Abstract
Background
Microvascular invasion (MVI) is a risk factor for postoperative recurrence of hepatocellular carcinoma (HCC), even in early-stage HCC. In small HCC ≤ 3 cm, treatment options include anatomical resection or non-anatomical resection, and MVI has a major effect on treatment decisions. We aimed to identify the predictors of MVI in small HCC ≤ 3 cm.
Methods
We retrospectively studied 129 patients with very early or early-stage HCC ≤ 3 cm who had undergone 18F-fluorodeoxyglucose positron emission tomography/computed tomography and subsequent hepatic resection from January 2016 to August 2023. These patients were divided into the derivation cohort (n = 86) and validation cohort (n = 43). We examined the risk factors for MVI using logistic regression analysis, and established a predictive scoring system in the derivation cohort. We evaluated the accuracy of our scoring system in the validation cohort.
Results
In the derivation cohort, a Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein (AFP-L3), prothrombin induced by vitamin K deficiency or antagonist-II (PIVKA-II), and metabolic tumor volume (MTV) were independent predictors of MVI. We established the scoring system using these three factors. In the validation test, there were no MVI-positive cases with a score of 0 and 1, and all cases were MVI-positive with a score of 4. Moreover, with a score ≥ 2, the sensitivity, specificity, and accuracy of our scoring system were 100%, 71.4%, and 81.4%, respectively.
Conclusions
Our scoring system can accurately predict MVI in small HCC ≤ 3 cm, and could contribute to establishing an appropriate treatment strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide. With recent improvements in surgical techniques, radiofrequency ablation (RFA), and systemic chemotherapy, treatment algorithms have been established according to the staging of HCC, and they have contributed to improved outcomes in HCC [1, 2]. However, even in very early and early-stage HCC, a high recurrence rate after treatment is still an ongoing issue [3, 4].
RFA is one of the first-line treatment options for very early and early-stage HCC. RFA is a minimally invasive local treatment for HCC compared with hepatic resection, and has comparable efficacy in patients with the largest tumor ≤ 3 cm in diameter and those with ≤ 3 nodules [5]. Hepatic resection is also a first-line treatment for HCC, and is one of the most effective and curative treatments in very early and early-stage HCC. To date, several risk factors for recurrence after resection have been reported, and microvascular invasion (MVI) was found to be a major risk factor [6, 7]. The presence of MVI implies local extension and is a risk factor for intrahepatic metastasis. In patients with HCC and MVI, anatomical resection of the liver may eradicate MVI confined to tumor-bearing portal territories and may be superior to partial resection in terms of a radical cure. In fact, anatomical resection for HCC with MVI results not only in the prevention of local recurrence but also in the long-term prognosis of recurrence-free survival and overall survival, compared with non-anatomical resection of the liver [8,9,10]. Therefore, MVI has a major effect on the decision between performing anatomical resection and non-anatomical resection for HCC. RFA is also a local treatment, and anatomical resection is theoretically reasonable for HCC with MVI. Therefore, MVI is a crucial factor regarding the decision between performing RFA and anatomical resection.
On the basis of the clinical question that small HCC ≤ 3 cm is an important borderline for treatment decisions such as anatomical or non-anatomical resection or RFA, we believe that the presence of MVI should be evaluated preoperatively. Information on MVI is only obtained from post-resection specimens, but some studies have predicted MVI preoperatively. Several studies have reported that 18F-fluorodeoxyglucose (18F-FDG) uptake reflects tumor aggressiveness, and the standardized uptake value (SUV) is associated with the presence of MVI [11, 12]. Moreover, we have focused on metabolic parameters shown by 18F-FDG-PET/CT, such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG). These are metabolic parameters that take into account the metabolic activity of the entire tumor. We have reported that these parameters more accurately predicted tumor aggressiveness [13, 14]. We considered that these metabolic parameters would be useful in HCC. However, studies on early HCC with a small tumor size are limited. Therefore, this retrospective study aimed to identify predictors of MVI in very early and early-stage HCC, especially with tumor diameters ≤ 3 cm, which could be important for optimal treatment decisions.
