Abstract
Background
Anal fissure has a very large number of treatment options. The choice is difficult. In an effort to assist in that, choice presented here is a systematic review and meta-analysis of all published treatments for anal fissure that have been studied in randomized controlled trials.
Methods
Randomized trials were sought in the Cochrane Controlled Trials Register, Medline, EMBASE and the trials registry sites clinicaltrials.gov and who/int/ictrp/search/en. Abstracts were screened, full-text studies chosen, and finally eligible studies selected and abstracted. The review was then divided into those studies that compared two or more surgical procedures and those that had at least one arm that was non-surgical. Studies were further categorized by the specific interventions and comparisons. The outcome assessed was treatment failure. Negative effects of treatment assessed were headache and anal incontinence. Risk of bias was assessed for each study, and the strength of the evidence of each comparison was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach.
Results
One hundred and forty-eight eligible trials were found and assessed, 31 in the surgical group and 117 in the non-surgical group. There were 14 different operations described in the surgical group and 29 different non-surgical treatments in the non-surgical group along with partial lateral internal sphincterotomy (LIS). There were 61 different comparisons. Of these, 47 were reported in 2 or fewer studies, usually with quite small patient samples. The largest single comparison was glyceryl trinitrate (GTN) versus control with 19 studies. GTN was more effective than control in sustained cure (OR 0.68; 95% CI 0.63–0.77), but the quality of evidence was very poor because of severe heterogeneity, and risk of bias due to inadequate clinical follow-up. The only comparison to have a GRADE quality of evidence of high was a subgroup analysis of LIS versus any medical therapy (OR 0.12; CI 0.07–0.21). Most of the other studies were downgraded in GRADE due to imprecision.
Conclusions
LIS is superior to non-surgical therapies in achieving sustained cure of fissure. Calcium channel blockers were more effective than GTN and with less risk of headache, but with only a low quality of evidence. Anal incontinence, once thought to be a frequent risk with LIS, was found in various subgroups in this review to have a risk between 3.4 and 4.4%. Among the surgical studies, manual anal stretch performed worse than LIS in the treatment of chronic anal fissure in adults. For those patients requiring surgery for anal fissure, open LIS and closed LIS appear to be equally efficacious, with a moderate GRADE quality of evidence. All other GRADE evaluations of procedures were low to very low due mostly to imprecision.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of anal fissure was varied and chaotic until 1951 when Eisenhammer proposed using partial lateral internal sphincterotomy (LIS). He also combined LIS with a rather liberal dilation of the anal canal after the sphincterotomy. He was the first to list the number of patients treated by this method and reported that none had any defecation difficulties afterward [1]. This procedure was enthusiastically adopted by surgeons around the world. It was widely believed that incontinence was not an issue after sphincter division [2]. An early study to quantify continence disturbance published in 1985 stated that of 306 patients who had undergone LIS at least 1 year earlier, only 15 suffered from any degree of incontinence and this was principally only incontinence to flatus. In none was it severe enough for the patient to wear a pad [3].
However, in 1989 everything changed. Khubchandani published a large case series of follow-up after LIS in which 36% of the patients were incontinent to flatus and 5% to solid stool [4]. In 1996, a group from the University of Minnesota, which had reported the low incontinence rate in 1985 [3], in a retrospective comparison of open versus closed LIS, found that 30.3% of their patients were incontinent to flatus and 11.8% to solid stool [5]. The age of glyceryl trinitrate (GTN) ointment, botulinum toxin (Botox) injection and calcium channel blockers (CCBs) was born. It appears that in many countries, LIS had been abandoned in favor of medical therapy [6]. In one systematic review of anal incontinence following LIS, 22 studies, mostly non-randomized case series or cohorts, found an overall incontinence rate of 14% with less than 1% having incontinence to solid stool [7]. Yet patient satisfaction with LIS has been reported to be high [8]. The often crippling pain of fissure is almost immediately relieved by LIS.
Nevertheless, the fear of incontinence has resulted in a rapid expansion in the number of treatment options for anal fissure. The goal was to find a medicine or surgical procedure that simulated the high success rate of LIS and avoided the presumed high risk of postoperative incontinence. We performed a systematic review of all the published treatment options for anal fissure that have been subjected to randomized clinical trials only. The review was divided structurally into two halves. The first is comparison of surgical procedures only. The second is a comparison of non-surgical (usually pharmacological) treatments to either best supportive care, to other non-operative treatments or to a surgical procedure, which in all cases was LIS.
This type of review and meta-analysis is needed because individual reports of surgical procedures and non-medical therapies are variable in their results and under-powered. In addition, this approach allows assessment of the risk of bias in each publication as well as a combined assessment of the strength of the evidence for each individual intervention.
Materials and methods
Surgical procedures
Trials in which participants were randomized to a surgical procedure and either no treatment or an alternative surgical procedure were eligible for inclusion in this part of the review. Studies that compared any surgical procedure to any non-surgical procedure were not included in this section, but in a separately searched and analyzed group described below. Cluster- and group-randomized trials were also eligible but were not found.
Participants eligible for this portion of the review were patients with chronic anal fissure. Chronic anal fissure is typically described as an anal fissure which lasts more than 4–6 weeks, or which has characteristic features such as a sentinel pile, bare internal sphincter, heaped up edges or hypertrophied anal papillae. As it is common practice among surgeons reporting this disease not to operate on acute fissures, or fissures in children, or atypical fissures (multiple, irregular, off the midline or not associated with sphincter spasm), these were not eligible for inclusion in this section of the review.
Non-operative therapy
Studies in which participants were randomized to non-surgical treatment for anal fissure are the focus of this part of the review. Comparison groups in each of these studies may include a surgical procedure, medical therapy, or a control group consisting of no treatment, supportive care or placebo. Supportive care may consist of dietary fiber, laxatives or warm baths, lubricants, and even topical anesthetics, applied sometimes equally to both groups and sometimes only to the control group. Similar to the above group, cluster- and group-randomized trials were also eligible but were not found. Acute fissure will be included in this part of the review (see “Discussion” section).
Outcome measures
The main outcome measures were the following: fissure non-healing
-
Fissure recurrence.
-
Anal incontinence incidence—assessed mostly but not exclusively in surgical studies. In most cases, it was specified that it was minor incontinence, to flatus or anal seepage.
-
Headache—assessed mostly but not exclusively in GTN and isosorbide mononitrate (ISMN) studies.
The primary outcome analyzed and reported in this entire review is fissure treatment failure, which is a combination of primary non-healing and recurrence after apparent healing. This is the inverse of sustained fissure healing. This combination of the two outcomes often reported as stated above was done because due to the waxing–waning nature of fissure, it was problematic to differentiate persistence from recurrence (see “Discussion” section).
Adverse events, also primary outcomes, were principally anal incontinence and headache.
A secondary outcome found is the anal incontinence score which was only rarely reported.
Other outcomes reported were pain, bleeding, infection, but all of these were only reported sporadically, in varying metrics, and so are not analyzed in this review.
Literature search
A literature search (Fig. 1) was conducted to identify all published and unpublished randomized controlled trials with no language restriction, using the following electronic databases to identify potential studies including:
-
The Cochrane Central Register of Controlled Trials (Issue 3, 2017).
-
Ovid Medline (1950 to January 18, 2017).
-
EMBASE (January 17, 2017).
-
ClinicalTrials.gov and the World Health Organization’s Internet clinical trial portal (ICTRP) were searched to March 7, 2017.
Study selection and review
Screening of each title and abstract and full text, data abstraction, data entry and risk of bias assessments were all conducted by at least 3 reviewers. All differences were resolved by the whole group discussion. The risk of bias of each study was assessed against key criteria: random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting [9]. Another most significant source of bias in this review was duration of follow-up after completing therapy (see “Discussion” section). The following judgments were used: low risk, high risk and unclear (either lack of information of uncertainty over the potential for bias).
Authors were contacted for missing data. When data on non-healing were unavailable, such as drop outs or losses to follow-up, the missing data, if they could be assigned to a treatment group, were treated as treatment failures, the last observation brought forward [10].
