Abstract
Purpose
We evaluated the prognostic value of pretreatment 18F-fluorodeoxyglucose positron emission tomography with computed tomography (FDG PET/CT) in patients with Barcelona Clinic Liver Cancer (BCLC) stage 0 or A hepatocellular carcinoma (HCC) who had received curative treatment or transarterial chemoembolization (TACE).
Methods
Between 2009 and 2010, 317 patients diagnosed with HCC at seven hospitals were enrolled. Among these, 195 patients underwent curative treatments including resection, liver transplantation, and radiofrequency ablation. TACE was performed in 122 patients. The tumor-to-normal liver standardized uptake value ratio (TLR) of the primary tumor was measured using pretreatment FDG PET/CT. The prognostic significance of TLR and other clinical variables was assessed using Cox regression models. Differences in the overall survival (OS) associated with TLR or other significant clinical factors were examined using the Kaplan-Meier method.
Results
Over a median follow-up period of 46 months, 77 patients died from cancer. In the curative cohort, higher TLR (≥2) was significantly associated with death (hazard ratio [HR] = 2.68; 95 % CI, 1.16–6.15; P = 0.020) in multivariable analysis. Patients with a higher TLR had significantly worse OS than patients with a lower TLR (5-year overall survival, 61 % vs. 79.4 %; P = 0.006). In the TACE cohort, the Model for End-Stage Liver Disease (MELD) score (≥8) was a significant independent prognostic factor for OS (HR = 3.34; 95 % CI, 1.49–7.48; P = 0.003), whereas TLR was not associated with OS. The Kaplan-Meier curves showed significantly poorer OS in patients with higher MELD scores (≥8) than in those with lower MELD scores (5-year survival rate, 33.1 % vs. 79.6 %; P < 0.001).
Conclusions
Pretreatment TLR measured using FDG PET/CT was an independent prognostic factor for OS in patients with BCLC stage 0 or A HCC undergoing curative treatment. In contrast, underlying liver function appeared to be important in predicting the prognosis of patients undergoing TACE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Staging systems in hepatocellular carcinoma (HCC) use variables related to tumor stage, liver function, and overall health status of patients. The Barcelona Clinic Liver Cancer (BCLC) system is an algorithmic approach for selecting the best treatment and predicting clinical outcomes in patients with HCC. Patients are classified into five categories: very early (stage 0), early (stage A), intermediate (stage B), advanced (stage C), and terminal (stage D). The staging system is an excellent tool for selecting early-stage patients who could benefit from curative treatment [1].
Surgical resection, liver transplantation, and local ablative therapy are available curative treatment options for patients with HCC diagnosed with BCLC stage 0 or A. However, some patients with early-stage HCC cannot undergo surgical resection or ablation therapy because of an unsuitable location of the lesion or impaired liver function. In these cases, transarterial chemoembolization (TACE) is an effective and alternative treatment option, with favorable long-term survival rates [2, 3].
18F-fluorodeoxyglucose positron emission tomography with computed tomography (FDG PET/CT), a molecular imaging tool, has been found useful for patients with malignancy in accurately assessing various clinical indications such as staging or restaging, therapy response, and prognostication. In patients with HCC, the degree of primary tumor FDG uptake observed on PET/CT varies widely [4]. Although larger tumors have a tendency to show positive FDG uptake, more than half of tumors 2–5 cm in diameter are observed as non-FDG-avid on PET/CT [5]. Previous studies have shown an association between tumor FDG uptake and aggressive biological characteristics and increased risk of recurrence following liver transplantation or surgical resection [6–12]. Therefore, rather than considering it a limitation of FDG PET/CT in tumor detection, the degree of FDG uptake in primary tumors can be seen as a potential new biological tumor factor associated with prognosis independent of tumor size. Because of the low sensitivity of FDG PET/CT, however, there are few studies on the prognostic value of FDG PET/CT in patients with early-stage HCC. The aim of this multicenter retrospective cohort study was to evaluate the role of tumor FDG uptake on PET/CT in predicting the prognosis of patients with BCLC stage 0/A HCC.
