Abstract
Human epidermal growth factor receptor 2 has been a pivotal biomarker for gastric cancer treatment strategies for many years. However, more than a decade after the ToGA trial demonstrated the efficacy of trastuzumab in improving survival, the development of treatments targeting human epidermal growth factor receptor 2 remains challenging. Several large-scale clinical trials of tyrosine kinase inhibitors, non-trastuzumab anti-human epidermal growth factor receptor 2 antibodies, and antibody–drug conjugates have failed to meet the primary endpoints. The concept of trastuzumab beyond progression and the complexity of resistance mechanisms to anti-human epidermal growth factor receptor 2 therapy after trastuzumab treatment presented significant obstacles, leading to trastuzumab being the sole therapy for human epidermal growth factor receptor 2-positive gastric cancer for some time. Nevertheless, the landscape has shifted in recent years, especially since the introduction of the antibody–drug conjugate trastuzumab deruxtecan in 2020. This has rekindled the interest in developing treatments targeting human epidermal growth factor receptor 2 in gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
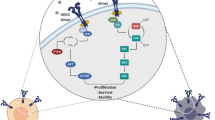
Human epidermal growth factor receptor 2 (HER2) has been considered a crucial biomarker and therapeutic target for over 2 decades. Coded by the ERBB2 gene on chromosome 17q21, HER2 is a transmembrane glycoprotein belonging to the epidermal growth factor receptor (EGFR) family, which includes EGFR (HER1), HER3, and HER4. These receptors play crucial roles in cellular processes including growth, differentiation, and survival. HER2 is unique among its family members because of its potent intrinsic kinase activity and ability to form active homodimers or heterodimers with other HER receptors without a ligand. This leads to amplified signaling pathways that drive oncogenesis. Although breast and gastric cancers are the primary malignancies associated with HER2-targeted therapies, recent findings have revealed HER2 positivity in other cancers, emphasizing its importance across different cancer types. However, the effectiveness of therapies and supporting evidence between breast and gastric cancers, highlighting that treatments effective for HER2-positive tumors are not universally applicable to all cancers. This article outlines the evidence gathered regarding gastric cancer and discusses future perspectives. Currently, HER2-positive gastric cancer, accounting for 15–20% of all gastric cancers, is defined by an immunohistochemistry (IHC) score of 3 + or 2 + in conjunction with HER2 gene amplification detected by in situ hybridization (ISH). HER2 positivity is more common in intestinal-type tumors than in diffuse-type tumors and often found in tumors originating from the gastroesophageal junction to the proximal stomach [1].
First-line treatment for HER2-positive gastric cancer
The ToGA trial was the first to provide significant evidence for treating HER2-positive gastric cancer. This open-label, phase III trial examined the additive effects of trastuzumab and capecitabine (or 5-fluorouracil, 5-FU) and cisplatin. The inclusion criteria for HER2 positivity were initially defined as IHC 3 + or fluorescence ISH (FISH)-positive cells. The median overall survival (mOS) was 11.1 months for the control group and 13.8 months for the trastuzumab group (hazard ratio [HR] 0.74, 95% confidence interval [CI] 0.60–0.91, p = 0.0046), showing a significantly prolonged survival in the trastuzumab group (Table 1) [2]. However, a subset analysis revealed that the additive effect of trastuzumab was unclear in the groups with IHC 0 or 1 + (despite being FISH-positive), leading to the current definition of HER2-positive gastric cancer as IHC 3 + or IHC 2 + /FISH-positive cells, for which trastuzumab treatment is indicated. Notably, in the IHC 3 + or IHC 2 + /FISH-positive group, the mOS was 11.8 months for the control group and 16.0 months for the trastuzumab group (HR 0.65, 95% CI 0.51–0.83).