Clinical heterogeneity was sought in the performance of the surgery, or administration of the treatment, the veracity of the diagnosis and the accuracy and timing of outcome assessment. That is, how comparable were data from the included studies for meta-analysis. Methodological heterogeneity was sought in the differential risks of bias between studies. Statistical heterogeneity was calculated in Revman [9]. It was defined as a Chi-square (p < 0.10) and I-square (I 2) (>60%).
Subgroup analyses were done to investigate sources of heterogeneity in meta-analyses. Sensitivity analyses were done after eliminating the studies found to be of poor quality to assess the robustness of the results of the meta-analysis.
There are three nitrous oxide donors that have been tested for their ability to heal anal fissure: glyceryl trinitrate or nitroglycerin (GTN), isosorbide dinitrate (ISDN) and isosorbide mononitrate (ISMN). These three have been combined in these analyses. Their mechanisms of action are the same, and in one small trial, they were found to have a similar effect on healing fissure (#24 in Table 2). Similarly nifedipine and diltiazem have been combined in the review as CCBs (calcium channel blockers).
Data were analyzed using Revman 5.3. Results were expressed as odds ratios (OR) and 95% confidence intervals(CI) for the dichotomous outcomes, using the random effects model because of the heterogeneity seen in most of the larger comparisons.
The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) [11, 12] approach was used to classify the quality of evidence for each intervention of the three primary outcomes (treatment failure, incidence of incontinence and other adverse events, principally headache) into one of the four grades:
-
1.
High: Further research is very unlikely to change our confidence in the estimate of effect;
-
2.
Moderate: Further research is likely to have an impact on our confidence in the estimate of effect and may change the estimate;
-
3.
Low: Further research is very likely to have an important impact on our confidence on the estimate of effect and is likely to change the estimate;
-
4.
Very low: Any estimate of effect is very uncertain.
Each intervention began with a high quality of evidence and was downgraded either one or two points to moderate, low or very low depending on whether any of the five factors listed below were present and how seriously (one or two steps down) the factor impacted the data.
-
1.
Risk of bias as described above;
-
2.
Inconsistency (unexplained heterogeneity and thus inconsistency of results) [13];
-
3.
Indirectness (indirect or atypical populations, interventions, controls, outcomes. For instance, if a surrogate outcome was measured in some studies, such as anal pain only, without examining for fissure healing);
-
4.
Imprecision, as made evident by wide confidence intervals, small study size, two or fewer studies and/or fewer than 100 events in that comparison. Random error in that case is too great [14].
-
5.
Publication bias: Evidence either graphically in funnel plots or from review of trials registries that many potentially included studies have not been published.
Absolute risk reduction was measured using GRADEPro software [12].
Results
Search results
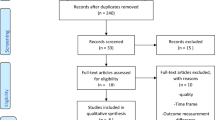
In the surgical review, 931 abstracts were found; 499 remained after duplicates were removed. From these, 462 studies were excluded and 37 reviewed in full text; 31 were chosen for inclusion and reported 2606 patients with fissure [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Results of the search for this update are shown in the PRISMA diagram (Fig. 2).
In the non-surgical portion of the review, 1221 abstracts were found and 663 remained after duplicates were removed. From these, 494 studies were excluded and 170 reviewed in full text, 117 being chosen for inclusion with 9456 participants [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162]. The study by Oueidat [125] was excluded as the abstract was never published in full text. Results for the search for this update are shown in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) diagram (Fig. 3).
Fifty-nine publications were read in full text and excluded from the review. The reasons for exclusion mostly fell into three categories:
-
They were non-randomized trials.
-
They were abstracts only from meeting presentations. There were therefore insufficient data in these for this review, and in most cases they were years old, so it is unlikely that a full publication will ever occur.
-
Fissure healing was not an endpoint of the study.
Interventions
The interventions described in the included studies of the surgical review are listed in Table 1, and for the non-surgical review in Table 2. Along with the interventions are:
-
The comparator for each test intervention,
-
The number of included randomized studies for that intervention,
-
The number of patients in each treatment group,
-
The number of events found in each treatment group, the event being the inverse of sustained fissure healing, i.e., failure of fissure therapy, or incontinence or headache in the case of adverse events,
-
The OR and CI obtained from a meta-analysis of the studies included in each intervention,
-
The GRADE classification of the strength of the evidence for that intervention and the reason for each GRADE.
Risk of bias
Table 3 shows the prevalence of the components of the risk of bias in the whole segments of the review, surgical and non-surgical, as well as several of the most important interventions examined in this review. In addition, statistical heterogeneity is displayed for each of the specific interventions, both the Chi-square and I-square.
The sensitivity analysis of manual anal stretch versus LIS, eliminating 2 studies with significant quality issues, is shown to eliminate the statistical heterogeneity of the whole group (Table 1, #2). A subgroup analysis of LIS versus any non-surgical therapy was done to investigate the heterogeneity found in the whole group of 32 studies, choosing only those studies with more than 6 months of follow-up. Once again this analysis resolved the heterogeneity (Table 2 #10).
Numerous subgroup analyses were done to investigate the extreme heterogeneity in GTN versus control including elimination of all but the 4 largest studies, which had more than 100 patients each. These included 896 patients and 498 events (non-healing). In addition, an influence analysis was done eliminating one study at a time to see whether any single study altered the heterogeneity. In all cases, the Chi-square remained <0.00001 and I-square > 75%. The remarkable feature of this intervention was that 17 of 19 (89%) of the studies had inadequate follow-up. This introduces a major bias because it fails to detect persistence in a disease known to wax and wane, and recurrence (which may in fact be the same thing). GTN gave a sustained cure in 47.5% of the patients, and the control group had sustained cure in 38.6%. In this comparison as well as many others in the non-surgical review, a “control” group is variously described as best supportive care, involving laxatives, baths and sometimes topical anesthetics such as lidocaine (see Table 2 comparison 2) or occasionally a true placebo. These were combined in the analyses as a control group.
Grades of recommendation, assessment, development and evaluation (GRADE)
In the majority of interventions, the evidence is rated low to very low because the studies included in the interventions are under-powered and have significant quality issues related to selection bias, blinding and length of follow-up. Only 1 was rated as high, the subgroup analysis of LIS versus any medical therapy (Table 2 #10). The only weakness in this group was a high attrition rate, which would be expected with the longer follow-up. It is offset because one can upgrade GRADE if the effect is extreme and the information size otherwise adequate. It is for both in the case here (Table 2 #10). The medical therapies included in Table 2 #s 9 and 10 were, in all but 2 cases, either GTN, CCBs or Botox. The other two were arginine (2#14) and posterior tibial nerve stimulation (2#31). Publication bias is difficult to ascertain and quantify. Funnel plots were done in this review when there were at least 10 studies in a specific intervention. However, they are open to wide interpretation. A more interesting means of assessing publication bias is, in this age of trials registries, the World Health Organization Internet Clinical Trials Registry Portal (ICTRP). Many trials were found there which were not apparent in publication search. Many investigated substances that were previously unheard of. No cluster was found that would substantially change the GRADE rating of any of these interventions in this review.
Effects of interventions
The effects of interventions are varied. They are presented in Tables 1 and 2. The most important include:
Table 1, #2 | LIS is superior to manual anal stretch |
Table 1, #3 | Open and closed LIS are equally efficacious |
Table 1, #4 7 #5 | Length of LIS is important, but the quality of the evidence makes it difficult to determine which is best |
Table 1, #8 | Posterior internal sphincterotomy seems no worse than LIS in healing, though nonsignificantly worse than LIS with incontinence (not shown in the table; OR 4.46, 95% CI 0.47–42) |
GTN is superior to control, but with very poor quality of evidence (see below) | |
Table 2, #2 | GTN is superior to lidocaine |
Table 2, #5 | GTN is roughly equivalent to Botox |
Table 2, #6 | CCBs are superior to GTN |
Table 2, #10 | LIS is far superior in healing to medical therapies, but LIS is associated with an increased risk of minor incontinence (see “Discussion” section) |
Many of these interventions about which only one or two studies have been done have yielded interesting results that should be pursued in further investigations. These are listed in “Discussion” section.
