Abstract
The discovery of human epidermal growth factor receptor 2 (HER2) overexpression in 15–20% of gastric adenocarcinomas has been a key advance in the global care of this disease. Validated by the ToGA trial in the first-line setting of advanced HER2-positive (+) gastric cancer (GC), trastuzumab, an anti-HER2 monoclonal antibody (mAb), was the first therapeutic agent to significantly improve the prognosis of these patients. Since these results, many attempts have been made to improve the clinical outcomes of patients with HER2+ GC. However, all the other HER2-targeting molecules have failed to show a survival benefit in large phase III studies. The value of continuing trastuzumab after disease progression has been suggested by several retrospective studies. However, recent results of a randomized phase II trial showed no benefit from this strategy. On the other hand, novel therapeutic methods, such as immunotherapy, are emerging as new tools in the strategy of care of advanced GC, even if their benefit in the specific HER2+ population remains undetermined. Furthermore, substantial progress has been made in the understanding of the mechanisms leading to resistance to anti-HER2 therapies, and in the screening methods to detect them, thus opening new perspectives. The aim of this review was firstly to summarize the existing data on the specific strategy of care of HER2+ advanced GC, and secondly, to describe current knowledge regarding the potential mechanisms of resistance to HER2-targeting therapies. Lastly, we report the prospects for overcoming these potential obstacles, from future therapeutic strategies to new detection methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Trastuzumab has allowed major progress in the treatment of advanced HER2-overexpressing gastric adenocarcinoma. |
All other HER2-targeting molecules have failed to provide benefit in first- or second-line treatment in large randomized phase III studies, and the value of continuing trastuzumab after disease progression remains uncertain. |
Our understanding of the mechanisms leading to resistance to HER2-targeting treatments has recently improved, paving the way for new therapeutic approaches. |
Emerging methods for detection of circulating tumor DNA, for example, and new treatments, such as combination with immunotherapy, have shown interesting results and could become an integral part of the strategy of care of this disease. |
1 Introduction
Gastric cancer (GC) is the fifth most commonly diagnosed cancer and the third most leading cause of cancer-related death in the world [1]. In the majority of cases, GC is diagnosed at a metastatic or unresectable stage. For these patients, systemic treatment based on cytotoxic chemotherapy improves quality of life and survival compared to best supportive care [2]. However, despite the improvement observed during the past decades, advanced GC has a very poor prognosis, with a median overall survival (OS) of 10–12 months [3, 4]. This emphasizes the need to identify new therapeutic targets and to develop new antitumor agents to improve patient outcomes.
GC is a heterogeneous disease, the initiation, growth, and dissemination of which result from different genomic alterations that can induce the activation of numerous molecular pathways. Among these molecular pathways, the identification of a subgroup of tumors that overexpress human epidermal growth factor 2 (HER2) has been a major advance in the therapeutic approach to GC. The aim of this article is first to review the existing data on the treatment of HER2+ advanced GC, then, we discuss current knowledge on the different mechanisms that result in primary or secondary resistance to anti-HER2 therapies. Lastly, we report prospects for the improvement of care for patients with HER2+ advanced GC, from new detection methods to future therapeutic strategies.
2 Human Epidermal Growth Factor 2 (HER2) Signaling Pathway
HER2 is a tyrosine kinase receptor, and a member of the epidermal growth factor receptor (EGFR) family [5], which is involved in numerous signaling pathways such as the PI3K/AKT/mTOR and RAS/RAF/MAP kinase pathways, which result in cellular growth, proliferation, differentiation, and migration. Many studies report overexpression or amplification of HER2 in GC. The reported proportion of GC cases with HER2 overexpression ranges from 6 to 30% [10]. In a large meta-analysis, higher HER2 expression rates were significantly correlated with intestinal-type GC according to Lauren’s classification, proximal tumor sites, and well-differentiated cancers [11]. HER2 overexpression is mainly due to amplification of the HER2 gene, though other molecular mechanisms have been reported [8, 9]. Although HER2 overexpression has been associated with a worse outcome in some studies, its role in prognosis remains uncertain in GC [6, 7].
Immunohistochemistry (IHC) and fluorescence in situ hybridization/in situ hybridization (FISH/ISH) methods have been validated in daily practice to identify HER2-overexpressing gastric tumors [12, 13]. The IHC score divides gastric tumors into three categories: negative (0+ or 1+), equivocal (2+), or positive (3+). The IHC score has several limitations, including the heterogeneous nature of HER2 expression often observed in GC (unlike breast cancer), which can lead to difficulties or errors in its interpretation. Evaluation of the IHC score in GC differs slightly from that in breast cancer, and therefore calls for an experienced pathologist.
In the case of an equivocal score of 2+, it is currently recommended that HER2 positivity be confirmed by detecting HER2 amplification by FISH/ISH [14]. Current guidelines define FISH/ISH positivity as a ratio of HER2 signal to centromere (control) CEP17 signal ≥ 2.0 [14]. Two studies have reported an optimal cut-off ratio of ≥ 4.0 as a predictive factor for response to trastuzumab and a prognostic factor of better survival [15, 16].
Although the results of IHC and FISH are well correlated, some studies have shown that disparities can occur [17]. These inconsistencies may be due to several factors, such as polysomy (increased copy number of the control gene signal leading to a normal signal ratio), intratumoral heterogeneity, or technical errors [18].
3 First-Line Treatment in HER2-Positive Advanced Gastric Cancer
3.1 Trastuzumab
Trastuzumab is an mAb that specifically targets HER2, and was first validated in the treatment of HER2-overexpressing breast cancer [19, 20], and also showed antitumor effects in HER2+ GC in combination with chemotherapy in preclinical studies, including one study based on a human HER2+ GC xenograft model [21, 22]. Based on these results, the ToGA phase III randomized trial compared the efficacy of chemotherapy (cisplatin and fluoropyrimidine) alone or with trastuzumab (at a dose of 8 mg/kg on the first day of first cycle, followed by 6 mg/kg every 3 weeks) in the first-line treatment of HER2-overexpressing (IHC3+ or FISH+) advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma. The primary outcome was OS. Median follow-up was 18.6 months (interquartile range (IQR) 11–25) in the trastuzumab group and 17.1 months (IQR 9–25) in the placebo group. In this trial, trastuzumab with chemotherapy led to a significant increase in OS compared to chemotherapy alone (13.8 vs. 11.1 months; P = 0.0046). Secondary endpoints including PFS, overall tumor response rate, time to progression, and duration of response were also significantly improved in the trastuzumab + chemotherapy group compared to the chemotherapy-alone group [23]. Apart from diarrhea, the frequency of grade 3 or 4 adverse events was similar between the two groups. In particular, there was no between-group difference in cardiac-related events, regardless of grade (6% in both groups). Post hoc subgroup analyses showed that patients with a high level of HER2 expression (IHC3+ or IHC2+ and FISH+) had a higher benefit from the addition of trastuzumab to chemotherapy, whereas no benefit was observed in those with a null or low level of HER2 expression (IHC 0 or 1+), even with a positive FISH. Based on these data, trastuzumab in combination with cisplatin and fluoropyrimidine has become the new standard first-line treatment for patients with advanced gastroesophageal adenocarcinoma overexpressing HER2, defined as IHC 3+ or IHC 2+/FISH+ [3, 4].
Following ToGA, two phase II studies and one retrospective study reported similar results regarding the efficacy of replacing cisplatin by oxaliplatin and/or fluorouracil by capecitabine in combination with trastuzumab [24,25,26]. Therefore, in many centers, oxaliplatin and fluoropyrimidine combined with trastuzumab are used in the first-line treatment of HER2-positive advanced GC, although no phase III study has evaluated oxaliplatin and capecitabine in this specific situation. In addition, Takahari et al. recently showed in a Japanese multicenter phase II study that the combination of S-1 and oxaliplatin with trastuzumab was associated with survival results comparable to those of ToGA [27].