Materials and methods
Patients
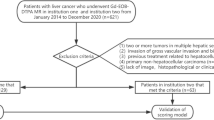
We retrospectively analyzed the data of 227 patients with HCC who underwent preoperative 18F-FDG-positron emission tomography/computed tomography (PET/CT) and surgical resection from January 2016 to August 2023 in the Division of Hepatobiliary and Pancreatic Surgery at Gunma University Hospital. The inclusion criteria were as follows: (a) Barcelona Clinic Liver Cancer (BCLC) staging of very early stage or early-stage HCC; (b) patients with the largest HCC diameter ≤ 3 cm; (c) patients with initial hepatic resection; and (d) patients with curative resection. The exclusion criteria were as follows: (a) patients with macroscopic vascular invasion and extrahepatic spread in preoperative imaging; (b) patients with preoperative treatment, such as systemic chemotherapy, RFA, and transcatheter arterial chemoembolization (TACE); (c) patients who take vitamin K or warfarin; and (d) patients with poorly controlled diabetes. On the basis of the above-mentioned criteria, 129 patients were included in the analysis. In the first 86 patients (patients who underwent surgery in the first two thirds of the study period: derivation cohort), independent risk factors for MVI were examined. The remaining 43 patients (patients who underwent surgery in the last one third of the study period: validation cohort) who later underwent hepatic resection were examined to determine the accuracy of our scoring system.
The clinical characteristics and treatment-related details of all patients were collected from the medical records. Clinical laboratory data, including the tumor markers alpha-fetoprotein (AFP), a Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), and prothrombin induced by vitamin K deficiency or antagonist-II (PIVKA-II), were collected within 1 month prior to hepatic resection. HCC lesions were preoperatively diagnosed by several imaging modalities, including dynamic CT, gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI), and abdominal ultrasonography (US). All imaging was performed within 1 month prior to surgery.
The final diagnosis of HCC was confirmed by a pathological examination of resected specimens. Pathological tumor characteristics were evaluated in accordance with the criteria of the Liver Cancer Study Group of Japan [15]. MVI was defined as the presence of tumor cells forming thrombi in the portal veins, intracapsular vessels, or vascular spaces lined by endothelial cells.
The study was approved by the Gunma University Ethics Committee (HS2023-017) and complied with institutional guidelines and the Declaration of Helsinki.
18F-FDG-PET and image analysis
Details of the 18F-FDG-PET/CT procedures have been previously reported [14]. We routinely performed 18F-FDG-PET/CT in all patients with HCC preoperatively. All PET imaging was performed within 1 month prior to surgery using a 18F-FDG-PET/CT scanner (Discovery STE, GE Healthcare, CA, USA; or Biograph 64, Siemens Medical Solutions, Knoxville, TN, USA) with a 700-mm field of view at Gunma University Hospital. The patients fasted for at least 6 h before 18F-FDG-PET imaging, and the peripheral blood glucose levels were < 120 mg/dl. Two experienced nuclear medicine physicians interpreted the PET images without using any patient’s clinical history or data. Any discrepancies were resolved by consensus.
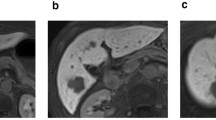
To analyze 18F-FDG uptake, the region of interest (ROI) was manually defined. In patients who did not show a high uptake, the ROI was drawn based on images from abdominal CT scans. The SUV was defined as follows: SUV = radioactive concentration in the ROI (kBq/ml)/injected dose (kBq)/patient’s body weight (kg). Siemens Syngo.via software VB60A (Siemens Healthcare Solutions, Erlangen, Germany) was used at Gunma University Hospital on a workstation to automatically calculate the metabolic tumor volume (MTV) (cm3) and total lesion glycolysis (TLG) (SUV x cm3). MTV was defined as the tumor volume inside the tumor boundaries using SUV thresholds that were 50% of the tumor maximum SUV (SUVmax). TLG was calculated by multiplying the MTV by the mean SUV determined in a selected contouring volume of interest. In multiple cases, tumors with the highest 18F-FDG uptake were selected, and MTV and TLG were calculated (Fig. 1).
