation of UV) [14]. Patients with increased UV greater than the MSC on the second day of TLV therapy compared with UV before the therapy began were defined as responders. In patients in whom we could not measure UV, we instead used body weight loss by one dose TLV. The optimal cut-off value for body weight change was determined using ROC analysis with twice the UV daily variance as an outcome. Changes in fractional excretion of sodium (FeNa) and urea nitrogen (FeUN), free water clearance (CH2O), and osmolar clearance (Cosm) were examined before and after the TLV therapy, and the performance in discriminating TLV responders was compared between % decrease in U-OSM and % decrease in [U-Cr].
Study in ADPKD patients
We retrospectively analyzed 13 ADPKD patients who started TLV between March 2014 and June 2015 at the Nephrology Department of Osaka University Hospital. Changes in FeNa, FeUN, blood pressure, and BUN were examined.
Data collection
Study in HF patients
In calculating the daily variance of UV, we excluded the data of patients in whom the prescription of diuretics, infusion volume, or peritoneal dialysis was changed; patients treated by steroid pulse therapy within 1 week before TLV administration; or patients who did not have UV data available for at least 3 days before TLV administration. UV before TLV therapy was defined as the most recent UV before TLV therapy, and UV after TLV therapy was defined as that of the second day of TLV administration. FeNa, FeUN, Cosm, CH2O, and the changes in U-OSM and [U-Cr] were calculated from blood and urine samples obtained prior to the administration of TLV and initial samples after TLV administration.
We calculated FeNa, FeUN, Cosm, and CH2O by the following formula:
where V is the urinary output (mL/min). Plasma osmolality was roughly estimated as (Serum Na × 2) + (Serum UN/2.8) in all patients, because data for blood glucose at individual time point were missing in most patients.
Study in ADPKD patients
FeNa and FeUN were calculated from blood and urine samples obtained prior to the administration of TLV and on the day following TLV was first administered. Average systolic blood pressure before and after TLV therapy was obtained from blood pressure measurements in the outpatient department for 3 months before and after TLV was first administered, respectively. Blood pressure data after the change in antihypertensives excluded for analyses. BUN was measured the day before, on the second day and at 3 months (with an allowance of 2 weeks) after starting TLV.
Statistical analysis
We tested for normal distribution of continuous variables using Shapiro–Wilk test and histograms. Continuous variables were analyzed using t tests for normally distributed variables, Mann–Whitney U test for non-normally distributed variables. Comparisons of paired continuous variables were made using paired t tests for normally distributed variables or Wilcoxon signed-rank tests for non-normally distributed variables. Fisher’s exact test was employed for comparisons of categorical variables. Results were presented as either mean ± standard deviation (SD), median and interquartile range (IQR), or number of patients. Linear regression analyses were used to assess the association between % decrease in U-OSM or [U-Cr] and the change in UV after TLV therapy. Receiver operating characteristic (ROC) curve, logistic regression analyses, category-free net reclassification improvement (cfNRI), and the integrated discrimination index (IDI) were performed to investigate the discriminating ability of % decrease in U-OSM and % decrease in [U-Cr] for TLV responders. The Akaike information criterion (AIC) and the Bayesian information criterion (BIC) were calculated to compare the relative quality of statistical models. All statistical analyses were performed with the Stata 12.1 statistical software (StataCorp LP, College Station, TX, USA). Two-tailed probability tests were used, with a p level below 0.05 regarded as statistically significant.
We conducted this study in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Ethics Committee of Osaka University Hospital approved the study protocol (approval number 16322). We provided all individual patients with the option to opt out of participation. The reporting here follows Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [15].