In a phase III randomized study, Shah et al. assessed the efficacy of a high-dose regimen of trastuzumab (loading dose of 8 mg/kg followed by 10 mg/kg every 3 weeks) in combination with cisplatin and capecitabine in a cohort of 248 patients with HER2 + untreated metastatic GC. Although the serum trough concentration of trastuzumab was significantly increased in the high-dose trastuzumab group, no benefit was observed in terms of OS (primary endpoint) or PFS [28].
3.2 Other Anti-HER2 Agents Evaluated in First-Line Chemotherapy by Phase III Randomized Trials
All phase III randomized studies assessing anti-HER2 agents in GC are summarized in Table 1.
3.2.1 Lapatinib
Lapatinib, a dual HER1 and HER2 tyrosine kinase inhibitor [29], was evaluated in the randomized LOGIC phase III study comparing capecitabine and oxaliplatin (CapeOx) alone or combined with lapatinib as first-line therapy in 487 patients with HER2 FISH-positive advanced GC [30]. In this trial, the addition of lapatinib to CapeOx was associated with improvement in PFS but not in OS, which was the primary endpoint. Note that, in this study, selection of HER2-overexpressing patients was based on FISH only, regardless of IHC status, which could explain, at least partly, the negative results, although subgroup analysis found no significant efficacy of lapatinib among IHC 2/3 + patients. Interestingly, pre-planned subgroup analyses found that in younger patients and in patients of Asian origin lapatinib was associated with a significantly increased OS, raising the question of better selection of the patients who would benefit from this drug. Moreover, 23% of patients included in this study had had prior gastrectomy removing their pylorus, which could also have negatively impacted the results by decreasing lapatinib absorption.
3.2.2 Pertuzumab
Pertuzumab is a recombinant humanized mAb that binds to the dimerization domain of HER2, thereby blocking ligand-induced HER2 heterodimerization. Pertuzumab is effective in HER2+ breast cancer at both the early and the advanced stages [31, 32]. A recent phase III study (the JACOB trial) evaluated the addition of pertuzumab versus placebo to trastuzumab with chemotherapy in 780 patients with advanced HER2+ GC (defined as IHC3+ or IHC2+ and FISH+) in a first-line setting [33]. Although patients treated with pertuzumab had a 3.3-month improvement of median OS compared to those who received the placebo, the results from this study did not reach statistical significance (14.2 vs. 17.5 months; HR = 0.84, P = 0.056). These negative results, in contradiction with those observed in breast cancer, underline the differences in HER2 biology between gastric and breast cancers and its role as an oncogenic driver. Notably, according to the authors, the heterogeneous pattern of HER2 expression observed in GC could negatively impact on pertuzumab activity.
4 Targeting HER2 in Second-Line Treatment
4.1 Anti-HER2 Agents Evaluated in Second-Line Therapy by Phase III Randomized Studies
4.1.1 Lapatinb
Lapatinib was evaluated in a second-line setting in the TyTAN phase III trial, which randomized 261 patients with advanced HER2 FISH + GC (regardless of IHC status) [34]. Note that in this trial, only 6% of patients received trastuzumab first line. The addition of lapatinib to paclitaxel was not associated with an improvement of clinical outcome. Interestingly, in a subgroup analysis of patients with baseline IHC3 + HER2-positive tumors from the TyTAN trial (39%), the addition of lapatinib to paclitaxel was associated with a significant improvement of OS (14.0 months vs. 7.6 months; HR = 0.59, P = 0.02), whereas no difference was observed in the population of IHC2+ HER2-positive tumors. Moreover, it is interesting to note that 35% of the patients of this study were IHC0/1+ and would now be considered as HER2-negative.
4.1.2 Trastuzumab Emtansine
Trastuzumab emtansine (T-DM1) is an antibody–drug conjugate of trastuzumab linked to the tubulin inhibitor emtansine. T-DM1 was recently evaluated in the randomized GATSBY phase II/III study including HER2+ GC patients (IHC3+ or IHC2+ and FISH+) who had progressed on first-line treatment. Most of the patients had received trastuzumab in first-line therapy (79% and 76% in the taxane and T-DM1 arms, respectively). Patients were randomized to receive docetaxel or T-DM1. In this study, T-DM1 was not superior to taxane in terms of PFS or OS [35]. Two factors should be noted in regard to the negative results of this trial: first, the choice of T-DM1 monotherapy might not have been the most appropriate notably in regard to its limited targeted mechanism of action, and, second, survival of patients from the control group was 2.6 months longer than expected at the time of study design. A phase I/II trial is currently ongoing to evaluate T-DM1 in combination with capecitabine in first-line treatment of HER2+ advanced GC (NCT01702558).
Beyond the previously discussed limitations that could have negatively impacted the results of these studies, the contradictory results provided by HER2-targeting drugs in a second-line setting in GC and in breast cancer seem to indicate a significant difference in changes in tumor biology during first-line HER2 blockade. Indeed, acquired resistance mechanisms specific to GC have recently been described and will be discussed further in this article. These discrepancies also underline the need for better selection of patients who would benefit from these therapies in second-line treatment.
4.2 Continuing Trastuzumab Beyond First-Line Progression
As presented above, trastuzumab is currently the only HER2-targeting agent that has shown efficacy in a randomized phase III study. In HER2-positive metastatic breast cancer, administration of an anti-HER2 therapy is recommended throughout disease progression, in order to continue suppression of the HER2 pathway in first-line or second-line treatment or beyond [36]. More recently, this strategy of trastuzumab beyond first-line progression has been evaluated in HER2+ GC by several studies, which have shown contradictory results (Table 2).
4.2.1 Retrospective and Non-Randomized Studies
Li et al. showed in a prospective observational Chinese cohort of patients with HER2+ advanced GC that the continuation of trastuzumab beyond progression in combination with chemotherapy was associated with an improvement of PFS compared to chemotherapy alone, while tumor response rate and OS were not significantly improved [37].
In a French retrospective multicenter study, Palle et al. assessed the value of maintaining or not trastuzumab in second-line treatment in 104 patients who had progressed on first-line therapy based on 5-FU plus platinum salt and trastuzumab [38]. Patients treated with trastuzumab beyond progression (37.5%) had a longer median PFS (4.4 vs. 2.3 months) and OS (12.6 vs. 6.1 months) than patients who had been treated with second-line chemotherapy alone (PFS: hazard ratio (HR) = 0.51; 95% confidence interval (CI) [0.33–0.79]; OS: HR = 0.44; 95% CI [0.27–0.73]).
Lastly, a multicenter retrospective Japanese study in 46 patients suggested that continuation of trastuzumab significantly improved PFS in selected subgroups of patients with tumors exhibiting an HER2 IHC expression score of 3 + (HR = 0.41; P = 0.04), intestinal-type histology (HR = 0.32; P < 0.01), and a first-line PFS > 6 months (HR = 0.44; P = 0.04) [39]. This impact of first-line response duration on survival benefit of trastuzumab beyond progression was also suggested in another Japanese retrospective study comparing tumor response and survival in 28 patients who received paclitaxel alone (n = 8) or with trastuzumab (n = 20) in second-line treatment of advanced HER2+ GC. No difference was observed in terms of objective response rate (ORR), PFS, or OS. However, OS and PFS were significantly improved in the subgroup of patients with a first-line PFS > 6 months [40].
This might emphasize the importance of better selection of patients who may benefit from trastuzumab beyond progression. However, given the small number of patients included in the above-cited studies and their retrospective/non-randomized, nature, these results should be considered with caution.