Treatment and follow-up strategy
We performed a dynamic CT scan, Gd-EOB-DTPA-enhanced MRI, and abdominal US in all surgical cases to evaluate the number of tumors, tumor diameter, and tumor localization. The optimal operative procedure and extent of resection were determined by consensus through team conferences. After surgery, all patients were examined for recurrence every month by tumor markers, US, and a CT scan every 6 months after discharge. When recurrence was suspected, multiple image-based modalities were performed as indicated. Recurrent cases were treated by repeated hepatectomy, RFA, TACE, systemic chemotherapy or radiotherapy, depending on the situation of recurrence.
Statistical analysis
We analyzed the associations between continuous and categorical variables and MVI using the Student t-tests and the chi-square tests, as appropriate. We also performed a logistic regression analysis using variables with p-values of < 0.05 in the univariate analysis to predict independent predictors of MVI. To avoid the problem of collinearity, factors with strong correlations, such as tumor SUVmax, MTV, and TLG, were excluded and analyzed in a multivariate analysis. In addition, to determine the optimal cut-off values for predicting MVI, receiver operating characteristic (ROC) curves were created, and the area under the curve (AUC) was calculated. The cut-off value for the probability of MVI was determined using the Youden index in the derivation set. The Akaike information criteria (AIC) statistic was used to show the most appropriate metabolic parameter as predictor of MVI among tumor SUVmax, MTV, and TLG [16]. All statistical analyses were carried out using JMP software, version 15 (SAS Institute, Cary, NC, USA). A p-value < 0.05 was considered to show statistical significance.
Results
Patient’s characteristics
According to the study criteria, we included 129 patients, with 86 in the derivation cohort and 43 in the validation cohort. Clinicopathological features in the overall cohort are shown in Table 1. According to the Child–Pugh classification, 128 (99.2%) patients were classified as A. According to BCLC staging, 47 (36.4%) patients were very early stage and 82 (63.6%) patients were early stage. Regarding the operative procedures, anatomical resection was performed in 49 (37.9%) patients and non-anatomical resection in 80 (62.1%) patients.
Identification of predictors for MVI
Clinicopathological features in the derivation cohort and validation cohort are shown in Supplementary Table 1. We examined the predictors of MVI in 86 patients in the derivation cohort (Table 2). In the derivation cohort, 24 (27.9%) patients had MVI. The univariate analysis revealed some univariate factors. About tumor-related factor, preoperative AFP-L3 (p = 0.0004) and PIVKA-II (p = 0.013) values were significantly higher in the MVI-positive group than in the MVI-negative group. Moreover, the rate of the poorly differentiated type was significantly higher in the MVI-positive group than in the MVI-negative group (p = 0.0008). Regarding metabolic parameters, tumor SUVmax (p = 0.0003), MTV (p < 0.0001), and TLG (p < 0.0001) values in the MVI-positive group were significantly higher than those in the MVI-negative group (Fig. 2). There were no other significant potential predictive factors for MVI.
We then performed multivariate analysis by a logistic regression model. Independent predictors of MVI were preoperative AFP-L3, PIVKA-II, tumor SUVmax, MTV, and TLG (Table 3). Regarding metabolic parameters as predictors of MVI, each parameter was compared based on the AUC and AIC. MTV showed the highest AUC and the lowest AIC statistic value (AUC 0.87, AIC 66.4), which indicated that it had the best ability to predict MVI compared with the other metabolic parameters (Fig. 3, Table 4). These results suggested that preoperative AFP-L3, PIVKA-II, and MTV were predictors of MVI for small HCC ≤ 3 cm.
To determine the optimum values for predicting MVI, the ROC curve was plotted. The best cut-off value of AFP-L3 was 9.9% (area under the curve [AUC] 0.68), and the best cut-off value of PIVKA-II was 50.0 mAU/ml (AUC 0.71). The best cut-off of MTV was 4.17 cm3 (AUC 0.87) (Supplementary Fig. 1).