Results
Baseline characteristics and classification of TLV responders/non-responders in HF patients
We retrospectively analyzed 53 HF patients with CKD stage G3–G5D who had received TLV administration. The dose of TLV per day was 1.875 mg in 1 patients, 3.5 mg in 5 patients, 7.5 mg in 46 patients, and 15 mg in 1 patient. Physicians reduced the dose of sodium-losing diuretics in 4 patients (7.5%) on the first day TLV was started to avoid excessive diuresis. The time interval between the first administration of TLV and the time of obtaining initial samples was less than 24 h in 19 patients, 24–48 h in 18 patients, and over 48 h in 16 patients. The histogram of % increase of UV after TLV administration is shown in Fig. 1a. The median daily variance of the daily UV before TLV administration was 13.9%; therefore, we set the MSC at 27.8% and responders to TLV were defined as those in whom the UV increased by greater than 27.8% after TLV therapy. Regarding patients in whom we could not measure UV, the Youden index indicated that the determination of responders corresponded to percent decrease of body weight ≥1.1% (sensitivity and specificity were 50.0 and 78.6%, respectively); therefore, responders to TLV were defined as those in whom the body weight decreased by greater than 1.1% during 24 h after TLV therapy. As a result, 30 patients (56.6%) were identified as responders. In the responders, median CH2O [IQR] increased significantly after starting TLV (−0.03 [−0.12 to 0.08] mL/min, 0.21 [0.08–0.44] mL/min, p = 0.0003), but not in the non-responders (Fig. 1b). We did not observe any study patients in whom hypernatremia, hypovolemic shock, or deteriorated liver function compelled us to stop administration of TLV.
Definition of tolvaptan responders and change in free water clearance by tolvaptan in patients with decompensated heart failure. a Distribution of % increase in urine volume after starting tolvaptan therapy in patients with decompensated heart failure is shown as a histogram. The minimum significant change was set at 27.8% based on the natural daily variation of urine volume before tolvaptan administration. b Box plots of free water clearance on the day before and after tolvaptan was first administered, stratified by response to tolvaptan in patients with decompensated heart failure. The upper and lower hinges of the box represent the 75th and 25th percentile, respectively
The baseline characteristics of the patients according to response to TLV are shown in Table 1. Thirteen patients received peritoneal dialysis. In responders, baseline serum sodium concentrations were lower (p = 0.04) and E/e′ ratio was higher (p = 0.02) compared with non-responders. Dietary sodium intake was restricted to less than 6 g per day from the day of hospitalization according to Evidence-based Clinical Practice Guideline for CKD 2013 [16] in 47 patients (88.7%) except for patients with hyponatremia or reduced dietary intake.
The effect of TLV on solute excretion in HF patients
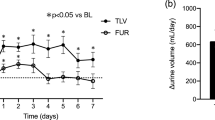
Analyses of the changes from baseline in FeNa and FeUN after TLV therapy in the responders are shown in Fig. 2. Median FeNa [IQR] increased by TLV therapy (but not significantly) over time from 2.8 [1.2–5.1]% to 4.3 [1.8–6.9]% (p = 0.27) (Fig. 2a). On the other hand, median FeUN [IQR] increased significantly from 36.1 [29.8–44.4]% to 44.2 [38.5–49.8]% (p = 0.04) (Fig. 2b). As a result, median Cosm [IQR] increased significantly from 0.55 [0.32–0.84] to 0.81 [0.49–1.16] mL/min (p = 0.002) in the responders, but not in the non-responders (Fig. 2c).