4.2.2 Randomized Studies
At the 2018 American Society of Clinical Oncology (ASCO) congress, Makiyama et al. presented the first results of a Japanese randomized phase II study that assessed the benefit of trastuzumab beyond progression [41]. In this study, 91 patients with advanced HER2+ gastroesophageal adenocarcinoma that had progressed on first-line treatment with fluoropyrimidine, cisplatin, and trastuzumab were randomized to receive either weekly paclitaxel alone or in combination with trastuzumab. The primary outcome was PFS. In this study, unlike the retrospective studies cited above, continuation of trastuzumab in combination with paclitaxel did not significantly improve PFS (3.19 vs. 3.68 months; HR = 0.91; 95% CI, [0.67–1.22]; P = 0.33) or OS (9.95 vs. 10.2 months; HR = 1.23; 95% CI, [0.75–1.99]; P = 0.2). In a subgroup analysis, patients who had a longer period (> 30 days) without trastuzumab prior to randomization (representing 31 patients), PFS (primary endpoint) was significantly longer in the paclitaxel + trastuzumab arm than in the paclitaxel alone arm (4.68 months vs. 2.98 months; HR = 0.45 [0.21–0.96]; P = 0.033) [41]. This could suggest a phenomenon of re-sensitization to trastuzumab after a treatment-free interval, possibly due to a decrease of pressure selection on HER2+ clones.
Furthermore, in this study, HER-2 status was reassessed at first progression in 16 patients. Interestingly, the authors observed that for 11 out of the 16 patients (69%), the HER2 positivity was lost, once more raising the question of the need to select patients who could benefit from maintaining trastuzumab beyond progression. The complete results of this study have not yet been published to our knowledge.
Given the contradictory results of the studies cited above, new trials testing this approach and considering the “plasticity” of HER2 status during treatment may still be relevant.
5 Mechanism of Primary Resistance to Anti-HER2 Agents
5.1 HER2 Heterogeneity
Unlike breast cancer, HER2 overexpression and/or amplification by GC can be heterogeneous (from 26 to 79% in IHC) [42, 43], which could affect response to anti-HER2 therapies such as trastuzumab. In two Japanese studies of patients with HER2 + metastatic or unresectable GC receiving trastuzumab-containing first-line therapy, HER2 heterogeneity (defined on endoscopic biopsy specimens) was an independent factor of worse prognosis [42, 44]. Similarly, in a cohort of 28 patients with HER2+ GC who had gastrectomy prior to receiving trastuzumab, heterogeneous expression of HER2 (defined on surgical specimens) was associated with a significantly worse outcome in a multivariate analysis, compared to homogeneous expression [45]. However, in a Chinese study including 48 patients, this correlation between HER2 heterogeneity and response to trastuzumab was not found [46]. Moreover, it is important to note that unlike breast cancer, HER2 heterogeneity in GC is not clearly defined, with different cut-off values between studies. Guidelines concerning the assessment of HER2 heterogeneity in IHC and/or FISH in GC are therefore needed.
5.2 Co-Existing Oncogenic Alterations
Oncogenic alterations can co-exist in HER2-amplified tumors. Indeed, several genomic alterations such as point mutations or amplifications have been described, leading to activation of tyrosine kinase receptors other than HER2 or downstream signaling pathways. In their study of point mutations and amplifications in the exome of 62 HER2-amplified GC samples, Kim et al. found that more than half of the HER2-amplified gastric tumors showed additional oncogenic alterations that could potentially hamper the growth-inhibitory effect of HER2-targeting drugs.
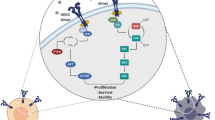
Oncogenic alterations associated with HER2 amplification/overexpression in GC are summarized in Fig. 1.
Oncogenic alterations associated with HER2 overexpression/amplification in gastric cancer conferring primary resistance to trastuzumab. Primary resistance to trastuzumab has mainly been attributed to oncogenic alterations that can co-occur in HER2-amplified tumors [48]. PTEN deficiency and/or PI3K-activating mutations, resulting in activation of AKT/mTOR signaling, have been described in vitro and in vivo in HER2-overexpressing tumors and in HER2 amplified GC cell lines conferring primary resistance to trastuzumab [47, 48]. Hyperactivation of the HGF/MET pathway, induced by MET amplification, MET mutations and elevated serum HGF levels, could also impact negatively on tumor response to trastuzumab [48, 49]. Lastly, EGFR overexpression has been found in a large proportion of HER2-amplified GC cases and could have a synergistic action on the HER signaling pathway and its transforming effect, leading to resistance to HER2-targeting therapies [48, 50]
5.2.1 PTEN and PI3K
Phosphatase and tensin homolog (PTEN) deficiency or PI3K mutations can lead to a constitutive activation of the phosphatidylinositol 3 kinase (PI3K) pathway. In a Japanese study, Deguchi et al. evaluated the co-occurrence of HER2 overexpression and PTEN loss or PI3K-mutations in 264 surgically removed gastric adenocarcinomas [47]. In this study, PTEN loss was observed in 34.5% of HER2-overexpressing tumors. In the same work, the authors evaluated PTEN expression in 23 patients receiving trastuzumab as first-line treatment. While a clinical response was observed in 50% of PTEN-positive tumors, none of the tumors with PTEN loss responded to first-line treatment with trastuzumab, therefore suggesting that PTEN loss could be associated with trastuzumab resistance. In this study, PI3K activating mutations were rarer (5.6%), whereas Kim et al. showed that it could confer resistance to HER2-targeting drugs in HER2-amplified cell line in vitro models [48].
5.2.2 HGF/MET
Hyperactivation of the hepatocyte growth factor (HGF)/mesenchymal epithelial transition factor (MET) pathway could also be involved in primary resistance to trastuzumab. In a study conducted by Takahashi et al. on 46 patients with metastatic GC treated with trastuzumab, high serum HGF was predictive of poor response [49]. In another study, amplification of MET was found in some HER2-amplified GC samples, and conferred resistance to HER2-targeting drugs in HER2-amplified cell-line in vitro models [48].
To our knowledge, no study has evaluated the feasibility and efficacy of combined HER2 and cMet/HGF targeting treatment.
5.2.3 EGFR
In a genomic study based notably on TCGA data, including 62 HER2-amplified untreated GC cases, Kim et al. found co-amplification of EGFR in 7.8% of cases. In a separate cohort of 12 HER2-amplified GC cases, co-existing EGFR overexpression was observed in 58.3% of cases [48]. However, in this small series no data were given regarding the concordance between EGFR amplification and EGFR expression in the context of GC. More recently, the synergistic transforming effects of EGFR and HER2 in GC were studied by Wang et al. using specific small interfering RNAs (siRNAs) to inhibit mRNA and protein expression of target genes [50]. In this study, EGFR-specific siRNA and EGFR/HER2 siRNAs inhibited cell growth, whereas HER2-specific siRNA had no effect. Resistance to HER2-siRNA was linked to compensatory activation of EGFR.
These preclinical data suggest that a dual anti-HER2 anti-EGFR could be beneficial in tumors showing both EGFR and HER2 overexpression.
Emphasizing the importance of concomitant oncogenic alterations in primary resistance to trastuzumab, Pietrantonio et al. proposed a panel of genomic alterations including EGFR/MET/KRAS/PI3K/PTEN mutations and EGFR/MET/KRAS amplifications that was significantly associated with a poorer response to trastuzumab in a prospective case–control study including 37 patients with previously untreated HER2+ metastatic GC [51].
6 Mechanism of Secondary Resistance to Anti-HER2 Agents
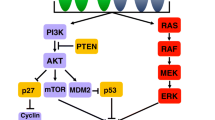
Mechanisms of acquired resistance to trastuzumab are summarized in Fig. 2.