Predictive scoring system for MVI
The predictive scoring system was determined according to the number of positive MVI predictors in each case. Points were assigned according to odds ratio in the multivariate analysis. (AFP-L3 ≥ 9.9%, score = 1; PIVKA-II ≥ 50.0 mAU/ml, score = 1; MTV ≥ 4.17 cm3, score = 2, with a total prediction score of 0 to 4 points). In addition, we examined 43 patients in the validation cohort to determine the accuracy of our scoring system (Fig. 4). There were 15 (34.9%) MVI-positive cases. No patients with a score of 0 (0/15) and score of 1 (0/5) were MVI-positive. More importantly, all (6/6) patients with a score of 4 were MVI-positive. Moreover, with a score ≥ 2, the sensitivity, specificity, and accuracy of our scoring system were 100%, 71.4%, and 81.4%, respectively.
In addition, we examined the Kaplan–Meier curves for recurrence-free survival (RFS) and overall survival (OS) by the classifications of scoring: 0 to 4 in the total cohort (Supplementary Fig. 3). These curves showed that patients with a score of 4 had significantly worse RFS (p = 0.022) and OS (p = 0.024) than those with a score of 0.
Discussion
In this study, we showed that high AFP-L3 and PIVKA-II values, and a high MTV were independent predictors of MVI in patients with small HCC ≤ 3 cm. Moreover, the predictive scoring system created from these factors could accurately identify MVI. In addition to tumor markers, this scoring system incorporated MTV with 18F-FDG-PET/CT, which was able to accurately evaluate tumor aggressiveness, and is considered to be more reliable. Currently, the treatment options are anatomical resection, non-anatomical resection, and RFA for small HCC ≤ 3 cm, and the presence of MVI is a major factor in determining the treatment strategy. Our novel scoring system could be useful for predicting MVI in very early or early-stage HCC ≤ 3 cm.
Among tumor markers, AFP-L3 and PIVKA-II are important factors in predicting the recurrence of HCC, but they are also important for predicting MVI [17]. Pote et al. reported the high diagnostic performance of PIVKA-II for MVI in early-stage HCC with BCLC staging [18]. Imura et al. found that high AFP-L3 values were an independent risk factors among tumor markers for MVI in HCC within the Milan criteria [19]. Furthermore, Hirokawa et al. reported that high AFP-L3 and PIVKA-II values were independent predictors of MVI, and the recurrence rate in patients with MVI was worse when these values were high [20]. Although these previous studies support the validity of our study, the cut-off values of tumor markers for predicting MVI are not constant. This variation may be due to the background liver or the tumor size in HCC. We limited the target HCC to the very early or early stage of BCLC staging and a tumor size ≤ 3 cm in our study. We believe that a strength of our study is that we established cut-off values of AFP-L3 and PIVKA-II, which are considered useful for predicting MVI in very early and early-stage HCC, and also created an accurate scoring system.
Regarding the association between tumor size and MVI, the incidence of MVI increases as the tumor size increases [21, 22]. Wang et al. confirmed this finding in a large analysis [23]. However, in very early or early-stage HCC in BCLC staging, the relationship between tumor size and MVI is controversial. Yamashita et al. found that, even in patients with HCC with a tumor size ≤ 3 cm, a larger tumor size was related to the incidence of MVI, and a tumor size ≥ 2 cm was an independent predictor of MVI [9]. However, Wang et al. reported no relationship between tumor size and the presence of MVI in HCC with a tumor size ≤ 2 cm [24]. In our study, we included HCC with a tumor size ≤ 3 cm, and the tumor size was not an independent predictor of MVI. Therefore, we believe that an unclear borderline of MVI exists between tumor diameters of 2 cm and 3 cm, even in early stage HCC of BCLC staging, and MVI should not be determined only by the tumor diameter. Instead of the tumor diameter, the usefulness of tumor volume has also been evaluated. The ADV score, which is composed of AFP, PIVKA-II, and tumor volume, is an accurate prognostic indicator for HCC and a useful predictor of MVI [25].