Effect of tolvaptan on solute excretion in patients with decompensated heart failure. FeNa (a) and FeUN (b) data on the day before and after the start of tolvaptan therapy in responders are illustrated in box plot diagrams. c Box plots of osmolar clearance on the day before and after tolvaptan were first prescribed, grouped by response to tolvaptan. The upper and lower hinges of the box represent the 75th and 25th percentile, respectively. FeNa fractional excretion of sodium, FeUN fractional excretion of urea nitrogen
Comparison of markers for early discrimination of TLV responders
We then compared the ability of % decrease in [U-Cr] and % decrease in U-OSM to discriminate TLV responders. We analyzed 43 HF patients by excluding patients with missing data of [U-Cr] or U-OSM. Percent decrease in [U-Cr] in responders after TLV administration was significantly greater than in non-responders (median 38.5 [IQR 11.1–60.0]% versus 4.7 [IQR −12.2 to 19.8]%; p = 0.02), while no significant difference was observed in the decrement of U-OSM after TLV administration (median 20.8 [IQR 10.8–37.9]% versus 9.0 [IQR −7.3 to 29.2]%; p = 0.09) (Fig. 3a, b). We subsequently confirmed that median serum creatinine [IQR], which influences the change in [U-Cr], remained unchanged both in responders (2.9 [1.7–5.4] mg/dL, 3.0 [1.7–5.9] mg/dL, p = 0.63) and non-responders (4.0 [2.1–9.4] mg/dL, 3.9 [2.0–9.5] mg/dL, p = 0.14) after TLV administration (Supplementary Fig. 1).
Decrease in urinary creatinine concentration and decrease in urinary osmolality by tolvaptan and their relationship with the increase in urine volume. Distribution of % decrease in urinary creatinine concentration (a) and % decrease in urinary osmolality (b) stratified by response to tolvaptan is depicted using a box plot. The upper and lower hinges of the box represent the 75th and 25th percentile, respectively. The scatterplots of % increase in urine volume with % decrease in urinary creatinine concentration (c) and % decrease in urinary osmolality (d) in all patients. The solid line indicates the linear regression line of the data, and R2 indicates coefficient of determination. [U-Cr] urinary creatinine concentration, U-Osm urinary osmolality
Figure 3c, d shows the analysis of the correlation between % increase of UV and % decrease in [U-Cr] or % decrease in U-OSM after TLV administration. Percent decrease in [U-Cr] was significantly correlated with % increase in UV (R 2 = 0.22, p = 0.01), as well as % decrease in U-OSM (R 2 = 0.30, p = 0.002). The area under the receiver operating characteristic curve (ROC-AUC) was compared to determine the superiority of % decrease in [U-Cr] or % decrease in U-OSM in discriminating TLV responders. The ROC-AUC of % decrease in [U-Cr] tended to be larger (0.72 versus 0.65; Fig. 4a). Figure 4b shows the ROC curves that were constructed incorporating % decrease in [U-Cr] or % decrease in U-OSM with clinical data, consisting of age, sex, and eGFR. The AUC-ROC was 0.72 with the addition of % decrease in [U-Cr], whereas the AUC-ROC was 0.71 with the addition of % decrease in U-OSM. In univariate logistic regression analysis, % decrease in [U-Cr] was an independent factor in the discrimination of TLV responders (odds ratio [OR] 2.1 per 1SD, 95% confidence interval (CI) 1.0–4.2, p = 0.04), whereas % decrease in U-OSM was not (Table 2). Percent decrease in [U-Cr] remained an independent factor in the discrimination of TLV responders after adjusting for clinical data, consisting of age, sex, and eGFR (OR 2.2 per 1SD, 95% CI 1.0–4.5, p = 0.04, Table 3). As shown in Table 3, the AIC decreased from 65.4 to 62.5 by adding % decrease in [U-Cr] to clinical data, whereas the AIC decreased to 63.6 by adding % decrease in U-OSM. Moreover, as indicated in model 4, AIC did not improve with the addition of % decrease in U-OSM to model 3. In NRI, by adding % decrease in [U-Cr] or % decrease in U-OSM as a continuous variable to the basic model consisting of age, sex, eGFR (Table 4), % decrease in [U-Cr] correctly reclassified 47.8% responders and 40.0% non-responders, whereas % decrease in U-OSM correctly reclassified 21.7% responders and 20.0% non-responders. As a result, % decrease in [U-Cr] improved the discriminative parameters [total cfNRI = 87.8% (95% CI 26.1–149.5)], whereas % decrease in U-OSM did not [total cfNRI = 41.7% (95% CI −20.0 to 103.4)].