The ToGA trial used cisplatin as the platinum compound; no phase III trial has demonstrated a survival prolongation by adding trastuzumab to a regimen of fluoropyrimidine and oxaliplatin. However, based on evidence from several phase II trials on HER2-positive gastric cancer [3,4,5,6], oxaliplatin use as a platinum compound in combination with trastuzumab would likely yield treatment outcomes that are not significantly different from those with cisplatin. The ESMO guidelines [7] mention that cisplatin and oxaliplatin have almost equivalent effects, and the NCCN guidelines [8] state that oxaliplatin is preferable to cisplatin from a toxicity perspective. However, they do not deeply mention the efficacy of combining trastuzumab with oxaliplatin.
Other agents in first- and second-line treatment for HER2-positive gastric cancer
Several agents have been investigated for treating HER2-positive gastric cancer. However, these drugs, which have shown effectiveness in HER2-positive breast cancer and are active in routine clinical practice, fail to meet the primary endpoints in gastric cancer, leading to a prolonged situation in which trastuzumab remains the only treatment option for HER2-positive gastric cancer.
Lapatinib
Lapatinib is a small-molecule compound that inhibits cell proliferation signaling by binding to the intracellular tyrosine kinase domains of EGFR and HER2, thereby inhibiting their autophosphorylation. The TyTAN trial, an open-label randomized phase III study, investigated the additive effect of lapatinib and weekly paclitaxel in the second-line treatment of HER2-positive (defined as FISH-positive in this trial rather than IHC) gastric cancer. The primary endpoint, overall survival (OS), did not show superiority in the lapatinib group, with 11.0 months compared with 8.9 months in the control group (HR 0.84, p = 0.1044) (Table 1) [9]. Subgroup analysis showed promising results for the IHC 3 + population (HR 0.59, mOS 14.0 m vs. 7.6 m, p = 0.0176); however, unlike in the ToGA trial, no additive effect was observed in the IHC 2 + population (HR 0.88). The LOGiC trial, conducted around the same time, was a double-blind, randomized, phase III study aimed at evaluating the effectiveness of adding lapatinib to capecitabine and oxaliplatin in untreated patients with gastric/esophagogastric junction/esophageal adenocarcinoma that was positive for HER2 by FISH [10]. The primary endpoint, OS, failed to demonstrate a significant difference between the lapatinib (12.2 months) and placebo (10.5 months; HR 0.91, 95% CI 0.73–1.12) groups (Table 1). Notably, even among patients with IHC 3 + HER2, adding lapatinib did not show substantial benefit (HR 0.90, 95% CI 0.69–1.18, p = 0.4603), indicating a lack of efficacy in this subgroup. These studies also provided significant opportunities to determine whether HER2 protein expression or HER2 gene amplification should be used to define HER2-positive gastric cancer.
Pertuzumab
Pertuzumab, similar to trastuzumab, is an anti-HER2 monoclonal antibody; however, it binds to a different HER2 domain. While trastuzumab binds to the extracellular domain IV of HER2, pertuzumab binds to the extracellular domain II. Trastuzumab inhibits the formation of homodimers or heterodimers with the HER family by binding to extracellular domain IV. However, it cannot inhibit heterodimer formation via extracellular domain II. Therefore, the combination with pertuzumab, which binds to extracellular domain II and inhibits dimerization, was expected to inhibit cell proliferation signaling more effectively [11]. The JACOB trial, a double-blind, placebo-controlled phase III trial, investigated the additive effect of pertuzumab and trastuzumab plus chemotherapy (fluoropyrimidine + cisplatin) on untreated HER2-positive gastric or gastroesophageal junction cancer with IHC3 + or IHC2 + /ISH + . The trial included 780 participants and did not demonstrate statistical superiority in mOS, with 17.5 months in the pertuzumab group and 14.2 months in the placebo group (HR 0.84, 95% CI 0.71–1.00, p = 0.057) (Table 1) [12]. Subsequent analyses suggested the potential for refining patient selection based on genetic alterations associated with resistance (e.g., mutations in EGFR/MET/KRAS/PIK3CA and amplification of EGFR/MET/KRAS) and HER2 copy number variations [13, 14]. These findings imply the necessity to reconsider the sole reliance on IHC to identify candidates for anti-HER2 therapy in future therapeutic developments for HER2-positive gastric cancer.
