Abstract
Background
There has been some controversy regarding the efficacy of sacral nerve stimulation (SNS) for the treatment of chronic constipation, due to less positive outcomes and concerns about cost-effectiveness in the long term. The aim of the present study was to evaluate the long-term outcomes of SNS in patients with chronic constipation.
Methods
A retrospective study was conducted on patients who had SNS for chronic constipation in 2008–2017 at our institution. Clinical factors, profile of constipation, physiology studies, and patient satisfaction with SNS therapy were investigated during a follow-up period up to 10 years after the implantation.
Results
Twenty-nine patients [86% female, median age 49 years (range 17–86)] were tested for SNS, and 24 received implants after a positive test phase [median 47 days (range 21–56 days)]. There were 27 bilateral and 2 unilateral implants, in S3 or S4 depending on best response. Mean follow-up was 59 months. Efficacy was considered as a score > 5 (on a scale of 1–10) in general symptom improvement. Nine (37.9%) implanted patients had a satisfaction score > 5. In 6 cases (25%), patient satisfaction was higher than 9. Due to the small sample size, there were no statistically significant variables considered as predictors of response.
Conclusions
Our results agree with current studies which describe around a 30% response of SNS for refractory constipation. However, there is a small group of patients highly satisfied with SNS therapy. More studies are needed to better understand this profile and optimize outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sacral nerve stimulation (SNS) has emerged as a treatment for a range of disorders, including chronic constipation. Constipation is a common condition with a prevalence in Spain of 14–30% [1]. It increases with age and has a male-to-female ratio ranging from 1.01 to 3.77 [2]. Although it is not a life-threatening condition, it has a considerable impact on quality of life and is responsible for a large economic burden [3,4,5]. The three main areas where it affects quality of life are general health, social functioning, and mental health [6]. Emotional well-being, perceived health, and vitality score are significantly lower in patients with constipation [7]. Based on colonic transit time and evacuation, constipation can be classified as slow-transit constipation, normal transit constipation, and defecatory disorders [8].
The initial management for chronic constipation involves behavioural therapy and lifestyle changes, such as increasing daily fibre and water intake, laxatives, enemas, and bowel irrigation [9,10,11]. Approximately 1% of these patients do not respond to conservative treatment [9] leaving surgery as the next therapeutic option. Before SNS, which is a minimally invasive surgical technique [12, 13], the only surgical options available were subtotal or partial colectomy or colostomy [14]. Subtotal colectomy with ileorectal anastomosis is the standard technique in most centres, although in a study by Dong Yang et al., subtotal colectomy with cecorectal (preserving the ileocecal valve) end to end anastomosis had optimal results [15].
SNS has been established as an adequate procedure for fecal incontinence since 1995 [16] and since then has also shown promising results in other defecation disorders, including constipation. However, results have been contradictory when used for constipation, and the effectiveness of SNS has been widely debated [17]. The procedure itself is minimally invasive, usually performed in two stages. In the first stage, a lead is placed through the selected sacral foramina (usually S3) and connected to an external battery which provides the stimulation. If the patient has symptomatic improvement during a trial period, the second stage is performed, and a permanent battery is connected to the lead and placed subcutaneously in the gluteal area.
In our centre, SNS has been used for chronic constipation since 2008 with mixed results. The aim of this study was to evaluate the long-term outcomes of SNS in patients with chronic constipation, following the Rome III criteria [18] as well as determining what clinical factors contribute to more accurate patient selection.
Materials and methods
Notes of patients with chronic constipation between 2008 and 2017 treated with SNS at University Hospital La Paz in Madrid were reviewed. Chronic constipation was defined following the Rome III Criteria:
-
Fewer than three bowel movements per week
-
Straining or sense of incomplete evacuation associated with at least 25% of bowel movements.
-
Lumpy, hard stools, or anal digitation for at least 25% of bowel movements.
These symptoms had to be present for at least 6 months before the diagnosis.