Mechanisms of acquired resistance to trastuzumab. In treatment with trastuzumab, several molecular mechanisms have been described in vitro and/or in vivo, resulting in secondary resistance to trastuzumab and other anti-HER2 treatments: HER2 de novo mutations [52], compensatory pathway activation such as activation of the PI3K/Akt pathway through HER3 signaling [52, 56], protein overexpression such as Src, involved in crosstalk between growth signaling pathways [54, 55], and IGQAP1, involved in HER2 phosphorylation [52], regulation by micro RNAs [57], increased capacities of epithelial and mesenchymal transition [59, 60]. Moreover, a loss of HER2 positivity and/or overexpression has been observed in several clinical studies and could occur in up to two-thirds of patients receiving trastuzumab in first-line treatment [61,62,63]
6.1 Acquired HER2 Mutations
HER2 de novo mutations have been described in vitro, using HER2 overexpressing GC-derived cell lines treated with trastuzumab. These mutations were located in the portion of the protein involved in the regulation of kinase activity and affected the conformation of the HER2 receptor, which could result in the maintenance of its active form [52].
6.2 Activation of Alternative Pathways
In a study by Sampera et al. conducted in vitro on HER2+ GC cell lines, acquired resistance to trastuzumab was associated with persistent activation of the MAPK/ERK and PI3K/mTOR pathways mediated by Src, a non-receptor tyrosine kinase involved in signaling and crosstalk between growth-promoting pathways [53]. The role of Src in trastuzumab-acquired resistance was also suggested in several preclinical studies conducted in vitro and in vivo in HER2-overexpressing breast cancer [54, 55].
In the same study, GC cell lines with secondary resistance to trastuzumab showed increased mRNA expression of other EGFR family receptors (EGFR, ERBB3, and ERBB4) as well as EGFR family ligands such as epidermal growth factor (EGF) and amphiregulin (AREG) compared with the trastuzumab-sensitive parental cells. Moreover, in the same study, in a mouse model of trastuzumab-resistant derived xenograft, triple inhibition of EGFR, HER2, and human epidermal receptor 3 (HER3) could overcome acquired resistance to trastuzumab. Lastly, in another study conducted in vitro, HER3 overexpression was observed in GC cell lines with secondary resistance to trastuzumab [52]. Consistently with what is observed in GC, several preclinical studies of HER2+ breast cancer suggested that overexpression of HER3 could be induced by HER2 blockade and might play an important role in trastuzumab-acquired resistance [56].
6.3 Protein Overexpression
IQGAP1 is a scaffold protein that governs HER2 expression, phosphorylation, and signaling. It has recently been shown in vitro that trastuzumab-resistant GC cell lines express an increased level of IQGAP1, and that this resistance could be reversed by blocking IQGAP1 production with the use of siRNAs [52].
6.4 Micro RNAs
Micro RNAs (miRNAs) are a group of small non-coding RNAs that regulate cellular biological processes by regulating target genes at the post-transcriptional level. Several miRNAs target HER2 signaling pathway components (such as PI3K, PTEN, AKT) or HER2 compensatory receptors (such as HER3) and may be involved in primary and acquired resistance to anti-HER2 therapies [57].
6.5 Epithelial to Mesenchymal Transition
Epithelial to mesenchymal transition (EMT) in epithelial cells results in the loss of polarity and cell–cell adhesion capacities and increases their migratory and invasive properties. This process has been described as one of the promoters of tumor initiation and metastatic dissemination in cancer and in particular in GC [58]. Several studies suggest that EMT could be involved in acquired resistance to HER2 therapies. Using HER2-positive GC cell lines treated with trastuzumab, Jiaolong et al. showed that cells that acquired resistance to trastuzumab showed a mesenchymal phenotype, increased migration, and invasive capacities as well as higher levels of mesenchymal markers and lower levels of epithelial markers, compared to the parental cells [59]. Another team showed that EMT observed in trastuzumab-resistant HER2+ cell lines was dependent on the TGFβ/ZEB/miR-200 axis and that overexpression of miR-200c alleviated the resistance of trastuzumab and inhibited invasion and migration in vitro [60].
6.6 Loss of HER2 Positivity
Recently, several authors have addressed the question of a possible loss of HER2 overexpression acquired during anti-HER2-containing therapy. In these studies, which used paired biopsies at baseline and after first progression, a loss of HER2 positivity (IHC < 3 + and absence of FISH amplification) and/or loss of HER2 overexpression (scoring down from 2+/3+ to 1+/0+ independently of FISH status) was observed in 28.6–69% of patients [61,62,63]. This loss of HER2 positivity could occur more frequently in tumors with an initial IHC score of 2+ [63].
However, if loss of HER2 overexpression could theoretically lead to progression, the clinical role in progression is not really known, and such loss of HER2 expression has also been described in two case reports of patients responding to trastuzumab-based chemotherapy [61, 64].
These results indicate that dynamic changes of HER2 status may potentially be induced by chemotherapy with trastuzumab and suggest that reassessment of HER2 status after progression could help to identify patients who may benefit from HER2 inhibitor maintenance or rechallenge.
7 New Screening Methods for HER2 Status and Therapeutic Impact
7.1 Circulating Tumor Cells
Tumor cells can be isolated in the blood stream of patients harboring numerous types of cancers including GC. In GC, circulating tumor cells (CTC) could have a prognostic impact and might become a noninvasive tool to monitor disease progression throughout treatment [65, 66]. The molecular characterization of CTC is challenging, notably because of their low levels in the blood. In a recent study using FISH, HER2 amplification detected in CTC was strongly concordant with tissue amplification in patient-matched samples [67]. Moreover, in a study using a combination of immunofluorescence and FISH, 13 out of 50 patients with GC identified as HER2- based on tissue assessment (IHC 0 or 1+ or 2+ and FISH-) showed HER2 amplification in CTC, and preliminary clinical data showed that such patients could benefit from trastuzumab like patients with HER2+ tumors (IHC3+ or IHC2+ and FISH+) [68].
7.2 Circulating Tumor DNA
Circulating tumor DNA (ctDNA) carries tumor-related genetic alterations released by apoptotic and necrotic tumor cells and can be detected in the bloodstream as well as in other body fluids [69]. In early studies of ctDNA in GC, the concordance rate of HER2 amplification from the DNA samples and tumor tissues was around 60% [70]. However, more recent studies have shown a higher concordance (between 75 and 90%) in HER2 detection between tumor tissues and plasma, with more modern techniques such as digital droplet PCR (ddPCR) and next-generation sequencing (NGS) suggesting that ctDNA could be used as an alternative method to screen targeted HER2- populations [71,72,73,74]. Differences between plasma and tumor in HER2 status could also be partially explained by the high heterogeneity of HER2 expression in gastric tumor cells. More data are needed to confirm the validity of ctDNA as a tool for HER2 screening, notably with current innovative methods of detection.
In addition, a study suggested that ctDNA could be a predictive marker for treatment efficacy in the first-line setting. Indeed, Wang et al. showed in a cohort of 56 patients with advanced GC given oxaliplatin-based first-line treatment, with or without trastuzumab, that the patients with plasma HER2 amplification who were treated with trastuzumab had a significantly higher response rate than those without amplification (P = 0.032) [72]. In another study using NGS, HER2 amplification detected in ctDNA at baseline was not associated with the outcome of HER2-targeting treatment. However, in the same study based on 23 patients, HER2 amplification in ctDNA adjusted for HER2 gene copy number and combined with tissue HER2 amplification was associated with a better prognosis (P = 0.004), suggesting that ctDNA could be a complementary tool to predict response to treatment [74]. Furthermore, in the Wang et al. study, the role of plasma HER2 amplification in dynamic monitoring was investigated in 20 patients treated with trastuzumab. As compared to HER2 copy number at baseline, most patients had a decrease in HER2 copy number in the case of survival benefit afforded by trastuzumab [72]. These results tend to indicate that HER2 ctDNA dynamic evaluation could be a surrogate marker of treatment efficacy, although this needs to be confirmed by further studies.