Receiver operating characteristic curve indicating performance for parameters in early discrimination of tolvaptan responders. a Area under the receiver operating characteristic curve (AUC-ROC) indicating performance for % decrease in urinary creatinine concentration or % decrease in urinary osmolality in discriminating tolvaptan responders. b AUC-ROC constructed incorporating % decrease in urinary creatinine concentration or % decrease in urinary osmolality with clinical data, consisting of age, sex, and eGFR. Black solid line indicates % decrease in urinary creatinine concentration, and black dashed line indicates % decrease in urinary osmolality, respectively. Ninety-five percent confidence intervals (CIs) are also shown. eGFR estimated glomerular filtration rate, [U-Cr] urinary creatinine concentration, U-Osm urinary osmolality
The effect of TLV on solute excretion in ADPKD patients
Table 5 shows the baseline characteristics of the ADPKD patients who received TLV. Figure 5a, b provides the analysis of changes from baseline in FeNa and FeUN. The median FeNa [IQR] increased from baseline after starting TLV (0.8 [0.5–1.1]%, 0.9 [0.7–1.7]%, p = 0.04). Similarly, the median FeUN [IQR] increased (34.8 [32.7–43.5]%, 47.3 [41.2–52.1]%, p = 0.02). TLV significantly lowered median systolic blood pressure [IQR] from 131 [119–157] mmHg to 119 [113–131] mmHg (p = 0.02, Fig. 5c); physicians were forced to reduce the dose of antihypertensives in 6 out of 13 ADPKD patients within 3 months after TLV prescription. In these 6 patients, baseline serum chloride concentrations were lower compared with those in the remaining 7 patients (p = 0.03). On the other hand, heart rate or hematocrit did not change; and significant hypernatremia (>145 mEq/L) was not observed during TLV therapy. The effect of TLV on blood pressure was generally maintained throughout 12-month follow-up. The dosage of antihypertensives was continued to be reduced in 7 out of 13 ADPKD patients despite the decrease of median eGFR [IQR] (52.3 [25.4–70.9] mL/min/1.73 m2, 43.7 [22.3–67.0] mL/min/1.73 m2, p = 0.004) at 12 months. TLV also lowered median BUN [IQR] from 19 [15–28] mg/dL to 14 [13–24] mg/dL after starting TLV (p = 0.001), and this effect persisted even after 3 months (16 [13–28] mg/dL, p = 0.03, Fig. 5d).
Effect of tolvaptan on solute excretion, systolic blood pressure, and blood urea nitrogen levels in autosomal dominant polycystic kidney disease patients. The degree of FeNa (a) and FeUN (b) in ADPKD patients on the day before and after tolvaptan was first administered. c Temporal changes in median ambulatory systolic blood pressure during 3 months before and after tolvaptan therapy. d Serial change in BUN levels over time after the start of tolvaptan therapy in ADPKD patients. The upper and lower hinges of the box represent the 75th and 25th percentile, respectively. ADPKD autosomal dominant polycystic kidney disease, FeNa fractional excretion of sodium, FeUN fractional excretion of urea nitrogen, BUN blood urea nitrogen
The effects of TLV on solute excretion, blood pressure, and BUN in HF patients with CKD and ADPKD patients are summarized in Table 6.
Discussion
This study revealed the following three points. First, high-dose TLV therapy in ADPKD patients increases the excretion of urinary sodium and consequently lowers blood pressure. Second, TLV increases the urinary urea excretion; high-dose TLV therapy in ADPKD patients lowered BUN for at least 3 months. Finally, % decrease in [U-Cr] after TLV administration outperforms % decrease in U-OSM in discriminating TLV responders among HF patients.