SNS was proposed to adults with chronic constipation (as defined above) at evaluation by a colorectal surgeon. Exclusion criteria were significant pelvic floor abnormality, constipation secondary to anorectal malformation, irritable bowel syndrome, and contraindication for SNS (pregnancy, anatomic limitations for the placement of the device, and skin disease with risk of infection).
The data collected at baseline included patient demographics, Wexner constipation score, bowel habit, time with symptoms, and use of medication.
Before having the SNS procedure, patients were assessed with a set of tests that included anorectal manometry, colonic-transit time, and endoanal ultrasound. Patients were seen in the outpatient clinic, where the surgical procedure was explained and informed consent was obtained.
Prior to beginning data collection and contacting patients, our Hospital’s Ethics Committee reviewed the protocol and gave its approval.
For the follow-up, medical records to date were revised. Notes collected at the appointments in the general surgery outpatient clinic were reviewed for all patients included in our study. Follow-up included weekly visits during the first month after surgery and yearly visits at the outpatient clinic for at least 5 years. Out of 29 patients included, 21 were contacted by phone by the first author (SGC) up to 10 years after surgery. They were interviewed about the use of laxatives or enemas after the implantation, the period during which the device was effective and the overall satisfaction with the device, as these data were not collected in the outpatient clinic. This last item was assessed on a scale of 1–10; 10 being the best score possible and 1 the worst. As a general rule in Spanish culture, 5 is considered the minimum passing score [19]. Those who gave the device a score of 5 or higher considered the therapy to be effective.
Surgical procedure
The SNS procedure was performed in the operating room (OR) under local anesthesia and sedation with basic anesthetic monitoring (electrocardiography, pulse oximetry, and blood pressure measurement). The local anesthetic (1% lidocaine and 0.5% levobupivacaine) was infiltrated around the whole planned surgical field, and where the battery placement site. For the implantation of the electrodes, we prefer the patient to remain alert to be able to communicate and locate the area of stimulation, so in the first phase only anxiolytics (1 or 2 mg midazolam) and analgesia (an infusion of remifentanil 0.05–0.1 mcg/kg/min) were administrated. After locating the sacral nerves and placing the electrodes, a deeper sedation was delivered by propofol infusion at 1–2 mg/kg/h. All patients received one dose of prophylactic antibiotics (amoxicillin-clavulanic acid 2 g or metronidazole and gentamicin if allergic to β-lactams) prior to surgery. No antibiotics were given postoperatively.
During the first procedure and under X-ray vision, a tetrapolar electrode (Medtronic InterStim model 3057, Minneapolis, MN, USA) was placed in the third or fourth sacral foramen (S3 or S4). The adequate sensory and/or motor response was tested at this point and the electrode was then connected to an external pulse generator (Medtronic model 3625) with an extension lead. Patients were discharged on the day of surgery.
Patients were reviewed at weekly appointments in the general surgery outpatient clinic. During these weekly check-ups, the patient’s response and symptomatic progress were evaluated by the surgeon.
A successful response to treatment is considered if the symptoms of chronic constipation have improved.
To be eligible for permanent neurostimulator implantation, the patient had to fulfil at least one of the following criteria: (a) a reduction to < 50% in the number of episodes of straining and/or a decrease by > 50% in the sensation of incomplete evacuation, as recorded in their bowel habit diary over the trial period; (b) a subjective improvement of symptoms in the absence of an increase in the use of laxatives, enemas or manual stimulation; and (c) an increase in frequency of evacuation to ≥ 3 bowel movements per week.
For the permanent implantation of the device, a second surgical procedure was performed in the OR under the same conditions previously described for the first stage. The electrode was then connected to the permanent neurostimulator battery (Interstim I, Interstim II or TWIN, Medtronic) which was placed in a subcutaneous gluteal pocket.
Adverse events after both procedures were recorded, as well as the need to replace or remove the device due to side effects of the therapy or loss of effectiveness.
Statistical analysis
Statistical analysis was performed using SPSS. The tests used for the analysis were Chi-square, Wilcoxon, and Pearson, according to whether data were categorical or ordinal and their distribution. A p value of less than 0.05 was considered statistically significant.