Materials and methods
Patients
We reviewed the medical records of 847 patients with newly diagnosed HCC and pretreatment FDG PET/CT at seven university hospitals from January 2009 to December 2010. Of these 847 patients, 317 were included in the study, according to following criteria: 1) diagnosis of HCC by pathology or diagnostic criteria of the American Association for the Study of Liver Diseases guidelines, 2) no prior treatment, 3) BCLC stage 0 or A, and 4) no evidence of extrahepatic metastasis or unexpected second malignancy at staging workup.
All clinical data of the enrolled patients were collected and managed using electronic case report forms provided by the Internet-based Clinical Research and Trial Management System (iCReaT) of the Korean National Institute of Health. Pathologic characteristics of the tumors were obtained from surgical pathology reports.
The institutional review boards of the seven participating university hospitals (Dongsan Medical Center, Incheon St. Mary’s Hospital, Kyung Hee University Hospital, Samsung Medical Center, Seoul St. Mary’s Hospital, Uijeongbu St. Mary’s Hospital, and Yonsei University Health System) approved this retrospective multicenter cohort study, and the requirement to obtain informed consent was waived.
FDG PET/CT procedures
All FDG PET/CT imaging was performed with dedicated PET/CT scanners (Discovery STE, GE Healthcare at Dongsan Medical Center, Incheon St. Mary’s Hospital, Samsung Medical Center, and Yonsei University Health System; Gemini TF-16, Philips Healthcare at Kyung Hee University Hospital; Biograph TruePoint, Siemens Healthcare at Seoul St. Mary’s Hospital, Uijeongbu St. Mary’s Hospital, and Yonsei University Health System; Biograph Duo, Siemens Healthcare at Seoul St. Mary’s Hospital). All patients fasted for at least 6 h prior to intravenous administration of FDG, and blood glucose levels ≤ 140 mg/dL were measured before administering FDG. A dose of approximately 5.5 MBq/kg of FDG was administered intravenously for Discovery STE, 6.0 MBq/kg for Biograph TruePoint and Biograph Duo, and 333 MBq for Gemini TF-16. In all institutions, the PET/CT was performed from the cerebellum to the proximal thighs, 60 min after intravenous injection of FDG. First, a CT transmission scan was performed without contrast enhancement. Immediately following CT, an emission scan was performed in 3D mode. PET images were reconstructed by an iterative reconstruction algorithm using CT images for attenuation correction.
Image analysis of FDG PET/CT
All FDG PET/CT and contrast-enhanced CT or magnetic resonance (MR) imaging data were transferred to the image archive server (National Cancer Center, Korea) using the Digital Imaging and Communications in Medicine (DICOM) format. All images were centrally reviewed by two board-certified nuclear medicine physicians using commercially available imaging software (MIM 6.4; MIM software Inc., Cleveland, OH, USA). For semi-quantitative analysis, maximum standardized uptake value (SUVmax) in the primary tumor was measured using a spherical volume of interest (VOI) over the primary tumor volume. In case of non-FDG-avid tumors, the location and extent of the primary tumor on PET/CT images was determined by correlation with contrast-enhanced CT or MR images. In patients with multiple HCC lesions, only the one showing the highest SUVmax was included for analysis. The mean SUV of the normal liver was obtained by taking the average of the three VOIs (two in the right lobe and one in the left lobe) 1 cm in diameter, placed at a location where HCC was not detected on liver CT or MRI. Precautions were taken when placing the VOIs to avoid beam-hardening artifacts, focal changes of fatty liver or fatty sparing, major vessels, bile ducts, and liver surface margins. The tumor-to-normal liver SUV ratio (TLR) was calculated with the following equation: TLR = maximum SUV of the tumor/mean SUV of the normal liver. Discrepancies between readers were resolved by consensus.
Statistical analyses
Overall survival (OS) was the primary endpoint and was measured from the date of PET/CT imaging to the date of death from any cause or the date of the last clinical follow-up. Recurrence-free survival (RFS) was defined as the time from initial curative treatment to the date of first tumor recurrence or the last clinical follow-up. Variables for survival analyses included age at diagnosis, sex, tumor size, number of tumor nodules, the Model for End-Stage Liver Disease (MELD) score, serum albumin and serum alpha-fetoprotein levels, and TLR.
The MELD score as a measure of liver disease severity was calculated using data from the initial assessment of HCC, which was based on serum creatinine and serum total bilirubin levels, and the international normalized ratio (INR) of prothrombin time [13]. We used the largest tumor diameter measured on contrast-enhanced CT or MR images as a surrogate for tumor size.