Adverse events
Adverse event rates for the two principal adverse events related to anal fissure therapy are shown in Table 4. Many other adverse events are reported in the 148 included trials, but they were of low frequency and the various ascertainment methods used made determination of the compilation and rate impossible. Headache dominated nitrous oxide donor reports and so was also reported in the comparators to these interventions. Incontinence was reported in all surgical trials and so in the comparators to surgical procedures. Incontinence, in almost all cases, was described as minor, which was specified to mean in many studies incontinence to flatus and anal seepage. This often resolved over a year, implying that the symptoms were caused by a healing anal wound.
Discussion
There was only 1 comparison for which the quality of evidence was high, indicating that future research is unlikely to alter this finding, the subgroup analysis of LIS versus any medical therapy with at least 6-month follow-up (Table 2 #10). Figure 4 shows the forest plot for the entire group of 29 studies of LIS versus medical therapy, the last colored column showing which studies had adequate follow-up data. Figure 4 shows forest plots of the subgroup of studies with more than 6 months of follow-up, Fig. 4 being non-healing, with the remarkable OR of 0.12. Figure 4 shows that in these studies, LIS is still more likely than medical therapy to result in minor incontinence. This comparison (Table 2 #10) also highlights the weakness of comparison seen in Table 2 #1: GTN versus control. The predominance of short follow-up (2#1) is clearly the source of the extreme heterogeneity that causes the quality of the evidence in this important comparison to be very low despite the large number of included studies. Very high recurrence rates have been reported in patients whose fissures were initially healed by GTN, if they had 1 year of follow-up: 51 [163] and 67% [164]. In many of these cases, healing may have just been the usual behavior of the anal fissure rather than healing due to GTN.
The GRADE ranking of GTN versus control (Table 2 #1) is quite problematic. The number of studies and participants is large, and, using GRADE’s own rating, it seems unlikely that further research will change the relations seen in Fig. 4. And so upgrading it a bit, at least to poor would seem justified. Better studies with adequate follow-up would solve this problem.
At the other end of the spectrum, there are studies, many of which have original innovative ideas, but for which only a single study exists, usually with few patients. Random error cripples the impact of these studies, in spite of statistical significance, and they yield unreliable results [14]. Therefore, comparisons in all of Table 1 and in Table 2, #7 and #s 11–27, 30–43, all were downgraded in GRADE for serious or very serious imprecision.
The ones with the more interesting results were:
-
More controlled anal dilation than the older manual anal stretch: Table 1, #s 11, 12; Table 2 #4.
GTN patch versus GTN ointment: Table 2 #36, i.e., a patch as for angina on the chest or shoulder.
-
Clove oil versus lidocaine: Table 2, #15.
-
Botox anterior versus Botox posterior: Table 2, #26.
Several comparisons yielded moderate quality of evidence and so are more reliable, but future research may alter the summary effect:
-
Open versus closed LIS: Table 1, #3.
GTN versus lidocaine: Table 2, #2.
-
High- versus low-dose GTN: Table 2, #3.
-
GTN versus patient self-dilation at home: Table 2, #4.
-
GTN versus Botox: Table 2, #5.
-
GTN for 40 or 80 days: Table 2, #28.
-
Oligo-antigenic diet versus control diet (again): Table 2, #29.
GTN versus lidocaine is significant because prior to the introduction of GTN, CCBs and Botox, medical therapy consisted essentially of lidocaine, hydrocortisone (Table 2, #30) and bran (Table 2, #43) [101, 102]. With the passage of time and the introduction of newer medications, lidocaine, bran and hydrocortisone became only best supportive care as stated above and were thought to be ineffective in obtaining a sustained cure of anal fissure.
Other systematic reviews
The other global reviews of fissure therapy were published last in the Cochrane Library in 2011 and 2012 [165, 166]. These have now been updated here with the addition of 47 new randomized trials. A series of systematic reviews examined GTN, Botox, CCBs and LIS [167,168,169,170,171] individually. They were limited to English language publications and also separated primary healing from recurrence.
Acute anal fissure
This is described as a specific clinical entity. There are certainly anatomical components of chronic fissure that make it easy to differentiate from acute fissure. But another definition that separates acute from chronic fissure is duration of disease and that is where matters gets difficult. The definition varies greatly in published reports from as short as 2–4 weeks to as long as 3–6 months. Occasionally short episodes of symptoms widely separated in time may also diagnose chronicity. Patients often cross the border from acute to chronic while getting appointments or initiating therapy. Many acute fissures resolve spontaneously (or may come back later as chronic fissures). So do chronic fissures. It is generally believed that children do not get chronic fissures nor should children or adults with acute fissure ever have surgery. But there are reports in this review where exactly that happened [87]. This is the rationale for the integration of acute and chronic fissure in this review. It seemed too difficult to separate them. When interventions were limited to patients with acute fissure, it is stated in the tables.
Incontinence
Reconciling the quite shocking data concerning incontinence risk with LIS and the description in those publications of LIS-induced acquired incontinence as permanent with the data presented here is not easy. All the data in this review were obtained from prospective randomized trials, all approved by ethics committees, which are very sensitive to potential harms in clinical trials and recording of them, and the trials themselves, especially trials with an LIS component, had focused on incontinence risk. The term permanent incontinence is particularly unfortunate, since all anal incontinence is eminently treatable [172,173,174]. Retrospective review numbers are certainly subject to selection bias, but even that does not explain the disparity with what is described here and those publications. In the first surgical review, published in 2000, the risk of incontinence was 10%. Within the current surgical review, when LIS was compared to other operations, for publications after 2000, the risk has dropped to 3.4%. This may be due to the advent of effective medical therapy, and better patient selection for surgery, or more care being taken in surgery to avoid excessive sphincter injury. It should be noted that the risk of post-therapy incontinence related to LIS in this review is not radically different from what is seen after therapy with GTN, Botox or CCBs (Table 4).
The purpose of this review is not to establish guidelines for the treatment of anal fissure. There are numerous bodies that have that responsibility [174,175,176,177,178]. It is instead to give as detailed and unbiased as possible a summary of the evidence for all studied treatments for anal fissure in order to facilitate the creation of guidelines and to assist patients and doctors who need to understand the risks and benefits of fissure therapy.
Conclusions
LIS is the most effective treatment for anal fissure, curing all but 6% of patients. Late recurrences are very rare after LIS versus with medical therapy [179]. Minor incontinence is more likely with LIS than medical therapy (Fig. 4). The difference between LIS and medical therapy is significant, but the absolute risk alteration is small, increasing from 3 cases per 1000 patients with medical therapy to 14 cases per 1000 with LIS (95% CI 6–31). Open and closed (a euphemism meaning less open) LIS are equally effective. Manual anal dilation is inferior to LIS, but recent small studies suggest that more controlled dilation, either pneumatic, by speculum or by patients at home are just as effective as LIS and are not associated with any risk of incontinence. GTN, Botox and CCBs have been extensively investigated as treatments for acute and chronic anal fissure. They appear to be effective, but most studies have been marred by inadequate follow-up, thus missing late recurrences, which are common. Of the three, CCBs may be the most effective. More research is needed for all three with adequate follow-up. There is virtually no research on sequencing these drugs, i.e., if one fails, what is best to try next? Many other medications have been tried because of the less-than-perfect track record of the three above, but none have proven better, and in any case, the studies are too small and too few. Clove oil and sildenafil may be worth further investigation. Many unpublished studies can be found in this field, especially in ICTRP, but no results are available.