Trastuzumab emtansine (T-DM1)
T-DM1 is an antibody–drug conjugate (ADC) that combines trastuzumab with a cytotoxic chemotherapeutic agent. Derivatives of maytansine, including DM1, showed efficacy in tumor reduction but were discontinued owing to their high toxicity; specifically, DM1 showed 24–270 times the cytotoxic effect of taxanes in vitro. However, by conjugating DM1 as a payload with an anti-HER2 antibody, DM1 could be effectively delivered to HER2-positive cancer cells, mitigating its toxicity. Nevertheless, in the GATSBY trial, a phase II/III study for gastric cancer, T-DM1 did not demonstrate superiority in mOS (7.9 months for the T-DM1 group vs. 8.6 months for the control taxane group, HR 1.15, 95% CI 0.87–1.51, p = 0.86) (Table 1), and survival curves slightly favored the control taxane group.
Although these agents demonstrate clinical efficacy in breast cancer, they do not show the same efficacy in gastric cancer owing to several factors, such as the heterogeneity of HER2 staining in gastric cancer and the blood concentration of antibody preparations. However, a clear answer is yet to be obtained. Additionally, the concept of trastuzumab beyond progression (TBP) [15] suggests that continuing anti-HER2 therapy could extend survival. Although this TBP strategy has reached consensus in breast cancer, it is not well-established in gastric cancer. The WJOG7112G (T-ACT) trial, a randomized phase II trial, compared paclitaxel alone with paclitaxel plus trastuzumab in patients who received trastuzumab as the first-line treatment for HER2-positive gastric cancer. The primary endpoint was progression-free survival (PFS); however, no significant difference was observed (3.2 months vs. 3.7 months, HR 0.91, p = 0.33), and response rates were similar (32% vs. 33%) [16]. Continuing trastuzumab as a second-line treatment may not be beneficial owing to decreased HER2-positive gastric cancer cells rather than acquiring resistance mechanisms to trastuzumab. However, the detailed cause remains unknown. An important finding was the importance of confirming the HER2 status immediately before starting second-line or later anti-HER2 therapy after trastuzumab as a first-line treatment [17].
Third-line treatment for HER2-positive gastric cancer
Trastuzumab deruxtecan (T-DXd) has become the first drug since trastuzumab to meet the primary endpoints for treating HER2-positive gastric cancer and is available for daily clinical practice. As an ADC, T-DXd is characterized by its payload, a topoisomerase I inhibitor known as DXd, and the use of a cleavable peptide-based linker. This structural feature facilitates the delivery of the cytotoxic payload to cells expressing HER2 and adjacent tumor cells with lower levels of HER2 expression, thereby inducing a bystander-killing effect. This has been demonstrated in preclinical studies, suggesting the potential for broader efficacy in the heterogeneous expression landscape of HER2-positive gastric tumors [18, 19]. The therapeutic development of T-DXd is advancing for various HER2-positive cancers; however, the first evidence for gastric cancer was demonstrated in the DESTINY-Gastric01 trial [20, 21]. This open-label, randomized phase II trial was conducted in Japan and South Korea. In the primary cohort, patients with a history of at least two prior treatment regimens, including trastuzumab, were allocated 2:1 to T-DXd versus the physician's choice of chemotherapy (paclitaxel or irinotecan). The primary endpoint was the objective response rate, as determined by an independent review, which was 51% in the T-DXd group and 14% in the physician’s choice chemotherapy group (p < 0.001) (Table 1). The secondary endpoints, OS and PFS, were 12.5 months vs. 8.4 months (HR 0.59, 95% CI 0.39–0.88, p = 0.01) and 5.6 months vs. 3.5 months (HR 0.47, 95% CI 0.31–0.71), respectively. Although this trial was not a phase III study, these results led to the approval of T-DXd for treating HER2-positive gastric cancer in a third-line setting in Japan. Meanwhile, in Europe and the United States, a single-arm phase II trial (DESTINY-Gastric02 trial) to examine the activity of T-DXd was conducted, achieving an objective response rate of 42% (95% CI 30.8–53.4) [22]. The expected response rate was 45%, and the threshold response rate was 27%; the lower bound of the 95% confidence interval exceeded this threshold, meeting the primary endpoint. Therefore, T-DXd has been approved as a second-line treatment for HER2-positive gastric cancer in Western countries.