We focused on MTV as an alternative and accurate factor to tumor size. Some reports have shown that metabolic parameters shown by 18F-FDG-PET/CT are useful for reflecting tumor aggressiveness [13, 14, 26]. Additionally, 18F-FDG accumulation is associated with a poor prognosis of HCC [27]. Shirabe et al. have focused on the usefulness of 18F-FDG-PET/CT in evaluating histological differentiation and found that SUVmax was an independent predictor of MVI [11]. They also created a new scoring system for the prediction of MVI. In our study, among the metabolic parameters, MTV was the best predictor with the lowest AIC value, and one of the criteria in the accurate predicting scoring system. SUVmax is an index that represents only one point of the highest concentration of 18F-FDG in the malignancy. Furthermore, this single point represents the value for one voxel in the ROI (normally < 0.1 ml); therefore, it has limited value for evaluating malignancy of the entire tumor. In this context, MTV has recently been used as a more accurate metabolic parameter of FDG uptake in the whole tumor. The association between 18F-FDG uptake and MVI has already been demonstrated, and tumor volume has also been shown to be an important factor in predicting MVI [11, 12, 25, 27]. Our results that MTV represented 18F-FDG uptake and tumor volume and was valid for predicting MVI are strongly supported by the conclusions of these studies.
We established a novel predictive scoring system that was useful for predicting MVI, but this was a single-center, retrospective, observational study. We used a validation cohort to confirm the accuracy of this scoring system, but total sample size was not enough because the study was limited to HCC ≤ 3 cm. It was difficult to plan multi-institutional study due to institutional differences in the measurement of metabolic parameters. Instead, future prospective studies at our institution could report more reliable results. We would like to consider prospective studies in the future.
In conclusion, we identified independent factors that predict MVI with very early or early-stage HCC ≤ 3 cm. Preoperative AFP-L3, PIVKA-II, and MTV values are strong predictors of MVI, and a novel scoring system using these factors is able to predict MVI. MVI-positive HCC recurs at a high rate. Therefore, anatomical resection is recommended over local therapy, such as RFA or non-anatomical resection, even for very early or early-stage HCC with a tumor diameter ≤ 3 cm. Our scoring system can accurately identify the presence or absence of MVI preoperatively and could be used to establish an appropriate treatment strategy, which may contribute to improving the outcome of HCC.
References
Reig M, Forner A, Rimola J et al (2022) BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 76:681–693
Hasegawa K, Takemura N, Yamashita T et al (2023) Clinical practice guidelines for hepatocellular carcinoma: the japan society of hepatology 2021 version (5th jsh-hcc guidelines). Hepatol Res 53:383–390
Lima HA, Moazzam Z, Endo Y et al (2023) TBS-based preoperative score to predict non-transplantable recurrence and identify candidates for upfront resection versus transplantation for hepatocellular carcinoma. Ann Surg Oncol 30:3363–3373
Heimbach JK, Kulik LM, Finn RS et al (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67:358–380
Izumi N, Hasegawa K, Nishioka Y et al (2019) A multicenter randomized controlled trial to evaluate the efficacy of surgery vs. radiofrequency ablation for small hepatocellular carcinoma (SURF trial). J Clin Oncol 37:4002
Erstad DJ, Tanabe KK (2019) Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol 26:1474–1493
Chan AWH, Zhong J, Berhane S et al (2018) Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol 69:1284–1293
Zhang X-P, Shuai Xu, Lin Z-Y et al (2023) Significance of anatomical resection and resection margin status in patients with HBV-related hepatocellular carcinoma and microvascular invasion: a multicenter propensity score-matched study. Int J Surg 109:679–688
Haoyu Hu, Qi S, Zeng S et al (2021) Importance of microvascular invasion risk and tumor size on recurrence and survival of hepatocellular carcinoma after anatomical resection and non-anatomical resection. Front Oncol 11:621622