Results
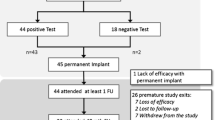
Between May 2008 and July 2017, 29 patients, 86.2% female (25), and 13.8% male (4) with a median age of 49 years (range 17–86 years) were eligible for the study (Fig. 1). Twenty-seven patients had idiopathic constipation and two had a cauda equina syndrome, which was the cause of constipation. The most frequent comorbidity was depression, which was present in four of the patients (13.7%).
During the preoperative assessment, endoanal ultrasound did not reveal any anatomical lesions in 6 (24.1%) of the patients. The most common lesion was a defect in the external anal sphincter (seven patients). The mean colonic-transit time was greater than the normal range in 20 out of the 29 patients: 2 patients had a lengthened transit of the left colon, 3 of the right colon, and 15 had pan colonic slow transit. Three patients had obstructed defecation syndrome (ODS) and 6 had constipation of undetermined causes. Anorectal manometry was performed in 19 patients and 16 (84.2%) had a decreased rectal sensitivity (Table 1).
Fourteen patients (48.2%) had lived with constipation for as long as they could recall, with 1 year being the minimum time of symptoms before being eligible for SNS (3.4%) (Fig. 2).
During the first procedure, electrodes were placed in the third or fourth sacral foramina. Depending on the motor response, they could be placed unilaterally or bilaterally. Bilateral electrodes were either placed at the same level (both in S3 or S4) or combining S3 and S4 of each side (Table 2).
Twenty-four (82.2%) patients fulfilled the criteria for permanent implantation after the trial period [median 47 days (range 21–56 days)]. Therefore, there was a 17.8% rate of early failure as five patients did not improve their symptoms after the trial period.
After implantation of the permanent device, 20 patients (85.7%) considered the treatment to be effective after 1 month. During follow-up, ten patients (47.6%) continued using laxatives and only three patients (14.3%) needed enemas.
The median duration of follow-up was 59 months (range 7–108 months). Eleven patients (37.9%) considered neuromodulation as an effective treatment option for constipation. After this period, out of the total number of patients (29), 2 died of conditions not related to the device implantation, 21 were contacted by phone for evaluation, and 1 patient could not be reached (Fig. 3).
Out of 12 patients with normal endoanal ultrasonography, 8 (66.7%) had to use laxatives after the SNS implantation. As for the 12 patients with a defect, only 3 (25%) had to use laxatives after SNS (p > 0.45).
No statistically significant correlation could be established between the clinical variables (sex, age, and time with symptoms) and the efficacy of neurostimulation.
Electrode position (S3, S4, or combined) did not influence the clinical outcome after the test phase nor the long-term efficacy.
The most common complications were infection of the surgical site 20.8% (n = 5) and discomfort/pain in the gluteal pocket 20.8% (n = 5).
A total of 10 (41.6%) SNS devices were removed by 51 months follow-up: four due to infection, two due to pain in the gluteal pocket, and four due to loss of efficacy. All patients who had signs of infection were treated with oral antibiotics. If the treatment failed to resolve the infection, both stimulator and electrode(s) were removed. Battery replacement was performed in one patient who reported pain in the gluteal pocket, with good results after the procedure. All complications were treated in an outpatient setting.
Discussion
We have demonstrated reasonable success for SNS in the treatment of chronic constipation. In our study, 24 patients were eligible for a permanent implant after a trial period, most of whom we deemed to have been treated effectively with SNS.
In 2016, Zerbib et al. [20] published a randomized clinical trial featuring 43 patients: only 20 of them responded after the trial period and got a permanent implant. After 1 year of follow-up, only 11 had a sustained response. Maeda et al. [13] published a multicentre study with a cohort of 62 patients with permanent implantation, where only 14 had sustained improvement after 60 months. Patton et al. [21] published a randomized trial, in which after 5.7 years, 47 patients out of 53 had withdrawn because of treatment failure. All three studies conclude that SNS cannot be recommended as a standard treatment for refractory constipation.