References
Eisenhammer S (1951) The surgical correction of chronic internal anal (sphincteric) contracture. S Afr Med J 25:486–489
Abcarian H (1980) Surgical correction of chronic anal fissure: results of partial internal sphincterotomy vs. fissurectomy—midline sphincterotomy. Dis Colon Rectum 23:31–36
Walker WA, Rothenberger DA, Goldberg SM (1985) Morbidity of internal sphincterotomy for anal fissure and stenosis. Dis Colon Rectum 28(11):832–835
Khubchandani IT, Reed JF (1989) Sequelae of internal sphincterotomy for chronic fissure in ano. Br J Surg 76(5):431–434
Garcia-Aguilar J, Belmonte C, Wong WD, Lowry AC, Madoff RD (1996) Open vs. closed sphincterotomy for chronic anal fissure: long-term results. Dis Colon Rectum 39(4):440–443
Ommer A (2015) Management of complications of fissure and fistula surgery. Chirurg 86(8):734–740
Garg P, Garg M, Menon GR (2013) Long-term continence disturbance after lateral internal sphincterotomy for chronic anal fissure: a systematic review and meta-analysis. Colorectal Dis 15(3):e104–e117
Hyman N (2004) Incontinence after lateral internal sphincterotomy: a prospective study and quality of life assessment. Dis Colon Rectum 47:35–38
Higgins JPT, Green S (ed) (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 the cochrane collaboration. www.cochrane-handbook.org
Armijo-Olivo S, Warren S, Magee D (2009) Intention to treat, compliance, drop outs and how to deal with missing data in clinical research: a review. Phys Ther Rev 14(1):36–49
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ (2011) GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64(4):383–394
GRADE Handbook. http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA, Norris S, Vist G, Dahm P, Shukla VK, Higgins J, Falck-Ytter Y, Schünemann HJ, GRADE Working Group (2011) GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol 64(12):1294–1302
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW Jr, Murad MH, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schünemann HJ (2011) GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol 64(12):1283–1293
Arroyo A, Perez F, Seerrano P, Candela F, Calpena R (2004) Open versus closed lateral sphincterotomy performed as an outpatient procedure under local anesthesia for chronic anal fissure: prospective randomized study of clinical and manometric results. J Am Coll Surg 199:361–367
Boulos PB, Araujo JG (1984) Adequate internal sphincterotomy for chronic anal fissure: subcutaneous or open technique? Br J Surg 71:360–362
Dudhamal TS, Baghel MS, Bhuyan C, Gupta SK (2014) Comparative study of Ksharasutra suturing and Lord’s anal dilatation in the management of Parikartika (chronic fissure-in-ano). Ayu 35(2):141–147
Ellis CN (2004) Anterior levatorplasty for the treatment of chronic anal fissures in females with a rectocele: a randomized controlled trial. Dis Colon Rectum 47:1170–1173
Elsebae MA (2007) A study of fecal incontinence in patients with chronic anal fissure: prospective, randomized, controlled trial of the extent of internal anal sphincter division during lateral sphincterotomy. World J Surg 31:2052–2057
Filingeri V, Gravante G (2005) A prospective randomized trial between subcutaneous lateral internal sphincterotomy with radiofrequency bistoury and conventional Parks’ operation in the treatment of anal fissure. Eur Rev Med Pharmacol Sci 9(3):175–178
Fischer M, Thermann M, Trobisch M, Sturm R, Hamelmann H (1976) Treatment of primary-chronic anal fissure through anal stretch or sphincterotomy [Die Behandlung der primar-chronischen analfissur durch dehnung des analkanales oder sphincterotomie]. Langenbeck’s Arch Chir 343:35–44
Gupta PJ, Kalaskar S (2003) Removal of hypertrophied anal papillae and fibrous anal polyps increases patient’s satisfaction after anal fissure surgery. Tech Coloproctol 7:155–158
Gupta PJ, Kalaskar S, Heda P (2008) Closed lateral internal sphincterotomy versus anal sphincterolysis for chronic anal fissure. Coloproctology 30:242–248
Gupta V, Rodrigues G, Prabhu R, Ravi C (2014) Open versus closed lateral internal anal sphincterotomy in the management of chronic anal fissures: a prospective randomized study. Asian J Surg 37(4):178–183
Hancke E, Schwaner S (2003) Chronische Analfissur—operative behandlung mit analdilatation, excision der analfissure versus laterale sphinkterotomie [Chronische Anafissur—oerative behandlung mit analdilatation, excision der analfissure versus laterale sphinkterotomie]. Coloproctology 25:95–105
Jensen SL, Lund F, Nielsen OV, Tange G (1984) Lateral subcutaneous sphincterotomy versus anal dilatation in the treatment of fissure in ano in outpatients: a prospective randomized study. Br Med J 289:528–530
Kang GS, Kim BS, Choi PS, Kang DW (2008) Evaluation of healing and complications after lateral internal sphincterotomy for chronic anal fissure: marginal suture of the incision vs. open left incision: prospective, randomized, controlled study. Dis Colon Rectum 51:329–333
Kortbeek JB, Langevin JM, Khoo RE, Heine JA (1992) Chronic fissure-in-ano: a randomized study comparing open and subcutaneous lateral internal sphincterotomy. Dis Colon Rectum 35:835–837
Leong AF, Seow-Choen F (1995) Lateral sphincterotomy compared with anal advancement flap for chronic anal fissure. Dis Colon Rectum 38:69–71
Magdy A, El Nakeeb A, Fouda EY, Youssef M, Farid M (2012) Comparative study of conventional lateral internal sphincterotomy, V-Y anoplasty, and tailored lateral internal sphincterotomy with V-Y anoplasty in the treatment of chronic anal fissure. J Gastrointest Surg 16:1955–1962
Marby M, Alexander-Williams J, Buchman P, Arabi Y, Kappas A, Minervini S, Gatehouse D, Keighley MR (1979) A randomized controlled trial to compare anal dilatation with lateral subcutaneous sphincterotomy for anal fissure. Dis Colon Rectum 22:308–311
Mentes BB, Ege B, Leventoglu S, Oguz M, Karadag A (2005) Extent of lateral internal sphincterotomy: up to the dentate line or up to the fissure apex. Dis Colon Rectum 48:365–370
Mentes BB, Guener MK, Leventoglu S, Akyuerek N (2008) Fine tuning of the extent of lateral internal sphincterotomy: spasm controlled vs. up to the fissure apex. Dis Colon Rectum 51:128–133
Mousavi SR, Sharifi M, Mehdikhah Z (2009) A comparison between the results of fissurectomy and lateral internal sphincterotomy in the surgical management of chronic anal fissure. J Gastrointest Surg 13:1279–1282
Olsen J, Mortensen PE, Krogh-Petersen I, Christiansen J (1987) Anal sphincter function after treatment of fissure-in-ano by lateral subcutaneous sphincterotomy versus anal dilatation. A randomized study. Int J Colorectal Dis 2:155–157
Pujahari AK (2010) Unilateral versus bilateral lateral internal sphincterotomy: a randomized controlled trial for chronic anal fissure in ano. Trop Gastroenterol 31(1):69–71
Ram E, Vishne T, Lerner I, Dreznik Z (2007) Anal dilation versus left lateral sphincterotomy for chronic anal fissure: a prospective randomized study. Tech Coloproctol. doi:10.1007/s10151-007-0373-7
Renzi A, Izzo D, Di Sarno G, Talento P, Torelli F, Izzo G, Di Martino N (2008) Clinical, manometric, and ultrasonographic results of pneumatic balloon dilatation vs. lateral internal sphincterotomy for chronic anal fissure: a prospective, randomized, controlled trial. Dis Colon Rectum 51(1):121–127