First, high-dose TLV therapy in ADPKD patients increases the excretion of urinary sodium and consequently lowers blood pressure. Previously, it has been pointed out that TLV can exert the excretion of urinary sodium [4, 8, 17] due to the following mechanisms: (1) attenuation of the resistance to concomitantly administered sodium-losing diuretics by ameliorating kidney congestion and (2) direct inhibition of the ENaC expression [5] and inactivation of NKCC2 [6]. However, only a few studies have shown the natriuretic effect of TLV, and these studies were limited to normal healthy people administered high-dose TLV [8] and to patients receiving peritoneal dialysis [17]. Our study is the first to demonstrate that TLV can exert natriuresis in ADPKD patients. Moreover, the direct effect of TLV on kidney tubules is suggested, because no ADPKD patients had previously been administered sodium-losing diuretics in our study.
Furthermore, our study clearly indicates that high-dose TLV therapy lowered blood pressure in ADPKD patients, and hence, antihypertensives had to be stopped or reduced in approximately half of the patients. There have been no reports of any blood pressure lowering effects of TLV therapy; the TEMPO study, which revealed a renoprotective effect of high-dose TLV therapy in 1445 ADPKD patients, reported that TLV therapy did not affect blood pressure [1]. The TEMPO study authors evaluated the addition of antihypertensive drugs and the exacerbation in blood pressure category; however, the investigators did not show any analyses regarding temporal change in blood pressure. The mechanism of lowering blood pressure by TLV is considered to occur through the suppression of renin angiotensin systems (RAS) in addition to the promotion of natriuresis. The natriuresis alone is unlikely to account for the blood pressure lowering effect of TLV, because sodium loss is small and will be easily compensated by the body, especially in the acute setting. The previous reports demonstrated that the activation of RAS was caused by rise in kidney interstitial pressure, which is induced by renal congestion in HF patients [18] or compression of renal vasculature by enlarged cysts in ADPKD patients [19]. We consider that TLV can suppress RAS by decreasing kidney interstitial pressure due to attenuation of renal congestion and cyst enlargement. Actually, the kidney volume decreased in 82% of ADPKD patients after TLV therapy (Supplementary Fig. 2). Moreover, mean serum potassium concentration ± SD was significantly increased from 4.0 ± 0.3 to 4.3 ± 0.4 mEq/L at 3 months after starting TLV in ADPKD patients who were compelled to reduce the dose of antihypertensives (p = 0.04), while potassium levels did not change in the remaining patients (4.0 ± 0.2 mEq/L, 4.1 ± 0.2 mEq/L, p = 0.78). These results are compatible with the putative suppressive effect of TLV on RAS. In ADPKD patients, strict blood pressure management is very important to delay the deterioration of kidney function [20, 21] and to avoid the formation and rupture of cerebral aneurysms, a life-threatening complication in ADPKD [22]. In this context, the blood pressure lowering effect of TLV might contribute to prevent these complications in ADPKD patients.
On the other hand, FeNa did not change after relatively low-dose TLV administration in HF patients. This coincides with a previous report that relatively low-dose TLV (≤30 mg/day) did not increase the excretion of urinary sodium in healthy people [8]. However, the possibility does exist that relatively low-dose TLV produces urinary sodium excretion, because we were able to diminish the dose of sodium-losing diuretics in 16 patients (30%) among the 53 enrolled HF patients within 1 week after starting TLV. Future prospective studies are needed to investigate the natriuretic effect of TLV in HF patients.
The second point revealed by the present study was that TLV increases the excretion of urinary urea, and high-dose TLV therapy lowers BUN. A previous study reported that vasopressin promoted the expression of the urea transporter UT-A1/3 in the kidney collecting ducts and, hence, increased the reabsorption of urea nitrogen [23] and another study revealed that BUN level in the UT-A1/3 knockout mice was significantly decreased on high protein intake [24]; therefore, we presume that TLV increases urinary urea excretion and decrease BUN level by inhibiting the expression of UT-A1/3. In fact, the EVEREST study, which enrolled 4133 admitted patients with HF, reported that TLV lowered BUN significantly, while serum creatinine was slightly elevated the next day when TLV was started [25]. Another study reported that the BUN-lowering effect of TLV persisted for 5 days [9]. However, our study is the first report that TLV therapy lowered BUN for at least 3 months. Recently, BUN was observed to be not only a marker of kidney function, but is also associated with arteriosclerosis, cardiovascular events and a poor life prognosis in CKD patients via protein carbamylation [26,27,28,29]. Future studies should evaluate whether TLV can prevent arteriosclerosis and cardiovascular events in CKD patients.