However, to our knowledge, no data have been published regarding the reliability of ctDNA as a predictive marker of response to anti-HER2 therapies in the specific setting of progression on first-line treatment.
7.3 HER2 Extracellular Domain
The extracellular domain (ECD) of HER2 can be measured in serum. In a study that included 133 cases of metastatic GC, there was a significant relationship between serum concentrations of HER2 ECD and tissue levels of HER2 protein, with an area under the curve for serum HER2 ECD of 0.77 (95% CI 0.68–0.86) [75]. Furthermore, several studies have evaluated changes in HER2 ECD level during HER2-targeting treatment in breast cancer, suggesting that it might predict response to HER2-targeted therapies [76, 77].
7.4 89Zr-Trastuzumab
89Zr-Trastuzumab is a novel imaging agent that combines trastuzumab radiolabeled with zirconium-89. A preclinical study using xenografts showed that 89Zr-trastuzumab positron emission tomography (PET) could identify and delineate HER2+ tumors and measure the pharmacodynamic effect of anti-HER2 therapies [78]. More recently, 89Zr-trastuzumab was assessed in a small cohort of ten patients with metastatic HER2+ GC and revealed the primary tumor site as well as known metastatic sites, with high-quality images and an acceptable safety profile, raising the possibility of noninvasive evaluation of variations of HER2 overexpression at both primary and metastatic sites [79].
8 Therapeutic Perspectives
8.1 Monoclonal Antibodies
Margetuximab is an Fc-modified chimeric anti-HER2 mAb that binds with high affinity to CD16A, an important receptor for antibody-dependent cell-mediated cytotoxicity. A phase I study has demonstrated its tolerability and promising antitumor activity [80]. One phase Ib/II study is currently evaluating the combination of margetuximab and pembrolizumab (an anti-PD1 mAb) in patients with refractory HER2-positive advanced gastric and gastroesophageal junction (GEJ) cancer (NCT02689284).
Trastuzumab deruxtecan (DS-8201a) is an antibody–drug conjugate that combines trastuzumab with a topoisomerase I inhibitor by a cleavable peptide linker [81]. Based on the results of a multicenter phase II study [82], trastuzumab deruxtecan was recently approved by the US Food and Drug Administration for the treatment of metastatic or unresectable HER2+ breast cancer, after two or more anti-HER2 agent-containing lines of treatment.
In GC, trastuzumab deruxtecan was recently evaluated in a dose-expansion/phase I trial conducted in the USA and Japan. In this study, 44 patients with HER2-positive (IHC 3+ or IHC 2+ and FISH-positive) advanced GC or GEJ carcinoma received at least one dose of trastuzumab deruxtecan. Patients had received a median of three previous lines. All patients had been previously treated with trastuzumab. The most frequent grade 3 or worse events were anemia, low platelet count, and low neutrophil count. The median follow-up was 5.5 months [2.8–13.1]. Nineteen patients (43.2%; 95% CI [28.3–59.0]) achieved an objective response and 35 (79.5%; 95% CI [64.7–90.2]) achieved disease control [83]. An international phase II trial is currently ongoing to further investigate these promising results (NCT04014075).
ZW25 is a novel bispecific antibody that targets two domains of HER2: ECD2 (pertuzumab binding site) and ECD4 (trastuzumab binding site). A phase I study presented at ASCO 2018 showed promising antitumor activity and an acceptable safety profile in patients with pretreated advanced HER2+ cancers, including GC and GEJ cancers [84]. A phase II trial is currently ongoing to evaluate the efficacy of ZW25 in combination with chemotherapy in first-line treatment of HER2+ unresectable or metastatic GC and GEJ cancers (NCT02892123).
8.2 Tyrosine Kinase Inhibitors (TKI)
Poziotinib is an irreversible pan-HER TKI that targets HER2, EGFR, and HER4. It has been evaluated in a prospective, multicenter, open-label, phase I/II study. Thirty-seven patients with advanced HER2+ GC who had received one prior line of chemotherapy (regardless of trastuzumab exposure) were enrolled to receive poziotinib in combination with paclitaxel and trastuzumab. Median PFS and OS were 13.0 weeks (95% CI [9.8–21.9]) and 29.5 weeks (95% CI [17.9–59.2]), respectively [85].
Dacomitinib is an irreversible panHER inhibitor that has shown preclinical antitumor activity in HER2+ GC. It a multicenter Korean phase II trial, 27 patients with advanced HER2+ GC, who had received from one to more than three prior chemotherapy regimens, were treated with dacomitinib monotherapy. Median PFS was 2.1 months (95% CI [2.3–3.4]) and median OS was 7.1 months (95% CI [4.4–9.8]). Note that only seven patients had received prior anti-HER2 therapy [86]. To our knowledge, no phase III trial is currently ongoing to further confirm these results.
Afatinib is an irreversible inhibitor of HER1, HER2, and HER3 that has shown efficacy in HER2-overexpressing GC in preclinical studies [87]. Afatinib is currently being evaluated in two ongoing phase II studies in combination with paclitaxel in second-line treatment of HER2+ advanced gastric adenocarcinoma, after progression during chemotherapy and trastuzumab (NCT02501603; NCT01522768).
8.3 Immune Checkpoint Inhibitors
Monoclonal antibodies targeting immune checkpoints such as programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), or cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) have shown significant benefit in various cancers by restoring an efficient antitumor immune response [88].
The efficacy of immunotherapy in GC has been assessed in four randomized phase III studies showing contradictory results. In the ATTRACTION-2 trial, patients with advanced, refractory GC treated by nivolumab (an anti-PD1 mAb) had a significantly improved OS compared to patients receiving placebo (5.26 vs. 4.14 months; HR = 0.63; 95% CI [0.51–0.78]; P < 0.0001) [89]. On the other hand, the JAVELIN 300 (avelumab, anti-PD-L1) and KEYNOTE-061 (pembrolizumab, anti-PD1) randomized phase III trials did not show significant survival benefits in third- and second-line chemotherapy, respectively [90, 91]. More recently, the KEYNOTE-062 randomized trial showed that addition of pembrolizumab to first-line chemotherapy was not associated with improvement of survival [92]. Based on these results, more studies are needed to improve selection of GC patients who may benefit from immune checkpoint inhibitors.
Data on the efficacy of immunotherapy in the specific context of HER2+ GC are scarce. In the ATTRACTION-2 trial, HER2 status was not collected at inclusion. However, an exploratory subgroup analysis later assessed the efficacy of nivolumab in the population of patients with HER2+ tumors, considering the prior use of trastuzumab as a surrogate for HER2 status. The median OS was significantly longer for patients receiving nivolumab, in the group of patients previously treated with trastuzumab and assumed to be HER2 + (8.3 months vs. 3.1 months; HR = 0.38; 95% CI [0.22–0.66]; P = 0.0006), and in those who did not receive trastuzumab and were assumed to be HER2- (4.8 months vs. 4.2 months; HR = 0.71 95% CI [0.57–0.88]; P = 0.0022) [93]. Even though one must be very cautious interpreting these exploratory data, these results may indicate that the benefit of immunotherapy might be greater in patients with HER2+ tumors compared to HER2-. Interestingly, trastuzumab stimulates HER2-specific T cell responses [94] and increases tumor PD-L1 expression [95], and anti-PD-1 antibody can help enhance the T-cell-specific immunity of trastuzumab. Moreover, oxaliplatin can further enhance T cells by activating dendritic cells. At the ASCO meeting in 2019, Janjigian et al. reported the results of a phase II study including 37 patients with HER2+ advanced GC treated in first-line chemotherapy by CAPOX and trastuzumab in combination with pembrolizumab. The ORR and 6-month PFS rate were 88.6% and 74%, respectively [96]. In order to confirm these preliminary efficacy results, the phase III KEYNOTE-811 trial comparing chemotherapy + trastuzumab with or without pembrolizumab in first-line treatment is currently ongoing (NCT03615326).