The DESTINY-Gastric01 trial included an exploratory cohort of patients with low HER2 expression [23]. Although evidence from a phase III trial has led to the approval of low HER2 expression in breast cancer [24], the DESTINY-Gastric01 trial showed some drug activity in gastric cancer with low HER2 expression (objective response rate of 26.3% in the HER2 IHC 2 + /ISH- population, 95% CI 9.1–51.2). However, this study was based on a few cases. Therefore, further evidence of gastric cancer with low HER2 expression is warranted.
Recent developments
Trastuzumab and immune checkpoint inhibitors
Immune checkpoint inhibitors have been used in combination for the first-line treatment of HER2-negative gastric cancer for a while [25, 26]. However, the appropriateness of combining anti-PD-1 antibodies with trastuzumab and cytotoxic chemotherapy for HER2-positive gastric cancer remains unclear. The potential combination of trastuzumab with immunotherapy is supported by reports of significant increases in NK cells, CD8-positive T cells, and B lymphocyte infiltration following treatment with trastuzumab, providing a rationale for expecting a synergistic effect in HER2-positive gastric cancer [27, 28], which has been further supported by single-arm phase II studies showing favorable objective response rates [29, 30].
The KEYNOTE-811 trial is a placebo-controlled, phase III comparative trial that evaluated the efficacy of adding pembrolizumab to chemotherapy (fluoropyrimidine plus platinum agents) and trastuzumab for HER2-positive gastric cancer. The primary endpoints were PFS and OS, but an interim analysis showed that the objective response rate was 74.4% in the pembrolizumab group and 51.9% in the placebo group, indicating a significant additive effect of 22.7% (95% CI 11.2–33.7, p = 0.00006) [31]. Consequently, the FDA rapidly approved a combination of pembrolizumab with chemotherapy and trastuzumab. However, the second interim analysis reported that the mPFS was 10.0 months in the pembrolizumab group and 8.1 months in the placebo group (HR 0.72, 95% CI 0.60–0.87, p = 0.0002), showing a significant prolongation regardless of PD-L1 expression level. In contrast, mOS was 20.0 months in the pembrolizumab group and 16.9 months in the placebo group (HR 0.87, 95% CI 0.72–1.06, p = 0.084) for all patients; it was 20.5 months versus 15.6 months (HR 0.79, 95% CI 0.64–0.98) for those with PD-L1 combined positive score (CPS) ≥ 1 (Table 1) [32]. In the third interim analysis, the respective hazard ratios were similar: PFS 0.73 and OS 0.84. Consequently, the FDA revised the approval criteria to include only patients with HER2-positive gastric cancer with a CPS of ≥ 1, and the EMA approved the combination for the same subset.
A trial combining margetuximab, an anti-HER2 antibody with an optimized Fc region, to enhance immune cell recognition and the destruction of cancer cells and the anti-PD-1 antibody retifanlimab (MAHOGANY trial; NCT04082364) was also conducted [33]. However, with an objective response rate of 53% (95% CI 36.1–68.5), the trial concluded that cytotoxic chemotherapy still plays a significant role and that immunotherapy alone is insufficient, leading to the termination of Part 2 of Cohort A without commencement [34]. Cohort B of the same trial involved a design that included margetuximab, chemotherapy, and tebotelimab (a bispecific antibody recognizing LAG-3 (lymphocyte-activation gene 3) and PD-1 checkpoint molecules). However, these results have not yet been reported.