Saad AM, Omer A (1992) Surgical treatment of chronic fissure-in-ano: a prospective randomized study. East Afr Med J 69:613–615
Tauro LF, Shindhe VV, Aithala PS, Martis JJ, Shenoy HD (2011) Comparative study of glyceryl trinitrate ointment versus surgical management of chronic anal fissure. Indian J Surg 73(4):268–277
Wang ZY, Sun JH, Chen XJ (2005) Prospective randomized trial of optimum anal canal release treatment for chronic anal fissure. J Chin Integr Med 3(3):190–206
Wang ZY, Liu H, Sun JH, Mao XM, Xu WX, Wu YG et al (2011) [Mucosa advancement flap anoplasty in treatment of chronic anal fissures: a prospective, multicenter, randomized controlled trial]. Zhong xi yi jie he xue bao = J Chin Integr Med 9(4):402–409
Weaver RM, Ambrose NS, Alexander-Williams J, Keighley MR (1987) Manual dilatation of the anus vs lateral subcutaneous sphincterotomy in the treatment of chronic fissure in ano. Results of a prospective, randomized clinical trial. Dis Colon Rectum 30:420–423
Wiley M, Day P, Rieger N, Stephens J, Moore J (2004) Open vs. Closed lateral internal sphincterotomy for idiopathic fissure-in-ano: a prospective randomized controlled trial. Dis Colon Rectum 47:847–852
Yucel T, Gonullu D, Oncu M, Koksoy FN, Ozkan SG, Aycan O (2009) Comparison of controlled-intermittent anal dilatation and lateral internal sphincterotomy in the treatment of chronic anal fissures: a prospective randomized study. Int J Surg 7(3):228–231
Abd Elhady HM, Othman IH, Hablus MA, Ismail TA, Aboryia MH, Selim MF (2009) Long-term prospective randomized clinical and manometric comparison between surgical and chemical sphincterotomy for treatment of chronic anal fissure. S Afr J Surg 47(4):112–114
Agrawal V, Kaushal G, Gupta R (2013) Randomized controlled pilot trial of nifedipine as oral therapy vs topical application in the treatment of fissure-in-ano. Am J Surg 206:748–751
Ahmad J, Andrabi SIH, Rathore MA (2007) Comparison of topical glyceryl trinitrate with lignocaine ointment for treatment of anal fissure: a randomized controlled trial. Int J Surg 5(6):429–432
Ahmad MS, Amin I, Kareemullah M, Bashir S, Sattar Z, Hanif A (2012) Outcome of botulinum toxin with lateral internal sphincterotomy for treatment of chronic anal fissure. Pak J Med Health Sci 8:901–904
Ala S, Saeedi M, Hadianamrei R, Ghorbanian A (2012) Topical diltiazem vs. topical glyceryl trinitrate in the treatment of chronic anal fissure: a prospective, randomized, double-blind trial. Acta Gastroenterol Belgica 75:438–442
Altomare DF, Rinaldi M, Milito G, Arcana F et al (2000) Glyceryl trinitrate for chronic anal fissure—healing or headache? Results of a multicenter, randomized, placebo-controlled double blind trial. Dis Colon Rectum 43(2):174–179
Antripoli C, Perrotti P, Rubino M, Martino A et al (1999) Nifedipine for local use in conservative treatment of anal fissures: preliminary results of a multicenter study. Dis Colon Rectum 42(8):1011–1015
Arroyo A, Perez F, Serrano P, Candela F, Lacueva J, Calpena R (2005) Surgical versus chemical (botulinum toxin) sphincterotomy for chronic anal fissure: long-term results of a prospective randomized clinical and manometric study. Am J Surg 189:429–434
Arslan K, Erenoglu B, Dogru O, Turan E, Eryilmaz MA, Atay A et al (2013) Lateral internal sphincterotomy versus 0.25% isosorbide dinitrate ointment for chronic anal fissures: a prospective randomized controlled trial. Surg Today 43:500–505
Asim M, Lowrie N, Stewart J, Lolohea S, Van Dalen R (2014) Botulinum toxin versus botulinum toxin with low-dose glyceryltrinitrate for healing of chronic anal fissure: a prospective, randomized trial. N Z Med J 127(1393):80–86
Aslam MI, Pervaiz A, Figueiredo R (2014) Internal sphincterotomy versus topical nitroglycerin ointment for chronic anal fissure. Asian J Surg 37:15–19
Bacher H, Mischinger HJ, Werkgartner G, Cerwenka H et al (1997) Local nitroglycerin for treatment of anal fissures: an alternative to lateral sphincterotomy? Dis Colon Rectum 40(7):840–845
Bailey HR, Beck DE, Billingham RP, Binderow SR et al (2002) A study to determine the nitroglycerin ointment dose and dosing interval that best promote the healing of chronic anal fissures. Dis Colon Rectum 45(9):1192–1199
Bansal AR, Yadav PK, Godara R, Pal N, Tripura R (2016) Comparative evaluation of 0.2% glyceryl trinitrate vs. 2% diltiazem ointment in treatment of chronic anal fissure treatment: a randomized trial. Hell J Surg 88(1):25–30
Berkel AEM, Rosman C, Koop R, Duijvendijk P, Palen J, Klaase JM (2014) Isosorbide dinitrate ointment vs botulinum toxin A (Dysport) as the primary treatment for chronic anal fissure: a randomized multicentre study. Colorectal Dis 16:360–366
Bielecki K, Kolodziejczak M (2003) A prospective randomized trial of diltiazem and glyceryltrinitrate ointment in the treatment of chronic anal fissure. Colorectal Dis 5:256–257
Boschetto S, Giovannone M, Tosoni M, Barberani F (2004) Hydropneumatic anal dilation in conservative treatment of Chronic anal fissure: clinical outcomes and randomized comparison with topical nitroglycerin. Tech Coloproctol 8(2):89–92
Brillantino A, Lacobellis F, Izzo G, Martino N, Grassi R, Renzi A (2014) Maintenance therapy with partially hydrolyzed guar gum in the conservative treatment of chronic anal fissure: results of a prospective randomized study. Biomed Res Int 1:1
Brisinda G, Maria G, Bentivoglio AR, Cassetta E, Gui D, Albanese A (1999) A comparison of injections of botulinum toxin and topical nitroglycerin ointment for the treatment of chronic anal fissure. New Engl J Med 341(2):65–69
Brisinda G, Maria G, Sganga G, Bentivoglio AR et al (2002) Effectiveness of higher doses of botulinum toxin to induce healing in patient with chronic anal fissures. Surgery 131(2):179–184
Brisinda G, Albanese A, Cadeddu F, Bentivoglio AR, Mabisonbi A, Maringa G, Maria G (2004) Botulinum neurotoxin to treat anal fissure: results of a randomized botox vs disport controlled trial. Aliment Pharm Ther 19(6):695–701
Brisinda G, Cadeddu F, Brandara F, Marniga G, Maria G (2007) Randomized clinical trial comparing botulinum toxin injections with 0.2 per cent nitroglycerin ointment for chronic anal fissure. Br J Surg 94(2):162–167
Bulus H, Varol N, Tas A, Coskun A (2013) Comparison of topical isosorbide mononitrate, topical diltiazem, and their combination in the treatment of chronic anal fissure. Asian J Surg 36:165–169
Buyukyavuz BI, Sava C, Duman L (2010) Efficacy of lanolin and bovine type I collagen in the treatment of childhood anal fissures: a prospective, randomized, controlled clinical trial. Surg Today 40(8):752–756
Carapeti EA, Kamm MA, McDonald PJ, Chadwick SJD et al (1999) Randomized controlled trial shows that glyceryl trinitrate heals anal fissures, higher doses are not more effective and there is a high recurrence rate. Gut 44:727–730
Carroccio A, Mansueto P, Morfino G, D’Alcamo A, Paola V, Iacono G et al (2013) Oligo-antigenic diet in the treatment of chronic anal fissures. Evidence for a relationship between food hypersensitivity and anal fissures. Am J Gastroent 108(5):825–832
Cevik M, Boleken ME, Koruk I, Ocal S, Balcioglu ME, Aydinoglu A et al (2012) A prospective, randomized, double-blind study comparing the efficacy of diltiazem, glyceryl trinitrate, and lidocaine for the treatment of anal fissure in children. Pediatr Surg Int 28:411–416