9 Conclusions
Since the ToGA trial, several anti-HER2 agents have been evaluated in randomized studies, but none of them have improved survival significantly enough to justify registration. Despite promising retrospective data on the strategy of maintaining trastuzumab beyond progression, the first results from a randomized phase II trial have failed to show a benefit of the addition of trastuzumab to paclitaxel after progression. These results emphasize the need for a better understanding of mechanisms leading to secondary resistance to anti-HER2. Notably, a loss of HER2 overexpression on trastuzumab treatment has been described in several studies and might occur in up to two-thirds of patients. Thus, it appears that the selection of patients who would benefit from the continuation of anti-HER2 therapy beyond progression will be the determinant factor in this approach. Although clinical and histological selection criteria have been suggested, more precise and noninvasive methods are necessary. In this context, the emergence of liquid biopsies containing plasma circulating DNA has been increasingly reported to yield genomic or epigenomic information about tumors. Regarding HER2 status, ctDNA seems to have a high concordance with HER2 amplification in tumor tissues and could serve as a surrogate marker to monitor the efficacy of anti-HER2 targeted agents. Furthermore, new therapeutic agents and combination therapies have recently emerged. In particular, novel antibody–drug conjugates and the combination of anti-HER2 mAb with immunotherapy could yield interesting results.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24:2903–9.
Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2016;27:v38–49.
Zaanan A, Bouché O, Benhaim L, Buecher B, Chapelle N, Dubreuil O, et al. Gastric cancer: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2018;50:768–79.
Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–9.
Yonemura Y, Ninomiya I, Yamaguchi A, Fushida S, Kimura H, Ohoyama S, et al. Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res. 1991;51:1034–8.
Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2005;16:273–8.
Lee JW, Soung YH, Seo SH, Kim SY, Park CH, Wang YP, et al. Somatic mutations of ERBB2 kinase domain in gastric, colorectal, and breast carcinomas. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12:57–61.
Hollywood DP, Hurst HC. A novel transcription factor, OB2-1, is required for overexpression of the proto-oncogene c-erbB-2 in mammary tumour lines. EMBO J. 1993;12:2369–75.
Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2008;19:1523–9.
Lei Y-Y, Huang J-Y, Zhao Q-R, Jiang N, Xu H-M, Wang Z-N, et al. The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: a meta-analysis of literature. World J Surg Oncol. 2017;15:68.
Wong NACS, Amary F, Butler R, Byers R, Gonzalez D, Haynes HR, et al. HER2 testing of gastro-oesophageal adenocarcinoma: a commentary and guidance document from the Association of Clinical Pathologists Molecular Pathology and Diagnostics Committee. J Clin Pathol. 2018;71:388–94.
Bartley AN, Washington MK, Colasacco C, Ventura CB, Ismaila N, Benson AB, et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35:446–64.
Bartley AN, Washington MK, Colasacco C, Ventura CB, Ismaila N, Benson AB, et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2016;35:446–64.
Ock C-Y, Lee K-W, Kim JW, Kim J-S, Kim T-Y, Lee K-H, et al. Optimal Patient Selection for Trastuzumab Treatment in HER2-Positive Advanced Gastric Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21:2520–9.
Gomez-Martin C, Plaza JC, Pazo-Cid R, Salud A, Pons F, Fonseca P, et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol. 2013;31:4445–52.
Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology. 2011;59:832–40.
Koro K, Swanson P, Yeh M. HER2 testing in gastric and gastroesophageal adenocarcinoma—review and update. Ajsp Rev Rep. 2019;24:179–87.
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2–positive operable breast cancer. J Clin Oncol. 2005;23:3676–85.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92.
Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIα gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–8.
Fujimoto-Ouchi K, Sekiguchi F, Yasuno H, Moriya Y, Mori K, Tanaka Y. Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother Pharmacol. 2007;59:795–805.
Bang Y-J, Cutsem EV, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. The Lancet. 2010;376:687–97.
Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer. 2016;16:68.
Ryu M-H, Yoo C, Kim JG, Ryoo B-Y, Park YS, Park SR, et al. Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer Oxf Engl. 1990;2015(51):482–8.
Soularue É, Cohen R, Tournigand C, Zaanan A, Louvet C, Bachet J-B, et al. Efficacy and safety of trastuzumab in combination with oxaliplatin and fluorouracil-based chemotherapy for patients with HER2-positive metastatic gastric and gastro-oesophageal junction adenocarcinoma patients: a retrospective study. Bull Cancer (Paris). 2015;102:324–31.
Takahari D, Chin K, Ishizuka N, Takashima A, Minashi K, Kadowaki S, et al. Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-naïve, HER2-positive advanced gastric cancer. Gastric Cancer. 2019;22:1238–46.
Shah MA, Xu R, Bang Y-J, Hoff PM, Liu T, Herráez-Baranda LA, et al. HELOISE: Phase IIIb Randomized Multicenter Study Comparing Standard-of-Care and Higher-Dose Trastuzumab Regimens Combined With Chemotherapy as First-Line Therapy in Patients With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma. J Clin Oncol. 2017;35:2558–67.
Xia W, Gerard CM, Liu L, Baudson NM, Ory TL, Spector NL. Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24:6213–21.
Hecht JR, Bang Y-J, Qin SK, Chung HC, Xu JM, Park JO, et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2–Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC—A Randomized Phase III Trial. J Clin Oncol. 2015;34:443–51.
Von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377:122–31.
Baselga J, Cortés J, Kim S-B, Im S-A, Hegg R, Im Y-H, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19.
Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372–84.
Satoh T, Xu R-H, Chung HC, Sun G-P, Doi T, Xu J-M, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN–a randomized, phase III study. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32:2039–49.
Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18:640–53.
Cardoso F, Costa A, Senkus E, Aapro M, André F, Barrios CH, et al. 3rd ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol. 2017;28:16–33.
Li Q, Jiang H, Li H, Xu R, Shen L, Yu Y, et al. Efficacy of trastuzumab beyond progression in HER2 positive advanced gastric cancer: a multicenter prospective observational cohort study. Oncotarget. 2016;7:50656–65.
Palle J, Tougeron D, Pozet A, Soularue E, Artru P, Leroy F, et al. Trastuzumab beyond progression in patients with HER2-positive advanced gastric adenocarcinoma: a multicenter AGEO study. Oncotarget. 2017;8:101383–93.
Narita Y, Kadowaki S, Masuishi T, Taniguchi H, Takahari D, Ura T, et al. Correlation between human epidermal growth factor receptor 2 expression level and efficacy of trastuzumab beyond progression in metastatic gastric cancer. Oncol Lett. 2017;14:2545–51.
Horita Y, Nishino M, Sugimoto S, Kida A, Mizukami A, Yano M, et al. Phase II clinical trial of second-line weekly paclitaxel plus trastuzumab for patients with HER2-positive metastatic gastric cancer. Anticancer Drugs. 2019;30:98–104.
Makiyama et al. A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT). J Clin Oncol 36 2018 Suppl Abstr 4011.
Kaito A, Kuwata T, Tokunaga M, Shitara K, Sato R, Akimoto T, et al. HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer. World J Clin Cases. 2019;7:1964–77.