In HER2-negative gastric cancer, a treatment arm in the CheckMate-649 trial did not use chemotherapy agents, consisting of nivolumab 1 mg/kg and ipilimumab 3 mg/kg; however, this arm was prematurely discontinued owing to side effects. What is the optimal combination of multiple immune checkpoint inhibitors and anti-HER2 therapy for HER2-positive gastric cancer? The AIO INTEGA trial was a randomized phase II trial conducted with a parallel non-comparative design that compared two regimens for HER2-positive gastric/gastroesophageal junction adenocarcinoma: trastuzumab + nivolumab (1 mg/kg) + FOLFOX and trastuzumab + nivolumab (1 mg/kg) + ipilimumab (3 mg/kg), each against historical controls. With a 12-month survival rate of 55% for the historical controls and 70% for the experimental arm of this trial, the results were 70% (95% CI 54–81%) for the FOLFOX group and 57% (95% CI 41–71%) for the ipilimumab group, with the FOLFOX group achieving the primary endpoint [35].
Other tyrosine kinase inhibitors (TKIs)
Apart from lapatinib, which binds to the intracellular domain of HER2, other TKIs, such as neratinib, afatinib, and tucatinib, exist [36]. Lapatinib is a reversible TKI, whereas afatinib and neratinib are irreversible inhibitors of EGFR, HER2, and HER4 and function as pan-HER inhibitors. Although these agents have been approved for lung and breast cancers, their application in gastric cancer remains largely preclinical [37]. Trials combining neratinib with T-DXd for the treatment of HER2-positive gastric cancer are currently underway (NCT05274048). In contrast, tucatinib is known for its high selectivity for HER2, and its combination with trastuzumab is expected to offer enhanced efficacy compared with their individual efficacies. A phase II trial (MOUNTAINEER trial) that administered trastuzumab and tucatinib to treat HER2-positive colorectal cancer has been conducted [38], whereas for HER2-positive gastric cancer, a phase II/III trial (MOUNTAINEER-02 trial) targeting second-line treatment is in progress [39]. The phase II trial evaluates the activity of combining tucatinib and trastuzumab with paclitaxel and ramucirumab. In contrast, the phase III trial is a placebo-controlled trial designed to compare paclitaxel and ramucirumab with or without tucatinib and trastuzumab. A notable feature of this trial is the determination of HER2 positivity using circulating tumor DNA (ctDNA). An ongoing phase 1b/2 trial of tucatinib in HER2-positive gastrointestinal cancers (SGNTUC-024 trial; NCT04430738) includes a cohort for first-line treatment of HER2-positive gastric cancer. Given the anticipated synergistic antitumor effects of combining tucatinib with an anti-PD-1 antibody [40], the regimen used in the SGNTUC-024 trial comprises tucatinib + trastuzumab + pembrolizumab + FOLFOX/CAPOX.
Anti-HER2 therapy in second-line treatment
Following the DESTINY-Gastric01 and 02 trials, a phase III trial comparing T-DXd with ramucirumab + paclitaxel in patients with HER2-positive gastric cancer with HER2 IHC 3 + or IHC2 + /ISH + after first-line treatment with trastuzumab is currently underway (DESTINY-Gastric04 trial; NCT04704934). The primary endpoint is OS, and one distinctive feature of this trial is the confirmation of HER2 positivity just before enrollment, which differs from the DESTINY-Gastric01 trial.
CD47 expression on tumor cells can impair phagocytosis by immune cells mediated by the Fc portion of antibodies like trastuzumab (the "Don't Eat Me" signal) [41]. Therefore, a phase II/III trial is ongoing to test the additive effect of combining trastuzumab and the anti-CD47 antibody evorpacept (ALX148) with ramucirumab and paclitaxel using standard treatment with ramucirumab and paclitaxel as the control arm for HER2-positive gastric cancer (ASPEN-06 trial; NCT05002127). In Part 2 of the preceding ASPEN-01 trial, a response rate of 72.2% (n = 18) was reported for a combination of these four drugs [42].