Chaudhuri S, Pal AK, Acharya A, Dey A et al (2001) Treatment of chronic anal fissure with topical glyceryl trinitrate: a double blind, placebo controlled trial. Indian J Gastroenterol 20(3):101–102
Chen J, Michowitz M, Bawnik JB (1992) Solcoderm as alternative conservative treatment for acute anal fissure: a controlled clinical study. Am Surg 58(11):705–709
Colak T, Ipek T, Kanik A, Aydin S (2002) A randomized trial of botulinum toxin vs lidocaine pomade for chronic anal fissure. Acta Gastroenterol Belgica 65(4):187–190
Colak T, Ipek T, Urkaya N, Kanik A, Dirlik M (2003) A randomized study comparing systemic transdermal treatment and local application of glyceryl trinitrate in the management of chronic anal fissure. Eur J Surg Suppl 168(588):18–22
deNardi P, Ortolano E, Radaelli G, Staudacher C (2006) Comparison of glycerine trinitrate and botulinum toxin-A for the treatment of chronic anal fissure: long term results. Dis Colon Rectum 49(4):427–432
Dinç T, Ege B, Karslı MF, Coşkun F (2014) Comparison of botox and lateral internal sphincterotomy treatment outcomes in chronic anal fissures [Kronik anal fissür tedavisinde, botoks ve lateral internal sfinkterotomi uygulanan hastaların tedavi sonuçlarının karşılaştırılması]. Dicle Med J 41(1):133–137
di Visconte MS, di Bella R, Munegato G (2006) Randomized prospective trial comparing 0.25% glycerin trinitrate ointment and anal cryothermal dilators with 0.25% glycerin trinitrate ointment alone and with anal cryothermal dilators alone in the treatment of chronic anal fissure: a two year follow up. Dis Colon Rectum 49(12):1822–1830
di Visconte MS, Munegato G (2009) Glyceryl trinitrate ointment (0.25%) and anal cryothermal dilators in the treatment of chronic anal fissures. J Gastrointest Surg 13(7):1283–1291
El-Labban G, El-Gazzaz G, Hokkam E (2010) Topical nitroglycerin versus lateral internal sphincterotomy for chronic anal fissure. Acta Chir Austriaca 42:49–52
Elwakeel HA, Moneim HA, Farid M, Gohar AA (2007) Clove oil cream: a new effective treatment for chronic anal fissure. Colorectal Dis 9(6):549–552
Emami MH, Sayedyahossein S, Aslani A (2008) Safety and efficacy of new glyceryl trinitrate suppository formula: first double blind placebo-controlled clinical trial. Dis Colon Rectum 51(7):1079–1083
Eshghi F (2007) The efficacy of l-arginine gel for treatment of chronic anal fissure compared to surgical sphincterotomy. J Med Sci 7(3):481–484
Evans J, Luck A, Hewett P (2001) Glyceryl trinitrate vs. lateral sphincterotomy for chronic anal fissure. Dis Colon Rectum 44(1):93–97
Ezri T, Susmallian S (2003) Topical nifedipine vs. topical glyceryl trinitrate for treatment of chronic anal fissure. Dis Colon Rectum 46(6):805–808
Farooq U, Farooq S, Zahir S, Chaudhry AM (2012) Comparison of surgical and chemical sphincterotomy in the management of acute anal fissures. Pak J Med Health Sci 6:24–31
Festen S, Gisbertz SS, Van Schaagen F, Gerhards MF (2009) Blinded randomized clinical trial of botulinum toxin versus isosorbide dinitrate ointment for treatment of anal fissure. Br J Surg 96(12):1393–1399
Fruehauf H, Fried M, Wegmueller B, Bauerfeind P, Thumshirn M (2006) Efficacy and safety of botulinum toxin A injection compared with topical nitroglycerin ointment for the treatment of chronic anal fissure: a prospective randomized study. Am J Gastroenterol 101(9):2107–2112
Gagliardi G, Pascariello A, Altomare DF, Arcana F, Cafaro D, La Torre F et al (2010) Optimal treatment duration of glyceryl trinitrate for chronic anal fissure: results of a prospective randomized multicenter trial. Tech Coloproctol 14:241–248
Gaj F, Trecca A, Crispino P (2006) Efficacy of anal dilators in the treatment of acute anal fissure. A controlled clinical trial. Chir Ital 58(6):761–765
Gandomkar H, Zeinodinni A, Heidari R, Amoli H (2015) Partial lateral internal sphincterotomy versus combined botulinum toxin A injection and topical diltiazem in the treatment of chronic anal fissure: a randomized clinical trial. Dis Colon Rectum 58(2):228–234
Giridhar CM, Babu P, Seshagiri Rao K (2014) A comparative study of lateral sphincterotomy and 2% diltiazem gel local application in the treatment of chronic fissure in ANO. J Clin Diagn Res. doi:10.7860/JCDR/2014/10480.4925
Golfam F, Golfam P, Khalaj A, Sayed Mortaz SS (2019) The effect of topical nifedipine in treatment of chronic anal fissure. Acta Med Iran 48:295–299
Grekova NM, Maleva EA, Lebedeva Y, Bordunovsky VN, Telesheva LF, Bychkovskikh VA (2015) The effects of topical application of metronidazole for treatment of chronic anal fissure: a randomized, controlled pilot study. Indian J Gastroenterol 34(2):152–157
Gupta P (2006) Randomized, controlled study comparing sitz-bath and no-sitz-bath treatments in patients with acute anal fissures. ANZ J Surg 76(8):718–721
Hanumanthappa MB, Suvarna R, Guruprasad Rai D (2012) Topical diltiazem is superior to topical lignocaine in the treatment of chronic anal fissure: results of a prospective comparative study. J Clin Diagn Res 6:1014–1017
Ho KS, Ho YH (2005) Randomized clinical trial comparing oral nifedipine with lateral anal sphincterotomy and tailored sphincterotomy in the treatment of chronic anal fissure. Br J Surg 92(4):403–408
Iswariah H, Stephens J, Reiger N, Rodda D, Hewett P (2005) Randomized prospective controlled trial of lateral internal sphincterotomy versus injections of botulinum toxin for the treatment of idiopathic fissure in ano. ANZ J Surg 75(7):553–555
Jawaid M, Masood Z, Salim M (2009) Topical diltiazem hydrochloride and glyceryl trinitrate in the treatment of chronic anal fissure. J Coll Phys Surg Pak 19(10):614–617
Jensen SL (1986) Treatment of first episodes of acute anal fissure: a prospective randomized study of lignocaine ointment versus hydrocortisone ointment or warm sitz baths plus bran. Br Med J 292:1167–1170
Jensen SL (1987) Maintenance therapy with unprocessed bran in the prevention of acute anal fissure recurrence. J R Soc Med 80(5):296–298
Jonas M, Neal KR, Abercrombie JF, Scholefield JH (2001) A randomized trial of oral vs. topical diltiazem for chronic anal fissures. Dis Colon Rectum 44:1074–1078
Jones OM, Ramalingam T, Merrie A, Cunningham C, George BD, Mortensen NJ, Lindsey I (2006) Randomized clinical trial of botulinum toxin plus glyceryl trinitrate vs botulinum toxin alone for medically resistant chronic anal fissure: overall poor healing rates. Dis Colon Rectum 49(10):1574–1580
Jost WH, Schrank B (1999) Chronic anal fissures treated with botulinum toxin injections: a dose-finding study with Dysport. Colorectal Dis 1:26–28
Katsinelos P, Papaziogas B, Koutelidakis I, Paroutoglou G, Dimiropoulos S, Souparis A, Atmatzidis K (2006) Topical 0.5% nifedipine versus lateral internal sphincterotomy for the treatment of chronic anal fissure. Int J Colorectal Dis 21(2):179–183
Kennedy ML, Sowter S, Nguyen H, Lubowski DZ (1999) Glyceryl trinitrate ointment for the treatment of chronic anal fissure: results of a placebo-controlled trial and long term follow-up. Dis Colon Rectum 42(8):1000–1006
Kenny SE, Irvine T, Driver CP, Nunn AT et al (2001) Double blind randomized controlled trial of topical glyceryl trinitrate in anal fissure. Arch Dis Child 85:404–407