In our study, 16 out of 19 patients who had anorectal manometry were found to have decreased rectal sensitivity. No significant differences could be found between patients with decreased rectal sensitivity and those with no abnormalities; although all patients (except 1 with ODS) responded to temporary implantation. In 2012 Knowles et al. published similar results with a small series of 13 female patients, all with rectal hyposensitivity, and all responded to temporary SNS. They concluded that patient selection should be approached selecting physiologically defined subgroups [22].
As already mentioned, constipation is not usually a life-threatening condition, but it is associated with poorer quality of life [7, 8]. Patients generally start with behavioural therapy escalating until daily laxatives or enemas and retrograde bowel irrigation [23]. After the SNS procedure, only 47% of the patients had to take laxatives compared to the 82% before SNS. The reduced need for medication to relieve the symptoms of constipation can improve patients’ quality of life. Carriero et al. in 2010, tested 45 patients with a psychological evaluation, excluding patients with psychological distress, with a success rate of 84% [24]. We did not find a clinical correlation between patients’ comorbidities and the response to SNS.
After 51 months of follow-up, four devices had to be removed due to infection, two due to pain in the gluteal pocket and four due to loss of efficacy. Maeda et al. in a systematic review reported that the most common complication after the permanent implant was pain around the stimulator site, infection being the second most common [25]. No major adverse events were recorded. Despite the use of prophylactic antibiotic as recommended by the recent European consensus [26], five devices had to be removed because of infection. In our institution, these cases were some of the initial ones, where the learning curve and absence of a specific protocol for prophylaxis and skin disinfection in SNS might have played a role in the high incidence of infection and need for removal.
An important limitation of our study is the small sample size and heterogeneous nature of the series, as well as a change in the SNS protocol and technique over time. With this retrospective analysis we could not find any significant correlations between the clinical factors recorded in our study and the success of the therapy. Furthermore, we were not able to find any correlation in the level at which electrodes were placed (S3, S4, or combined) and the effectiveness of the therapy.
Further randomized trials with larger cohort should be carried out to identify different factors that could lead to better patient selection. Denmark is already enrolled in a randomized multicentre controlled trial that should end in 2021, with the primary objective of assessing the effectiveness of SNS compared to personalized treatment [9].
Conclusions
SNS could be a valid therapy for refractory constipation in carefully selected cases, as seen for 37.9% of the patients treated in our study. Improvement of patient selection is needed.
References
Serra J et al (2017) Guía de práctica clínica sobre el manejo del estreñimiento crónico en el paciente adulto: Parte 2: diagnóstico y tratamiento. Gastroenterol. Hepatol. 40:303–316
Knowles CH (2017) Constipation. In: Herold A, Lehur PA, Matzel KE, O’Connell PR (eds) Coloproctology, 2nd edn. Springer, Germany, pp 103–120
Miller LE, Ibarra A, Ouwehand AC (2017) Normative values for colonic transit time and patient assessment of constipation in adults with functional constipation: systematic review with meta-analysis. Clin Med Insights Gastroenterol. https://doi.org/10.1177/1179552217729343
Carrington EV et al (2014) A systematic review of sacral nerve stimulation mechanisms in the treatment of fecal incontinence and constipation. Neurogastroenterol Motil 26:1222–1237
Dinning PG et al (2015) Treatment efficacy of sacral nerve stimulation in slow transit constipation: a two-phase, double-blind randomized controlled crossover study. Am J Gastroenterol 110:733–740
Serra J et al (2017) Guía de práctica clínica sobre el manejo del estreñimiento crónico en el paciente adulto: parte 1: definición, etiología y manifestaciones clínicas. Gastroenterol Hepatol 40:132–141