Bispecific antibody (BsAb) targeting HER2
Antibody drugs, such as trastuzumab, typically recognize one antigenic site; however, BsAbs that recognize two different epitopes have recently gained attention [43]. One such antibody is zanidatamab (ZW256), a biparatopic antibody that recognizes both the extracellular domains IV and II of HER2 using a single antibody. Zanidatamab is expected to form large clusters of HER2 on the cell surface, potentially inducing complement-dependent cytotoxicity more than when trastuzumab and pertuzumab are administered [44]. Currently, a phase III trial is comparing three regimens for the first-line treatment of HER2-positive (IHC 3 + or IHC 2 + /ISH +) gastric cancer: trastuzumab + chemotherapy, zanidatamab + chemotherapy, and zanidatamab + tislelizumab + chemotherapy (HERIZON-GEA-01 trial; NCT05152147) [45].
Conclusion
The ToGA trial established a combination of trastuzumab as the standard treatment for HER2-positive gastric cancer; however, subsequent therapeutic developments have not advanced sufficiently. Although ADCs have achieved some success, continued efforts are necessary regarding the combination of anti-PD-1 antibodies and other immunotherapies and a thorough investigation of the resistance mechanisms of anti-HER2 therapies. The importance of HER2 as a critical therapeutic target for gastric cancer is undeniable, and further examination of treatment strategies for HER2-positive gastric cancer is essential.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
Van Cutsem E, Bang YJ, Feng-yi F et al (2015) HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18:476–484. https://doi.org/10.1007/S10120-014-0402-Y
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697. https://doi.org/10.1016/S0140-6736(10)61121-X
Rivera F, Romero C, Jimenez-Fonseca P et al (2019) Phase II study to evaluate the efficacy of trastuzumab in combination with Capecitabine and Oxaliplatin in first-line treatment of HER2-positive advanced gastric cancer: HERXO trial. Cancer Chemother Pharmacol 83:1175–1181. https://doi.org/10.1007/S00280-019-03820-7
Yuki S, Shinozaki K, Kashiwada T et al (2020) Multicenter phase II study of SOX plus trastuzumab for patients with HER2+ metastatic or recurrent gastric cancer: KSCC/HGCSG/CCOG/PerSeUS 1501B. Cancer Chemother Pharmacol 85:217–223. https://doi.org/10.1007/S00280-019-03991-3
Takahari D, Chin K, Ishizuka N et al (2019) Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-naïve, HER2-positive advanced gastric cancer. Gastric Cancer 22:1238–1246. https://doi.org/10.1007/S10120-019-00973-5
Ryu MH, Yoo C, Kim JG et al (2015) Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer 51:482–488. https://doi.org/10.1016/J.EJCA.2014.12.015
Lordick F, Carneiro F, Cascinu S et al (2022) Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 33:1005–1020. https://doi.org/10.1016/J.ANNONC.2022.07.004
Lisa Hang N, McMillian N, MaryElizabeth Stein M et al (2024) NCCN Guidelines version 1.2024 gastric cancer continue NCCN guidelines panel disclosures
Satoh T, Doi T, Ohtsu A et al (2014) Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN–a randomized, phase III study. J Clin Oncol 32:2039–2049. https://doi.org/10.1200/JCO.2013.53.6136
Hecht JR, Bang YJ, Qin SK et al (2016) Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–a randomized phase III trial. J Clin Oncol 34:443–451. https://doi.org/10.1200/JCO.2015.62.6598
Richard S, Selle F, Lotz JP et al (2016) Pertuzumab and trastuzumab: the rationale way to synergy. An Acad Bras Cienc 88:565–577. https://doi.org/10.1590/0001-3765201620150178
Tabernero J, Hoff PM, Shen L et al (2018) Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol 19:1372–1384. https://doi.org/10.1016/S1470-2045(18)30481-9