Khaledifar B, MahMoudi MYA, Mobasheri M (2015) A double-blind randomized trial comparing the effectiveness and safety of nifedipine and isosorbide dinitrate in chronic anal fissure. Malays J Med Sci 22(5):42–49
Kocher HM, Steward M, Leather AJM, Cullen PT (2002) Randomized clinical trial assessing the side effects of glyceryl trinitrate and diltiazem hydrochloride in the treatment of chronic anal fissure. Br J Surg 89:413–417
Libertiny G, Knight JS, Farouk R (2002) Randomized trial of topical 0.2% glyceryl trinitrate and lateral internal sphincterotomy for the treatment of patients with chronic anal fissures: long term follow-up. Eur J Surg 168(7):418–421
Lund JN, Scholefield JH (1997) A randomized, prospective, double-blind, placebo-controlled trial of glyceryl trinitrate in treatment of anal fissure. Lancet 349(9044):11–14
Maan MS, Mishra R, Thomas S, Hadke N (2004) Randomized double blind trial comparing topical nitroglycerin with xylocaine and Proctosedyl in idiopathic chronic anal fissure. Indian J Gastroenterol 23:91–93
Maria G, Cassetta E, Gui D, Brisinda G et al (1998) A comparison of botulinum toxin and saline for the treatment of chronic anal fissure. N Engl J Med 338:217–220
Maria G, Brisinda G, Bentivoglio AR, Cassetta E, Gui D, Albanese A (2000) Influence of botulinum toxin site of injections on healing rate in patients with chronic anal fissure. Am J Surg 179:46–50
MacDonald P, Driscoll AM, Nicholls RJ (1983) The anal dilator in the conservative management of acute anal fissure. Br J Surg 70(1):25–26
Mentes BB, Irkorucu O, Akin M, Leventoglu S et al (2003) Botulinum toxin injection compares favorably with lateral internal sphincterotomy in the treatment of chronic anal fissure. Dis Colon Rectum 46:232–237
Mishra R, Thomas S, Maan MS, Hadke NS (2005) Topical nitroglycerin versus lateral internal sphincterotomy for chronic anal fissure: prospective randomized trial. ANZ J Surg 75:1032–1035
Moghimi M, Ghodosi I (2006) Topical sildenafil (Viagra) in the treatment of chronic anal fissure: a randomized double blind controlled trial. Int J Pharmacol 2(6):608–612
Motie MR, Hashemi P (2016) Comparative evaluation of 0.2% glyceryl trinitrate vs. 2% diltiazem ointment in treatment of chronic anal fissure treatment: a randomized trial. Acta Med Iran 54(7):437–440
Mustafa NA, Cengiz S, Türkyilmaz S, Yücel Y (2006) Comparison of topical glyceryl trinitrate ointment and oral nifedipine in the treatment of chronic anal fissure. Acta Chir Belgica 106(1):55–58
Muthukumarassamy R, Robinson SS, Sarath SC, Raveendran R (2005) Treatment of anal fissures using a combination of minoxidil and lignocaine: a randomized, double blind trial. Indian J Gastroenterol 24(4):158–160
Nasr M, Ezzat H, Elsebae M (2010) Botulinum toxin injection versus lateral internal sphincterotomy in the treatment of chronic anal fissure: a randomized controlled trial. World J Surg 34:2730–2734
Oettle GJ (1997) Glyceryl trinitrate vs. sphincterotomy for treatment of chronic fissure in ano: a randomized controlled trial. Dis Colon Rectum 40(11):1318–1320
Oueidat D (1999) A comparative study in anal fissure treatment. J Med Liban 47(3):164–168
Othman I (2010) Bilateral versus posterior injection of botulinum toxin in the internal anal sphincter for the treatment of acute anal fissure. S Afr J Surg 48(1):20–22
Parellada C (2004) Randomized prospective trial comparing 0.2 percent isosorbide dinitrate ointment with sphincterotomy in treatment of chronic anal fissure: two year follow up. Dis Colon Rectum 47(4):437–443
Peng H, Wang JP, Yang XQ, Zheng Y, Ding YJ, Ding SQ et al (2013) A multi-center, randomized, double-blind, placebo-controlled trial of glyceryl trinitrate ointment in the treatment of anal fissure [Chinese]. Zhonghua Weichang Waike Zazhi 16(7):654–657
Perrotti P, Bove A, Antropoli C, Molino D et al (2002) Topical nifedipine with lidocaine vs. active control for the treatment of anal fissure. Dis Colon Rectum 45(11):1468–1475
Peshala KKVS, Sahu M (2014) A study of anal sphincter tone in acute fissure in ano patients treated with mahanarayana oil. Int J Res Ayurveda Pharm 5:318–321
Pitt J, Dawson PM, Hallan RI, Boulos PB (2001) A double-blind randomized placebo controlled trial of oral indoramin to treat chronic anal fissure. Colorectal Dis 3(3):165–168
Prudente AC, Melo VA, Torres Neto JR, Santiago RR (2011) Vidal MA [Evaluation of the treatment of chronic anal fissure with topical isosorbide 1%]. Rev Bras Coloproctol 30:409–413
Richard CS, Gregroire R, Plewes EA, Silverman R et al (2000) Internal sphincterotomy is superior to topical nitroglycerin in the treatment of chronic anal fissure: results of a randomized controlled trial. Dis Colon Rectum 43(8):1048–1057
Rosa M, Gentile M (2012) Conservative vs. surgical treatment for chronic anal idiopatic fissure: a prospective randomized trial. Europaische Chirurgische Forschung Recherches Chirurgicales Europeennes 49:161–162
Ruiz-Tovar J, Llavero C (2017) Percutaneous posterior tibial nerve stimulation vs perianal application of glyceryl trinitrate ointment in the treatment of chronic anal fissure: a randomized clinical trial. Dis Colon Rectum 60(1):81–86
Sahakitrungruang C, Vivatvakin B, Chongsrisawat V, Rojanasakul A (2011) Long-term outcome of botulinum toxin injection for the treatment of chronic anal fissure: a randomized controlled trial. Asian Biomed 5:397–401
Samad A, Khanzada TW, Sushel C (2007) Cost effectiveness of topical glyceryl trinitrate versus lateral internal sphincterotomy for chronic anal fissure. J Postgrad Med Inst 21(1):16–20
Samim M, Twigt B, Stoker L, Pronk A (2012) Topical diltiazem cream versus botulinum toxin a for the treatment of chronic anal fissure: a double-blind randomized clinical trial. Ann Surg 255:18–22
Sanei B, Mahmoodieh M, Masoudpour H (2009) Comparison of topical glyceryl trinitrate with diltiazem ointment for treatment of chronic anal fissure. A randomized clinical trial. Ann Ital Chir 80(5):379–383
Scholefield JH, Bock JU, Marla B, Richter HJ, Athanasiadis S, Prois M, Herold A (2003) A dose finding study with 0.1%, 0.2%, and 0.4% glyceryl trinitrate ointment in patients with chronic anal fissure. Gut 52:264–269
Shrivastava UK, Jain BK, Kumar P, Saifee Y (2007) A comparison of the effects of diltiazem and glyceryl trinitrate ointment in the treatment of chronic anal fissure: a randomized clinical trial. Surg Today 37(6):482–485
Siddique MI, Murshed KM, Majid MA (2008) Comparative study of lateral internal sphincterotomy versus local 0.2% glyceryl trinitrate ointment for the treatment of chronic anal fissure. Bangladesh Med Res Counc Bull 34(1):12–15
Simpson J, Lund JN, Thompson RJ, Kapila L, Scholefield JH (2003) The use of glyceryl trinitrate (GTN) in the treatment of chronic anal fissure in children. Med Sci Monit 9(10):P1123–P1126
Siproudhis L, Sebille V, Pigot F, Hemerey P, Juguet F, Bellissant E (2003) Lack of efficacy of botulinum toxin in chronic anal fissure. Aliment Pharmacol Ther 18(5):515–524
Sonmez K, Demmirogullan B, Ekingen G, Turkyilmaz Z et al (2002) Randomized placebo controlled treatment of anal fissure by lidocaine, EMLA and GTN in children. J Pediatr Surg 37(9):1313–1316
Suknaic S, Patrlj L, Steresinic M, Skopljanac Macina A, Erdelez L (2008) Surgical or biologic sphincterotomy in the treatment of chronic anal fissure. Acta Med Croat 62(1):73–80