Patients were divided into two cohorts according to curative or non-curative initial treatment modality. Differences in clinical characteristics between the two groups were tested using the chi-square test (categorical variables) or the Mann–Whitney U test (continuous variables). A multivariate Cox proportional hazards model was used to assess the potential independent effects of the prognostic variables after adjusting for other risk factors. We also performed an internal validation by a bootstrap method, using 1000 bootstrap replications. Survival curves were estimated using the Kaplan-Meier method, and differences between subgroups were compared with the log-rank test. For comparison of survival curves, the continuous variables were stratified using optimal cutoffs derived from maximally selected rank statistics [14]. Data were analyzed using the open source statistical software R version 3.2.0 (http://www.R-project.org) and IBM SPSS version 20.0 (IBM Corp., Armonk, NY, USA). The R package ‘maxstat’ was used for the maximally selected rank statistics. All tests were two-sided, and P values < 0.05 were considered statistically significant.
Results
Clinical characteristics of patients according to initial treatment
The patient clinical characteristics are summarized in Table 1. The curative cohort included 195 patients who underwent curative therapy including surgical resection (n = 145, 45.7 %), liver transplantation (n = 15, 4.7 %), and radiofrequency ablation (n = 35, 11 %) as an initial treatment option. The TACE cohort included 122 patients treated by TACE (n = 122, 38.5 %). Patients who underwent TACE had significantly shorter survival than those who underwent curative treatment (5-year survival rate, 54.2 % vs. 77.0 %; P < 0.001; Fig. 1). Liver disease severity as measured by the levels of total bilirubin, INR, and albumin, and MELD score was significantly different between the two treatment cohorts. The number of tumor nodules in the TACE cohort was slightly greater than that in the curative cohort. There were no significant differences in TLR, tumor size, and the other clinical variables (Table 1). The median TLR was 1.5 (range, 0.9 to 7.6), and the median MELD score was 7.8 (range, 6.4 to 27.7) for the entire cohort.
Overall survival curves for 317 patients with BCLC stage 0/A HCC stratified by initial treatment modality. The curative cohort includes patients who underwent surgical resection, liver transplantation, and radiofrequency ablation as initial treatment. BCLC Barcelona Clinic Liver Cancer, HCC hepatocellular carcinoma, TACE transarterial chemoembolization
Univariable and multivariable survival analyses
Over a median follow-up of 46 months, 156 patients (49.2 %) showed tumor recurrence or progression, and 77 patients (24.3 %) died from cancer. In univariable Cox regression analyses, TLR and tumor size were significant predictors of OS for the curative cohort, whereas albumin levels and MELD score were significant predictors of OS for the TACE cohort (Table 2). In the curative cohort, a higher TLR (≥2) was significantly associated with poor OS (hazard ratio [HR] = 2.68; 95 % CI, 1.16–6.15; P = 0.020) after adjusting for potential prognostic variables (Table 3). In addition, each doubling of TLR (one unit increase on the log2 scale) was associated with a 2.25-fold increase in the risk of death (HR = 2.25; 95 % CI, 1.13–4.47; P = 0.021; Table S1). Tumor size was an additional prognostic factor in multivariable analysis using continuous variables (Table S1). In the TACE cohort, a higher MELD score (≥8; HR = 3.34; 95 % CI, 1.49–7.48; P = 0.003) was a significant independent prognostic factor for OS, whereas TLR and tumor size were not significant predictors of OS (Table 3, S1). Albumin level was an additional prognostic factor in multivariable analyses using continuous variables (Table S1). The validation cohort confirmed the prognostic performance of TLR for the curative cohort (HR = 2.77; 95 % CI, 1.22–6.28; P = 0.015) and MELD score for the TACE cohort (HR = 3.38; 95 % CI, 1.52–7.51; P = 0.003; Table S2).
Kaplan-Meier analyses according to TLR and MELD score
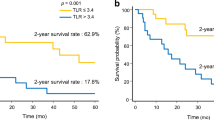
Kaplan-Meier analyses of the curative cohort stratified by TLR demonstrated worse OS in patients with a higher TLR (≥2) than those with a lower TLR (5-year survival rate, 61.6 % vs. 79.4 %; P = 0.006; Fig. 2a). In the TACE cohort, there was no survival difference according to TLR (Fig. 3a).