Tabernero J, Hoff PM, Shen L et al (2023) Pertuzumab, trastuzumab, and chemotherapy in HER2-positive gastric/gastroesophageal junction cancer: end-of-study analysis of the JACOB phase III randomized clinical trial. Gastric Cancer 26:123–131. https://doi.org/10.1007/S10120-022-01335-4/TABLES/2
Pietrantonio F, Manca P, Bellomo SE et al (2023) HER2 copy number and resistance mechanisms in patients with HER2-positive advanced gastric cancer receiving initial trastuzumab-based therapy in JACOB trial. Clin Cancer Res 29:571–580. https://doi.org/10.1158/1078-0432.CCR-22-2533/711129/AM/HER2-COPY-NUMBER-AND-RESISTANCE-MECHANISMS-IN
Rayson D, Lutes S, Walsh G et al (2014) Trastuzumab beyond progression for HER2 positive metastatic breast cancer: progression-free survival on first-line therapy predicts overall survival impact. Breast J 20:408–413. https://doi.org/10.1111/TBJ.12284
Makiyama A, Sukawa Y, Kashiwada T et al (2020) Randomized, phase II study of trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT Study). J Clin Oncol 38:1919–1927. https://doi.org/10.1200/JCO.19.03077
Saeki H, Oki E, Kashiwada T et al (2018) Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604). Eur J Cancer 105:41–49. https://doi.org/10.1016/J.EJCA.2018.09.024
Ogitani Y, Aida T, Hagihara K et al (2016) DS-8201a, a novel HER2-targeting ADC with a novel DNA Topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 22:5097–5108. https://doi.org/10.1158/1078-0432.CCR-15-2822
Ogitani Y, Hagihara K, Oitate M et al (2016) Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 107:1039. https://doi.org/10.1111/CAS.12966
Shitara K, Iwata H, Takahashi S et al (2019) Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol 20:827–836. https://doi.org/10.1016/S1470-2045(19)30088-9
Shitara K, Bang Y-J, Iwasa S et al (2020) Trastuzumab Deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 382:2419–2430. https://doi.org/10.1056/NEJMOA2004413
Van Cutsem E, di Bartolomeo M, Smyth E et al (2023) Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol 24:744–756. https://doi.org/10.1016/S1470-2045(23)00215-2
Yamaguchi K, Bang YJ, Iwasa S et al (2023) Trastuzumab deruxtecan in anti–human epidermal growth factor receptor 2 treatment–naive patients with human epidermal growth factor receptor 2–low gastric or gastroesophageal junction adenocarcinoma: exploratory cohort results in a phase II trial. J Clin Oncol 41:816. https://doi.org/10.1200/JCO.22.00575
Modi S, Jacot W, Yamashita T et al (2022) Trastuzumab Deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387:9–20. https://doi.org/10.1056/NEJMOA2203690/SUPPL_FILE/NEJMOA2203690_DATA-SHARING.PDF
Janjigian YY, Shitara K, Moehler M et al (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398:27–40. https://doi.org/10.1016/S0140-6736(21)00797-2
Kang YK, Chen LT, Ryu MH et al (2022) Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 23:234–247. https://doi.org/10.1016/S1470-2045(21)00692-6
Ramamoorthi G, Kodumudi K, Snyder C et al (2022) Intratumoral delivery of dendritic cells plus anti-HER2 therapy triggers both robust systemic antitumor immunity and complete regression in HER2 mammary carcinoma. J Immunother Cancer 10:e004841. https://doi.org/10.1136/JITC-2022-004841
Jiang L, Zhao X, Li Y et al (2023) The tumor immune microenvironment remodeling and response to HER2-targeted therapy in HER2-positive advanced gastric cancer. IUBMB Life. https://doi.org/10.1002/IUB.2804
Janjigian YY, Maron SB, Chatila WK et al (2020) First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol 21:821–831. https://doi.org/10.1016/S1470-2045(20)30169-8
Kun LC, Rha SY, Kim HS et al (2022) A single arm phase Ib/II trial of first-line pembrolizumab, trastuzumab and chemotherapy for advanced HER2-positive gastric cancer. Nat Commun 13:6002. https://doi.org/10.1038/S41467-022-33267-Z
Janjigian YY, Kawazoe A, Yañez P et al (2021) The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 600:727–730. https://doi.org/10.1038/S41586-021-04161-3