Suvarna R, Panchami Guruprasad Rai D (2012) Chemical sphicterotomy versus surgical sphicterotomy in the management of chronic fissure in ANO: a prospective, randomized trial. J Clin Diagn Res 6:1018–1021
Tander B, Guven A, Demirbag S, Ozkan Y et al (1999) A prospective, randomized, double-blind, placebo-controlled trial of glyceryl-trinitrate ointment in the treatment of children with anal fissure. J Pediatr Surg 34(12):1810–1812
Tankova L, Yoncheva K, Muhtarov M, Kadyan H, Draganov V (2002) Topical mononitrate treatment in patients with anal fissure. Aliment Pharmacol Ther 16(1):101–103
Tankova L, Yoncheva K, Kovatchki D, Doytchinova I (2009) Topical anal fissure treatment: placebo-controlled study of mononitrate and trinitrate therapies. Int J Colorectal Dis 24(4):461–464
Tauro LF, Shindhe VV, Aithala PS, Martis JJ, Shenoy HD (2011) Comparative study of glyceryl trinitrate ointment versus surgical management of chronic anal fissure. Indian J Surg 73(4):268–277
Torrabadella L, Salgado G (2006) Controlled dose delivery in topical treatment of anal fissure: pilot study of a new paradigm. Dis Colon Rectum 49(6):865–868
Uluutku H, Akin ML, Erenglu C, Yildiz M, Ukraya N, Celen K (2001) Efficacy of nifedipine, glyceryl trinitrate and botulinum toxin in treatment of chronic anal fissure. Turk J Surg 17(6):343–350
Vaithianathan R, Panneerselvam S (2015) Randomized prospective controlled trial of topical 2% diltiazem versus lateral internal sphincterotomy for the treatment of chronic fissure in Ano. Indian J Surg 77(Suppl 3):1484–1487
Valizadeh N, Jalaly NY, Hassanzadeh M, Kamani F, Dadvar Z, Azizi S et al (2012) Botulinum toxin injection versus lateral internal sphincterotomy for the treatment of chronic anal fissure: randomized prospective controlled trial. Langenbeck’s Arch Surg 397:1093–1098
Weinstein D, Halevy A, Negri M, Levy N, Gelertner I, Ziv Y (2004) A prospective randomized double-blind study on the treatment of anal fissures with nitroglycerin ointment. Harefuah 143(10):713–717
Werre AJ, Palamba HW, Bilgen EJS, Eggink WF (2001) Isosorbide dinitrate in the treatment of anal fissure: a randomized, prospective double blind placebo controlled trial. Eur J Surg 167:382–385
Yakoot M, Salaam MA (2009) Study of efficacy and safety of a new local cream (“healer”) in the treatment of chronic anal fissure. A prospective, randomized, single-blind, comparative study. Arq Gastroenterol 46(3):179–182
Yetisir F, Salman E, Yurekli B, Aksoy M, Yildirim MB, Kilic M (2012) Comparison of lateral internal sphincterotomy with topical nitro-glycerin treatment in patients with chronic anal fissure: a prospective randomized study. Surg Curr Res 2(4):1
Youssef T, Youssef M, Thabet W, Lotfy A, Shaat R, Abd-Elrazek E et al (2015) Randomized clinical trial of transcutaneous electrical posterior tibial nerve stimulation versus lateral internal sphincterotomy for treatment of chronic anal fissure. Int J Surg (Lond Engl) 22:143–148
Zuberi BF, Rajput MR, Abro H, Shaikh S (2000) A randomized trial of glyceryl trinitrate ointment and nitroglycerin patch in healing of anal fissures. Int J Colorectal Dis 15(4):243–245
Gough MJ, Lewis A (1983) The conservative treatment of fissure in ano. Br J Surg 70(3):175–176
Jonas M, Lund JN, Scholefield JH (2002) Topical 0.2% glyceryl trinitrate ointment for anal fissure: long-term efficacy in routine clinical practice. Colorectal Dis 4(5):317–320
Graziano A, Svidler Lopez L, Lencinas S, Masciangioli G, Gualdrini U, Bisisio O (2001) Long-term results of topical nitroglycerin in the treatment of chronic anal fissures are disappointing. Tech Coloproctol 5(3):143–147
Nelson RL, Chattopadhyay A, Brooks W, Platt I, Paavana T, Earl S (2011) Operative procedures for fissure in ano. Cochrane Database Syst Rev 11:CD002199
Nelson RL, Thomas K, Morgan J, Jones A (2012) Non-surgical therapy for anal fissure. Cochrane Database Syst Rev 2:CD003431
Sajid MS, Vijaynagar B, Desai M, Cheek E, Baig MK (2008) Botulinum toxin vs glyceryltrinitrate for the medical management of chronic anal fissure: a meta-analysis. Colorectal Dis 10(6):541–546
Sajid MS, Hunte S, Hippolyte S, Kiri VA, Maringe C, Baig MK (2008) Comparison of surgical vs chemical sphincterotomy using botulinum toxin for the treatment of chronic anal fissure: a meta-analysis. Colorectal Dis 10(6):547–552
Sajid MS, Rimple J, Cheek E, Baig MK (2008) The efficacy of diltiazem and glyceryltrinitrate for the medical management of chronic anal fissure: a meta-analysis. Int J Colorectal Dis 23(1):1–6
Yiannakopoulou E (2012) Botulinum toxin and anal fissure: efficacy and safety systematic review. Int J Colorectal Dis 27:1–9
Sajid MS, Whitehouse PA, Sains P, Baig MK (2013) A systematic review on the use of topical application of diltiazem vs glyceryltrinitrate for the pharmacological management of chronic anal fissure. Colorectal Dis 15(1):19–26
Paquette IM, Varma MG, Kaiser AM, Steele SR, Rafferty JF (2015) The American society of colon and recta surgeons’ clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum 58(7):623–636
Italian Society of Colorectal Surgery (SICCR), Pucciani F, Altomare DF, Dodi G, Falletto E, Frasson A, Giani I, Martellucci J, Naldini G, Piloni V, Sciaudone G, Italian Association of Hospital Gastroenterologists (AIGO), Bove A, Bocchini R, Bellini M, Alduini P, Battaglia E, Galeazzi F, Rossitti P, Usai Satta P (2015) Diagnosis and treatment of faecal incontinence: consensus statement of the Italian society of colorectal surgery and the italian association of hospital gastroenterologists. Dig Liver Dis 47(8):628–645
Vitton V, Soudan D, Siproudhis L, Abramowitz L, Bouvier M, Faucheron JL, Leroi AM, Meurette G, Pigot F, Damon H, French National Society of Coloproctology (2014) Treatments of faecal incontinence: recommendations from the French national society of coloproctology. Colorectal Dis 16(3):159–166
Wald A, Bharucha AE, Cosman BC, Whitehead WE (2014) ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol 109(8):1141–1157 ((Quiz) 1058)
Perry WB, Dykes SL, Buie WD, Rafferty JF (2010) Standards practice task force of the American society of colon and rectal surgeons. Practice parameters for the management of anal fissures (3rd revision). Dis Colon Rectum 53(8):1110–1115
Cross KL, Massey EJ, Fowler AL, Monson JR, ACPGBI (2008) The management of anal fissure: ACPGBI position statement. Colorectal Dis 10(Suppl 3):1–7
American Gastroenterological Association (2003) American Gastroenterological Association medical position statement: diagnosis and care of patients with anal fissure. Gastroenterology 124(1):233–234
Rotholtz NA, Bun M, Mauri MV, Bosio R, Peczan CE, Mezzadri NA (2005) Long-term assessment of fecal incontinence after lateral internal sphincterotomy. Tech Coloproctol 9:115–118
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study does not contain studies with human or animals performed by any of the authors.
Informed consent
Informed consent is not required for review articles.
Rights and permissions
About this article
Cite this article
Nelson, R.L., Manuel, D., Gumienny, C. et al. A systematic review and meta-analysis of the treatment of anal fissure. Tech Coloproctol 21, 605–625 (2017). https://doi.org/10.1007/s10151-017-1664-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-017-1664-2