Marquis P, De La Loge C, Dubois D, McDermott A, Chassany O (2005) Development and validation of the patient assessment of constipation quality of life questionnaire. Scand J Gastroenterol 40:540–551
Belsey J, Greenfield S, Candy D, Geraint M (2010) Systematic review: Impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther 31:938–949
SCM H et al. (2017) Sacral neuromodulation versus personalized conservative treatment in patients with idiopathic slow-transit constipation: study protocol of the No. 2-trial, a multicentre randomized controlled trial. Color. Disease Conference 12th Scientific Annual Meeting European Society of Coloproctology Gastroenterology, pp 493–501. doi: 10.1111/codi.13799
Schiano-di-Visconte M et al (2018) Effectiveness of sacral nerve stimulation in fecal incontinence after multimodal oncologic treatment for pelvic malignancies: a multicenter study with 2-year follow-up. Tech Coloproctol 22:97–105
Thomas GP, Dudding TC, Rahbour G, Nicholls RJ, Vaizey CJ (2013) Sacral nerve stimulation for constipation. Br J Surg 100:174–181
Zeiton M, Faily S, Nicholson J, Telford K, Sharma A (2016) Sacral nerve stimulation—hidden costs (uncovered). Int J Colorectal Dis 31:1005–1010
Maeda Y et al (2017) Long-term outcome of sacral neuromodulation for chronic refractory constipation. Tech Coloproctol 21:277–286
Frattini JC, Nogueras JJ (2008) Slow transit constipation: A review of a colonic functional disorder. Clin Colon Rectal Surg 21:146–152
Yang D, He L, Su T-R, Chen Y, Wang Q (2018) Outcomes of laparoscopic subtotal colectomy with cecorectal anastomosis for slow-transit constipation: a single center retrospective study. Acta Chir Belg 119:1–5
Lumi CM et al (2016) Neuromodulación sacra: resultados a largo plazo. Acta Gastroenterol Latinoam 46:82–94
Pilkington SA et al (2017) Surgery for constipation: systematic review and practice recommendations: Results V: sacral Nerve stimulation. Color Dis 19:92–100
Xin HW et al (2014) Diagnosis of functional constipation: agreement between Rome III and Rome II criteria and evaluation for the practicality. J Dig Dis 15:314–320
Ley Orgánica 2/2006, de 3 de mayo, de Educación. BOE 22-06-2007, núm. 149, pág. 27049.
Zerbib F et al (2017) Randomized clinical trial of sacral nerve stimulation for refractory constipation. Br J Surg 104:205–213
Patton V, Stewart P, Lubowski DZ, Cook IJ, Dinning PG (2016) Sacral nerve stimulation fails to offer long-term benefit in patients with slow-transit constipation. Dis Colon Rectum 59:878–885
Knowles CH, Thin N, Gill K, Bhan C (2012) Prospective randomized double-blind study of temporary sacral nerve stimulation in patients with rectal evacuatory dysfunction and rectal hyposensitivity. Ann Surg 255(4):643–649
Kamm MA et al (2010) Sacral nerve stimulation for intractable constipation. Gut 59:333–340
Carriero A, Martelluci J, Talenento P, Ferrari CA (2010) Sacral nerve stimulation for constipation: do we still miss something? Int J Colorectal Dis 25:1005–1010
Maeda Y, Matzel K, Lundby L, Buntzen S, Laurberg S (2011) Postoperative issues of sacral nerve stimulation for fecal incontinence and constipation: a systematic literature review and treatment guideline. Dis Colon Rectum 54:1443–1460
Maeda Y et al (2015) Sacral nerve stimulation for faecal incontinence and constipation: a European consensus statement. Color Dis 17:O74–O87
Funding
The authors did not receive grants or funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. IRP, IPM, JLMM report personal fees from Medtronic related to the organization of educational workshops or educational lectures on Sacral Nerve Stimulation, outside the submitted work.
Ethical approval
Prior to beginning data collection and contacting patients, our Hospital’s Ethics Committee reviewed the protocol and gave its approval.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Gortazar de las Casas, S., Rubio-Pérez, I., Saavedra Ambrosy, J. et al. Sacral nerve stimulation for constipation: long-term outcomes. Tech Coloproctol 23, 559–564 (2019). https://doi.org/10.1007/s10151-019-02011-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-019-02011-z