Yamashita Y, Imai K, Yusa T et al (2018) Microvascular invasion of single small hepatocellular carcinoma ≤3 cm: Predictors and optimal treatments. Ann Gastroenterol Surg 2:197–203
Shirabe K, Toshima T, Kimura K et al (2014) New scoring system for prediction of microvascular invasion in patients with hepatocellular carcinoma. Liver Int 34:937–941
Yoh T, Seo S, Ogiso S et al (2021) Quantitative assessment of microvascular invasion in hepatocellular carcinoma using preoperative serological and imaging markers. HPB (Oxford) 23(7):1039–1045
Harimoto N, Hoshino K, Muranushi R et al (2019) Impact of metabolic parameters of 18F-fluorodeoxyglucose positron emission tomography after hepatic resection in patients with intrahepatic cholangiocarcinoma. Anticancer Res 39:971–977
Fukushima R, Harimoto N, Kawai S et al (2024) Total lesion glycolysis by 18F-fluorodeoxyglucose positron emission tomography predicts tumor aggressiveness in patients with extrahepatic bile duct carcinoma. J Hepatobiliary Pancreat Sci. https://doi.org/10.1002/jhbp.1421
Liver Cancer Study Group of Japan (2010) General rules for the clinical and pathological study of primary liver cancer. Kanehara & Co., Ltd, Tokyo
Akaike H (1974) A new look at statistical model identification. IEEE Trans Automatic Control 19(6):716–723
Norman JS, Li PJ, Kotwani P et al (2023) AFP-L3 and DCP strongly predict early hepatocellular carcinoma recurrence after liver transplantation. J Hepatol 79:1469–1477
Poté N, Cauchy F, Albuquerque M et al (2015) Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol 62:848–854
Imura S, Teraoku H, Yoshikawa M et al (2018) Potential predictive factors for microvascular invasion in hepatocellular carcinoma classified within the milan criteria. Int J Clin Oncol 23:98–103
Hirokawa F, Hayashi M, Miyaoto Y et al (2014) Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol Res 2014(44):846–853
Mao S, Yu X, Yang Y et al (2021) Preoperative nomogram for microvascular invasion prediction based on clinical database in hepatocellular carcinoma. Sci Rep 11:13999
Hwang S, Lee YJ, Kim KH et al (2015) The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg 19:1281–1290
Wang X, Fu Y, Zhu C et al (2022) New insights into a microvascular invasion prediction model in hepatocellular carcinoma: a retrospective study from the SEER database and China. Front Surg 9:1046713
Wang H, Wu MC, Cong WM (2019) Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res 49:344–354
Kang WH, Hwang S, Kaibori M et al (2023) Validation of quantitative prognostic prediction using ADV score for resection of hepatocellular carcinoma: a Korea-Japan collaborative study with 9200 patients. J Hepatobiliary Pancreat Sci 30:993–1005
Nose Y, Makino T, Tatsumi M et al (2023) Risk stratification of oesophageal squamous cell carcinoma using change in total lesion glycolysis and number of PET-positive lymph nodes. Br J Cancer 128:1879–1887
Hyun SH, Eo JS, Lee JW et al (2016) Prognostic value of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with Barcelona Clinic Liver Cancer stages 0 and A hepatocellular carcinomas: a multicenter retrospective cohort study. Eur J Nucl Med Mol Imaging 43:1638–1645
Acknowledgements
We thank Ellen Knapp, PhD, from Edanz (http://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
R.F., N.H., and K.S. contributed to the conception and design of this study. R.F., T.O., T.S., K.H., S.K., K.H. and H.T. contributed to the acquisition of data. R.F., N.H., N.I., M.T., T.I., and M.S. contributed to the analysis and interpretation of data. R.F., N.H., K.A., and K.S. contributed to the drafting of the manuscript. R.F., N.H., K.A., H.T., T.H., M.S., and K.S. contributed to the revision of the manuscript. All authors contributed to the final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Ethical approval
The study was approved by the Gunma University Ethics Committee (HS2023-017) and complied with institutional guidelines and the Declaration of Helsinki. Patient consent for participation was obtained using the opt-out method.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Fukushima, R., Harimoto, N., Okuyama, T. et al. New predictors of microvascular invasion for small hepatocellular carcinoma ≤ 3 cm. Int J Clin Oncol 29, 1182–1190 (2024). https://doi.org/10.1007/s10147-024-02553-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02553-9