Kaplan-Meier curves for 195 patients with BCLC stage 0/A HCC who underwent curative treatment. Overall survival curves according to TLR (a) and MELD score (b). BCLC Barcelona Clinic Liver Cancer, HCC hepatocellular carcinoma, TLR tumor-to-normal liver standardized uptake value ratio, MELD Model for End-Stage Liver Disease
Kaplan-Meier curves for 122 patients with BCLC stage 0/A HCC who underwent transarterial chemoembolization. Overall survival curves according to TLR (a) and MELD score (b). BCLC Barcelona Clinic Liver Cancer, HCC hepatocellular carcinoma, TLR tumor-to-normal liver standardized uptake value ratio, MELD Model for End-Stage Liver Disease
In the TACE cohort, OS was significantly improved in patients with lower MELD scores (Fig. 3b). Patients with higher MELD scores (≥8) had poorer OS than patients with lower MELD scores (5-year survival rate, 33.1 % vs. 79.6 %; P < 0.001). In the curative cohort, there was no survival difference according to MELD score (Fig. 2b).
Recurrence-free survival in patients with curative resection
We performed a subgroup analysis for the patients with BCLC stage 0/A HCC undergoing curative resection (n = 145) to examine the correlation between TLR and tumor recurrence. Over a median follow-up of 44 months, 49 patients (33.8 %) experienced intrahepatic recurrence (n = 37) or extrahepatic metastases (n = 12), and 24 patients (16.6 %) died from cancer. The Cox regression analysis for these patients showed that a higher TLR (≥2) was a significant prognostic factor for RFS (HR = 2.28; 95 % CI, 1.15–4.52; P = 0.018) after adjusting for potential prognostic variables (Table 4).
Discussion
Curative treatments such as surgical resection, liver transplantation, and local ablative therapy are available for patients with HCC diagnosed as BCLC stage 0 or A. Median survival of 70–90 % is seen at 5 years after curative treatment in BCLC stage 0 and 50–70 % in BCLC stage A. Despite the excellent prognosis, the aggressive behavior of some tumors leads to early recurrence [15]. Consensus is lacking regarding appropriate ways to individualize adjuvant treatment such as immune-based or molecular-targeted drug therapy in this group of patients [1], as well as regarding methods of identifying significant tumor- or liver function-related factors associated with prognosis.
Liver function-related factors such as portal vein hypertension, bilirubin levels, and Child–Pugh class are considered prognostic in patients with HCC [1]. With preserved liver function, tumor-related factors such as tumor size become important prognostic indicators [1, 16]. In this study, we evaluated the prognostic role of FDG uptake in patients with early-stage HCC. In the curative cohort, high TLR ≥ 2 on pretreatment FDG PET/CT was observed as an independent poor prognostic indicator for RFS, with a 2.3-fold increase in the risk of recurrence, and for OS, with a 2.7-fold increase in the risk of death. As such, patients with high TLR should be closely monitored postoperatively and are likely to be potential candidates for prospective clinical trials of novel treatment approaches.
There have been no previous studies with data correlating TLR and OS in patients with very early- or early-stage HCC undergoing TACE. The role of TLR on PET/CT has been evaluated mainly in patients with intermediate-stage HCC undergoing TACE. Although most studies have suggested high TLR as a poor prognostic factor [17–20], a recent study of 59 patients with HCC treated with TACE showed metabolic tumor volume, number of tumors, and tumor stage as prognostic factors, but did not show TLR as a prognostic factor [21]. In patients with HCC treated by TACE, the prognostic role of tumor FDG uptake on PET/CT is controversial, and further validation with a higher level of evidence is needed. In this study, TACE was given to patients with early-stage HCC that could not be managed by curative treatment due to an unsuitable location of the lesion or impaired liver function. We found that MELD score was significantly associated with OS: patients with higher MELD scores (≥8) had poorer OS than patients with lower scores (5-year survival rate, 33.1 % vs. 79.6 %). Unlike the curative cohort, pretreatment TLR in the TACE cohort was not significantly associated with survival. Further studies are needed to investigate whether PET imaging biomarkers such as volume-based parameters rather than TLR would have prognostic value in this cohort.