Yang J, Luo H, Li Y, Li J, Cai Z, Su X, et al. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem Biophys. 2012;62:221–8.
Yagi S, Wakatsuki T, Yamamoto N, Chin K, Takahari D, Ogura M, et al. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2019;22:518–25.
Wakatsuki T, Yamamoto N, Sano T, Chin K, Kawachi H, Takahari D, et al. Clinical impact of intratumoral HER2 heterogeneity on trastuzumab efficacy in patients with HER2-positive gastric cancer. J Gastroenterol. 2018;53:1186–95.
Xu C, Liu Y, Jiang D, Li Q, Ge X, Zhang Y, et al. Poor efficacy response to trastuzumab therapy in advanced gastric cancer with homogeneous HER2 positive and non-intestinal type. Oncotarget. 2017;8:33185–96.
Deguchi Y, Okabe H, Oshima N, Hisamori S, Minamiguchi S, Muto M, et al. PTEN loss is associated with a poor response to trastuzumab in HER2-overexpressing gastroesophageal adenocarcinoma. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2017;20:416–27.
Kim J, Fox C, Peng S, Pusung M, Pectasides E, Matthee E, et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J Clin Invest. 2014;124:5145–58.
Takahashi N, Furuta K, Taniguchi H, Sasaki Y, Shoji H, Honma Y, et al. Serum level of hepatocyte growth factor is a novel marker of predicting the outcome and resistance to the treatment with trastuzumab in HER2-positive patients with metastatic gastric cancer. Oncotarget. 2015;7:4925–38.
Wang L, Zhang H, Zheng J, Wei X, Du J, Lu H, et al. Dual silencing of EGFR and HER2 enhances the sensitivity of gastric cancer cells to gefitinib. Mol Carcinog. 2018;57:1008–16.
Pietrantonio F, Fucà G, Morano F, Gloghini A, Corso S, Aprile G, et al. Biomarkers of Primary Resistance to Trastuzumab in HER2-Positive Metastatic Gastric Cancer Patients: the AMNESIA Case-Control Study. Clin Cancer Res. 2018;24:1082–9.
Arienti C, Zanoni M, Pignatta S, Del Rio A, Carloni S, Tebaldi M, et al. Preclinical evidence of multiple mechanisms underlying trastuzumab resistance in gastric cancer. Oncotarget. 2016;7:18424–39.
Sampera A, Sánchez-Martín FJ, Arpí O, Visa L, Iglesias M, Menéndez S, et al. HER-Family Ligands Promote Acquired Resistance to Trastuzumab in Gastric Cancer. Mol Cancer Ther. 2019;18:2135–45.
Zhang S, Huang W-C, Li P, Guo H, Poh S-B, Brady SW, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17:461–9.
Peiró G, Ortiz-Martínez F, Gallardo A, Pérez-Balaguer A, Sánchez-Payá J, Ponce JJ, et al. Src, a potential target for overcoming trastuzumab resistance in HER2-positive breast carcinoma. Br J Cancer. 2014;111:689–95.
Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sánchez V, Chakrabarty A, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci USA. 2011;108:5021–6.
Mao L, Sun A, Wu J, Tang J. Involvement of microRNAs in HER2 signaling and trastuzumab treatment. Tumor Biol. 2016;37:15437–46.
Peng Z, Wang C-X, Fang E-H, Wang G-B, Tong Q. Role of epithelial-mesenchymal transition in gastric cancer initiation and progression. World J Gastroenterol. 2014;20:5403–10.
Shi J, Li F, Yao X, Mou T, Xu Z, Han Z, et al. The HER4-YAP1 axis promotes trastuzumab resistance in HER2-positive gastric cancer by inducing epithelial and mesenchymal transition. Oncogene. 2018;37:3022–38.
Zhou X, Men X, Zhao R, Han J, Fan Z, Wang Y, et al. miR-200c inhibits TGF-β-induced-EMT to restore trastuzumab sensitivity by targeting ZEB1 and ZEB2 in gastric cancer. Cancer Gene Ther. 2018;25:68–76.
Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: Results of GASTric cancer HER2 reassessment study 3 (GASTHER3).: Journal of Clinical Oncology: Vol 35, No 4_suppl. J Clin Oncol [Internet]. [cited 2017 Jun 8]; https://vpn.igr.fr/doi/abs/10.1200/,DanaInfo==ascopubs.org+JCO.2017.35.4_suppl.27.
Saeki H, Oki E, Kashiwada T, Arigami T, Makiyama A, Iwatsuki M, et al. Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604). Eur J Cancer Oxf Engl. 1990;2018(105):41–9.
Pietrantonio F, Caporale M, Morano F, Scartozzi M, Gloghini A, De Vita F, et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. Int J Cancer. 2016;139:2859–64.
Ikari N, Nakajima G, Taniguchi K, Sasagawa T, Narumiya K, Yamada T, et al. HER2-positive gastric cancer with paraaortic nodal metastasis successfully resected after chemotherapy with trastuzumab: a case report. Anticancer Res. 2014;34:867–72.
Liu Y, Ling Y, Qi Q, Lan F, Zhu M, Zhang Y, et al. Prognostic value of circulating tumor cells in advanced gastric cancer patients receiving chemotherapy. Mol Clin Oncol. 2017;6:235–42.
Wang S, Zheng G, Cheng B, Chen F, Wang Z, Chen Y, et al. Circulating tumor cells (CTCs) detected by RT-PCR and its prognostic role in gastric cancer: a meta-analysis of published literature. PLoS One. 2014;9:e99259.
Nevisi F, Yaghmaie M, Pashaiefar H, Alimoghaddam K, Iravani M, Javadi G, et al. Correlation of HER2, MDM2, c-MYC, c-MET, and TP53 copy number alterations in circulating tumor cells with tissue in gastric cancer patients: a pilot study. Iran Biomed J. 2020;24:47–53.
Mishima Y, Matsusaka S, Chin K, Mikuniya M, Minowa S, Takayama T, et al. Detection of HER2 amplification in circulating tumor cells of HER2-negative gastric cancer patients. Target Oncol. 2017;12:341–51.
Moati E, Taly V, Didelot A, Perkins G, Blons H, Taieb J, et al. Role of circulating tumor DNA in the management of patients with colorectal cancer. Clin Res Hepatol Gastroenterol [Internet]. 2018; http://linkinghub.elsevier.com/retrieve/pii/S221074011830041X.
Kinugasa H, Nouso K, Tanaka T, Miyahara K, Morimoto Y, Dohi C, et al. Droplet digital PCR measurement of HER2 in patients with gastric cancer. Br J Cancer. 2015;112:1652–5.
Shoda K, Masuda K, Ichikawa D, Arita T, Miyakami Y, Watanabe M, et al. HER2 amplification detected in the circulating DNA of patients with gastric cancer: a retrospective pilot study. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2015;18:698–710.
Wang H, Li B, Liu Z, Gong J, Shao L, Ren J, et al. HER2 copy number of circulating tumour DNA functions as a biomarker to predict and monitor trastuzumab efficacy in advanced gastric cancer. Eur J Cancer Oxf Engl. 1990;2018(88):92–100.
Gao J, Wang H, Zang W, Li B, Rao G, Li L, et al. Circulating tumor DNA functions as an alternative for tissue to overcome tumor heterogeneity in advanced gastric cancer. Cancer Sci. 2017;108:1881–7.
Maron SB, Chase LM, Lomnicki S, Kochanny S, Moore KL, Joshi SS, et al. Circulating tumor DNA sequencing analysis of gastroesophageal adenocarcinoma. Clin Cancer Res. 2019;25:7098–112.