Furthermore, the previous studies using UT-A1/3 knockout mice or urea transporter inhibitors demonstrated that increased urinary urea excretion via the inhibition of UT-A1/3 leads to a marked decrease of osmolality in the medulla and a urinary concentrating defect and promotes free water excretion [30, 31]. Since TLV increases urinary urea excretion, we speculate that TLV promotes free water clearance by the decrement of medullary urea concentration gradient in addition to by the suppression of AQP-2 expression.
The third and final points of the present study were that % decrease in [U-Cr] outperforms % decrease in U-OSM after TLV administration in discriminating TLV responders among HF patients. Previously, the U-OSM decrement was reported to be important in the short-term discrimination of TLV responders [13]. It is true that TLV decreases U-OSM by increasing free water excretion; however, the effect of decreasing U-OSM is partially attenuated by increased solute excretion by TLV. On the other hand, % decrease in [U-Cr] is thought to reflect the increased excretion of free water by TLV directly, because the excretion of urinary creatinine is not affected by TLV. In fact, the superiority of % decrease in [U-Cr] over % decrease in U-OSM in the discrimination of TLV responders was shown in the present study. For a more exact evaluation of aquaresis by TLV, CH2O is useful, but the measurement is slightly cumbersome, because simultaneous measurement of serum and urinary osmolality and urinary output is needed. On the other hand, % change in [U-Cr] can be easily calculated by two spot urine samples, and measurements of osmolality and urine output are not required.
The limitations of the present study are as follows. First, it was a retrospective study of a small number of patients in a single center. Second, we could not distinguish between 24-h urine samples and spot urine samples, which probably lead to decreased statistical power. Finally, we cannot exclude the possibility of the influence of dietary protein or sodium intake on BUN level or blood pressure, especially in an outpatient setting, although we did not change the recommended dietary allowance for protein or sodium intake before and after TLV therapy.
In conclusion, our data showed that: (1) high-dose TLV therapy increases the urinary sodium excretion and consequently lowers blood pressure in ADPKD patients; (2) TLV increases the urinary urea excretion; high-dose TLV therapy in ADPKD patients lowered BUN for at least 3 months; and (3) % decrease in [U-Cr] is more useful than % decrease in U-OSM after TLV administration in the discrimination of TLV responders among HF patients. Based on the effects of TLV on the excretion of these solutes, care must be taken in the interpretation of U-OSM changes produced by TLV in HF patients, and hypotension must be carefully monitored in ADPKD patients who receive TLV.
References
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–18.
Gattone VH 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–6.
Wang X, Gattone V 2nd, Harris PC, Torres VE. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol. 2005;16:846–51.
Perucca J, Bichet DG, Bardoux P, Bouby N, Bankir L. Sodium excretion in response to vasopressin and selective vasopressin receptor antagonists. J Am Soc Nephrol. 2008;19:1721–31.
Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, et al. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol. 2000;279:F46–53.
Sun A, Grossman EB, Lombardi M, Hebert SC. Vasopressin alters the mechanism of apical Cl− entry from Na+:Cl− to Na+:K+:2Cl− cotransport in mouse medullary thick ascending limb. J Membr Biol. 1991;120:83–94.
Bankir LT, Trinh-Trang-Tan MM. Renal urea transporters Direct and indirect regulation by vasopressin. Exp Physiol. 2000;85:243s–52s.
Kim SR, Hasunuma T, Sato O, Okada T, Kondo M, Azuma J. Pharmacokinetics, pharmacodynamics and safety of tolvaptan, a novel, oral, selective nonpeptide AVP V2-receptor antagonist: results of single- and multiple-dose studies in healthy Japanese male volunteers. Cardiovasc Drugs Ther. 2011;25(Suppl 1):S5–17.