Janjigian YY, Kawazoe A, Bai Y et al (2023) Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 402:2197–2208. https://doi.org/10.1016/S0140-6736(23)02033-0
Catenacci DVT, Rosales M, Chung HC et al (2021) MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol 17:1155–1164. https://doi.org/10.2217/FON-2020-1007/FORMAT/EPUB
Catenacci DVT, Kang YK, Yoon HH et al (2022) Margetuximab with retifanlimab as first-line therapy in HER2+/PD-L1+ unresectable or metastatic gastroesophageal adenocarcinoma: MAHOGANY cohort A. ESMO Open 7:100563. https://doi.org/10.1016/J.ESMOOP.2022.100563
Stein A, Paschold L, Tintelnot J et al (2022) Efficacy of Ipilimumab vs FOLFOX in combination with nivolumab and trastuzumab in patients with previously untreated ERBB2-positive esophagogastric adenocarcinoma: the AIO INTEGA randomized clinical trial. JAMA Oncol 8:1150–1158. https://doi.org/10.1001/JAMAONCOL.2022.2228
Conlon NT, Kooijman JJ, van Gerwen SJC et al (2021) Comparative analysis of drug response and gene profiling of HER2-targeted tyrosine kinase inhibitors. Br J Cancer 124:1249–1259. https://doi.org/10.1038/S41416-020-01257-X
Yoshioka T, Shien K, Namba K et al (2018) Antitumor activity of pan-HER inhibitors in HER2-positive gastric cancer. Cancer Sci 109:1166–1176. https://doi.org/10.1111/CAS.13546
Strickler JH, Cercek A, Siena S et al (2023) Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): a multicentre, open-label, phase 2 study. Lancet Oncol 24:496–508. https://doi.org/10.1016/S1470-2045(23)00150-X
Catenacci DVT, Strickler JH, Nakamura Y et al (2022) MOUNTAINEER-02: Phase 2/3 study of tucatinib, trastuzumab, ramucirumab, and paclitaxel in previously treated HER2+ gastric or gastroesophageal junction adenocarcinoma—trial in progress. J Clin Oncol. https://doi.org/10.1200/JCO.2022.40.4_SUPPL.TPS371
Li R, Sant S, Brown E et al (2023) Tucatinib promotes immune activation and synergizes with programmed cell death–1 and programmed cell death–ligand 1 inhibition in HER2-positive breast cancer. J Natl Cancer Inst 115:805–814. https://doi.org/10.1093/JNCI/DJAD072
Van Duijn A, Van Der Burg SH, Scheeren FA (2022) CD47/SIRPα axis: bridging innate and adaptive immunity. J Immunother Cancer 10:e004589. https://doi.org/10.1136/JITC-2022-004589
Lakhani NJ, Chow LQM, Gainor JF et al (2021) Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): a first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study. Lancet Oncol 22:1740–1751. https://doi.org/10.1016/S1470-2045(21)00584-2
Niquille DL, Fitzgerald KM, Gera N (2024) Biparatopic antibodies: therapeutic applications and prospects. MAbs 16:2310890. https://doi.org/10.1080/19420862.2024.2310890
Meric-Bernstam F, Beeram M, Hamilton E et al (2022) Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol 23:1558–1570. https://doi.org/10.1016/S1470-2045(22)00621-0
Tabernero J, Shen L, Elimova E et al (2022) HERIZON-GEA-01: Zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol 18:3255–3266. https://doi.org/10.2217/fon-2022-0595
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The author was responsible for all aspects of the study.
Corresponding author
Ethics declarations
Conflict of interest
Toshihiro Kudo received lecture fees from MSD Co., Ltd., DAIICHI SANKYO CO., LTD., ONO PHARMACEUTICAL CO., LTD., TAIHO PHARMACEUTICAL CO., LTD. Bristol-Myers Squibb K.K., Merck Biopharma Co., Ltd., and received a research grant from Astellas Pharma Inc., AstraZeneca K.K., Amgen inc., DAIICHI SANKYO CO., LTD., Incyte Biosciences Japan G.K., MSD Co., Ltd., Novartis Pharma K.K., ONO PHARMACEUTICAL CO., LTD., Pfizer Inc.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kudo, T. Advances in the treatment of human epidermal growth factor receptor 2-positive gastric cancer. Int J Clin Oncol 29, 1220–1227 (2024). https://doi.org/10.1007/s10147-024-02587-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02587-z