Peng Z, Liu Y, Li Y, Zhang X, Zhou J, Lu M, et al. Serum HER2 extracellular domain as a potential alternative for tissue HER2 status in metastatic gastric cancer patients. Biomark Med. 2014;8:663–70.
Witzel I, Loibl S, von Minckwitz G, Eidtmann H, Fehm T, Khandan F, et al. Predictive value of HER2 serum levels in patients treated with lapatinib or trastuzumab—a translational project in the neoadjuvant GeparQuinto trial. Br J Cancer. 2012;107:956–60.
Lipton A, Leitzel K, Ali SM, Carney W, Platek G, Steplewski K, et al. Human epidermal growth factor receptor 2 (HER2) extracellular domain levels are associated with progression-free survival in patients with HER2-positive metastatic breast cancer receiving lapatinib monotherapy. Cancer. 2011;117:5013–20.
Janjigian YY, Viola-Villegas N, Holland JP, Divilov V, Carlin SD, Gomes-DaGama EM, et al. Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and 89Zr-trastuzumab PET. J Nucl Med Off Publ Soc Nucl Med. 2013;54:936–43.
O’Donoghue JA, Lewis JS, Pandit-Taskar N, Fleming SE, Schöder H, Larson SM, et al. Pharmacokinetics, biodistribution, and radiation dosimetry for 89Zr-trastuzumab in patients with esophagogastric cancer. J Nucl Med. 2018;59:161–6.
Bang YJ, Giaccone G, Im SA, Oh DY, Bauer TM, Nordstrom JL, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol Off J Eur Soc Med Oncol. 2017;28:855–61.
Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, a novel HER2-targeting ADC with a novel dna topoisomerase i inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22:5097–108.
Modi S, Saura C, Yamashita T, Park YH, Kim S-B, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–21.
Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:827–36.
Meric-Bernstam F, Beeram M, Mayordomo JI, Hanna DL, Ajani JA, Blum Murphy MA, et al. Single agent activity of ZW25, a HER2-targeted bispecific antibody, in heavily pretreated HER2-expressing cancers. J Clin Oncol. 2018;36:2500.
al KT et. A phase I/II study of poziotinib combined with paclitaxel and trastuzumab in patients with HER2-positive advanced gastric cancer.—PubMed—NCBI [Internet]. [cited 2019 Apr 14]. Available from: https://vpn.gustaveroussy.fr/pubmed/,DanaInfo=www.ncbi.nlm.nih.gov,SSL+30945121#.
Oh D-Y, Lee K-W, Cho JY, Kang WK, Im S-A, Kim JW, et al. Phase II trial of dacomitinib in patients with HER2-positive gastric cancer. Gastric Cancer. 2016;19:1095–103.
Yoshioka T, Shien K, Namba K, Torigoe H, Sato H, Tomida S, et al. Antitumor activity of pan-HER inhibitors in HER2-positive gastric cancer. Cancer Sci. 2018;109:1166–76.
Coutzac C, Pernot S, Chaput N, Zaanan A. Immunotherapy in advanced gastric cancer, is it the future? Crit Rev Oncol Hematol. 2019;133:25–32.
Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2017;390:2461–71.
Bang Y-J, Ruiz EY, Van Cutsem E, Lee K-W, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol Off J Eur Soc Med Oncol. 2018;29:2052–60.
Shitara K, Özgüroğlu M, Bang Y-J, Di Bartolomeo M, Mandalà M, Ryu M-H, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet Lond Engl. 2018;392:123–33.
Tabernero J, Van Cutsem E, Bang Y-J, Fuchs CS, Wyrwicz L, Lee KW, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study. J Clin Oncol. 2019;37:LBA4007.
Satoh T, Kang Y-K, Chao Y, Ryu M-H, Kato K, Cheol Chung H, et al. Exploratory subgroup analysis of patients with prior trastuzumab use in the ATTRACTION-2 trial: a randomized phase III clinical trial investigating the efficacy and safety of nivolumab in patients with advanced gastric/gastroesophageal junction cancer. Gastric Cancer. 2020;23:143–53.
Kono K, Takahashi A, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Impaired antibody-dependent cellular cytotoxicity mediated by herceptin in patients with gastric cancer. Cancer Res. 2002;62:5813–7.
Chaganty BKR, Qiu S, Gest A, Lu Y, Ivan C, Calin GA, et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion. Cancer Lett. 2018;430:47–56.
Janjigian YY, Maron SB, Chou JF, Gabler A, Simmons M, Momtaz P, et al. First-line pembrolizumab (P), trastuzumab (T), capecitabine (C) and oxaliplatin (O) in HER2-positive metastatic esophagogastric adenocarcinoma. J Clin Oncol. 2019;37:4011.
Makiyama A, Shimokawa M, Kashiwada T, Takahashi I, Emi Y, Kusumoto T, et al. Trastuzumab beyond first progression in cases of HER2-positive advanced gastric or gastro-esophageal junction cancer: initial results from KSCC1105, a trastuzumab observational cohort study. J Clin Oncol. 2017;35(4_suppl):93–3.
Al-Shamsi HO, Fahmawi Y, Dahbour I, Tabash A, Rogers JE, Mares JE, et al. Continuation of trastuzumab beyond disease progression in HER2-positive metastatic gastric cancer: the MD Anderson experience. J Gastrointest Oncol. 2016;7:499–505.
Sun J, Pan S, Chen Q, Gao X, Li W. Efficacy of trsatuzumab (Herceptin) combined with FOLFIRI regimen in the treatment of HER2-positive advanced gastric cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31:1458–60.
Nishikawa K, Sakai D, Kawada J, Kawabata R, Kawase T, Oka Y, et al. A phase II trial of trastuzumab combined with irinotecan in patients with advanced HER2-positive chemo-refractory gastric cancer: OGSG1203 (HERBIS-5). J Clin Oncol. 2016;34:128.
Kawamoto Y, Meguro T, Yuki S, Nakatsumi H, Sasaki T, Hatanaka K, et al. Phase II study of trastuzumab with irinotecan in HER2-positive metastatic or advanced gastric cancer patients previously treated with trastuzumab and failed: HGCSG 1201/OGSG1205. J Clin Oncol. 2017;35:151.
Zaanan A, Palle J, Soularue E, Leroy F, Louafi S, Tougeron D, et al. Trastuzumab in Combination with FOLFIRI in Patients with Advanced HER2-Positive Gastro-Esophageal Adenocarcinoma: A Retrospective Multicenter AGEO Study. Target Oncol. 2018;13:107–12.
Nishikawa K, Takahashi T, Takaishi H, Miki A, Noshiro H, Yoshikawa T, et al. Phase II study of the effectiveness and safety of trastuzumab and paclitaxel for taxane- and trastuzumab-naïve patients with HER2-positive, previously treated, advanced, or recurrent gastric cancer (JFMC45-1102). Int J Cancer. 2017;140:188–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
JP has served on consulting boards for Servier; CG has served on consulting boards for Servier; SP has served on consulting and/or advisory boards for Amgen and Sanofi; JT has served on consulting and/or advisory boards for Merck, Roche, Amgen, Eli Lilly, Sanofi, Celgene, Servier, Pierre Fabre, and Sirtex; AZ has served on consulting and/or advisory boards for Amgen, Baxter, Lilly, Merck Serono, MSD, Sanofi, Roche and Servier. AR declares he has no conflicts of interest that might be relevant to the contents of this article.
Rights and permissions
About this article
Cite this article
Palle, J., Rochand, A., Pernot, S. et al. Human Epidermal Growth Factor Receptor 2 (HER2) in Advanced Gastric Cancer: Current Knowledge and Future Perspectives. Drugs 80, 401–415 (2020). https://doi.org/10.1007/s40265-020-01272-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01272-5