Kajimoto K, Abe T. Blood urea nitrogen as a marker of the acute response to addition of tolvaptan to standard therapy in patients hospitalized for acute heart failure syndromes. Int J Cardiol. 2014;177:589–91.
Gilotra NA, Russell SD. Arginine vasopressin as a target in the treatment of acute heart failure. World J Cardiol. 2014;6:1252–61.
Konstam MA, Gheorghiade M, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–31.
Matsuzaki M, Hori M, Izumi T, Fukunami M, Tolvaptan I. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study). Cardiovasc Drugs Ther. 2011;25(Suppl 1):S33–45.
Imamura T, Kinugawa K, Shiga T, Kato N, Muraoka H, Minatsuki S, et al. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients. Circ J. 2013;77:397–404.
Nishizawa Y, Nakamura T, Ohta H, Kushida K, Gorai I, Shiraki M, et al. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004). J Bone Miner Metab. 2005;23:97–104.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7.
Japanese Society of Nephrology. Evidence-based Clinical Practice Guideline for CKD 2013. Clin Exp Nephrol. 2014;18:346–423.
Mori T, Oba I, Koizumi K, Kodama M, Shimanuki M, Tanno M, et al. Beneficial role of tolvaptan in the control of body fluids without reductions in residual renal function in patients undergoing peritoneal dialysis. Adv Perit Dial. 2013;29:33–7.
Afsar B, Ortiz A, Covic A, Solak Y, Goldsmith D, Kanbay M. Focus on renal congestion in heart failure. Clin Kidney J. 2016;9:39–47.
Schrier RW. Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2009;20:1888–93.
Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2255–66.
Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142:342–51.
Rinkel GJ. Natural history, epidemiology and screening of unruptured intracranial aneurysms. J Neuroradiol. 2008;35:99–103.
Yang B, Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Renal Physiol. 2005;288:F881–96.
Fenton RA, Flynn A, Shodeinde A, Smith CP, Schnermann J, Knepper MA. Renal phenotype of UT-A urea transporter knockout mice. J Am Soc Nephrol. 2005;16:1583–92.
Gheorghiade M, Konstam MA, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–43.
Koeth RA, Kalantar-Zadeh K, Wang Z, Fu X, Tang WH, Hazen SL. Protein carbamylation predicts mortality in ESRD. J Am Soc Nephrol. 2013;24:853–61.
Drechsler C, Kalim S, Wenger JB, Suntharalingam P, Hod T, Thadhani RI, et al. Protein carbamylation is associated with heart failure and mortality in diabetic patients with end-stage renal disease. Kidney Int. 2015;87:1201–8.
Kalim S, Karumanchi SA, Thadhani RI, Berg AH. Protein carbamylation in kidney disease: pathogenesis and clinical implications. Am J Kidney Dis. 2014;64:793–803.
Pietrement C, Gorisse L, Jaisson S, Gillery P. Chronic increase of urea leads to carbamylated proteins accumulation in tissues in a mouse model of CKD. PLoS One. 2013;8:e82506.
Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA. 2004;101:7469–74.
Li F, Lei T, Zhu J, Wang W, Sun Y, Chen J, et al. A novel small-molecule thienoquinolin urea transporter inhibitor acts as a potential diuretic. Kidney Int. 2013;83:1076–86.
Acknowledgements
This study received no specific external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HI., T.H., and Y.I. have received grant support and lecture fees from Otsuka Pharmaceutical Company, which sells tolvaptan in Japan. The company played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; nor in the preparation, review, or approval of the manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB approval number 16322) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
We provided all individual patients with the option to opt out of participation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Minami, S., Hamano, T., Iwatani, H. et al. Tolvaptan promotes urinary excretion of sodium and urea: a retrospective cohort study. Clin Exp Nephrol 22, 550–561 (2018). https://doi.org/10.1007/s10157-017-1475-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1475-9