TACE is most commonly used as primary treatment in patients with intermediate-stage HCC. Because of the strong cytotoxic and ischemic effects of TACE, preservation of liver function is a critical aspect in patient selection [1]. In this study, liver disease severity as measured by the levels of total bilirubin, INR, and albumin, and MELD score was significantly different between the curative and TACE cohorts, with the latter having more severe liver disease. The OS in patients with preserved liver function in the TACE cohort was comparable to those with low TLR < 2 in the curative cohort, suggesting that TACE may be an effective alternative treatment with favorable survival outcome in certain patients with very early- or early-stage HCC.
A major limitation of this study is its retrospective nature. Selection bias can occur due to treatment-dependent variables such as tumor size, location, multiplicity, and underlying liver function. However, it is the largest study to date in a multicenter setting, comprising 317 patients with BCLC stage 0/A HCC who had undergone pretreatment FDG PET/CT. There are several technical issues that may have caused inaccuracy in SUV measurement. Different PET/CT scanners and imaging protocols were used among the various hospitals; however, an FDG uptake period of 60 min was used in all institutions, and semi-quantitative analysis of the primary tumor was performed using the tumor-to-normal liver SUV ratio to reduce the problem of inter-institutional variability. Partial volume effects and respiratory motion correction are important in SUV measurement of small hepatic lesions. Unfortunately, this was not done, because there was no widely accepted solution at the time of the study. In this study, the tumor FDG avidity was measured by SUVmax, which might not have reflected the true disease activity in the entire lesion. Measurement of global tumor burden such as total lesion glycolysis or metabolic tumor volume would be a more robust method, and further studies are needed to validate this hypothesis. Finally, routine 60-min imaging may be inadequate for accurate evaluation of HCC. With delayed imaging, the combination of increased activity in the tumor and decreased activity in the liver could have resulted in better contrast, especially in hepatic lesions [22].
Conclusions
Pretreatment TLR measured using FDG PET/CT is an independent prognostic factor for survival in patients with BCLC stage 0 or A HCC undergoing curative treatment. Further prospective studies are needed to confirm the prognostic value of TLR in these patients. In contrast, underlying liver function appears to be important in predicting prognosis for patients undergoing TACE.
References
European Association for The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43.
Bargellini I, Sacco R, Bozzi E, Bertini M, Ginanni B, Romano A, et al. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012;81:1173–8.
Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35:2155–66.
Eo JS, Paeng JC, Lee DS. Nuclear imaging for functional evaluation and theragnosis in liver malignancy and transplantation. World J Gastroenterol. 2014;20:5375–88.
Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008;49:1912–21.
Lee JW, Paeng JC, Kang KW, Kwon HW, Suh KS, Chung JK, et al. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med. 2009;50:682–7.
Ahn SG, Jeon TJ, Lee SD, Kim SH, Cho HJ, Yun M, et al. A survival benefit of major hepatectomy for hepatocellular carcinoma identified by preoperative [18F] fluorodeoxyglucose positron emission tomography in patients with well-preserved hepatic function. Eur J Surg Oncol. 2013;39:964–73.
Ahn SG, Kim SH, Jeon TJ, Cho HJ, Choi SB, Yun MJ, et al. The role of preoperative [18F]fluorodeoxyglucose positron emission tomography in predicting early recurrence after curative resection of hepatocellular carcinomas. J Gastrointest Surg. 2011;15:2044–52.
Kitamura K, Hatano E, Higashi T, Seo S, Nakamoto Y, Yamanaka K, et al. Preoperative FDG-PET predicts recurrence patterns in hepatocellular carcinoma. Ann Surg Oncol. 2012;19:156–62.
Kornberg A, Freesmeyer M, Barthel E, Jandt K, Katenkamp K, Steenbeck J, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9:592–600.
Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427–33.
Pant V, Sen IB, Soin AS. Role of 18F-FDG PET CT as an independent prognostic indicator in patients with hepatocellular carcinoma. Nucl Med Commun. 2013;34:749–57.
Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–6.
Lausen B, Schumacher M. Maximally selected rank statistics. Biometrics. 1992;48:73–85.
Kojiro M. Focus on dysplastic nodules and early hepatocellular carcinoma: an eastern point of view. Liver Transpl. 2004;10:S3–8.
Park SK, Jung YK, Chung DH, Kim KK, Park YH, Lee JN, et al. Factors influencing hepatocellular carcinoma prognosis after hepatectomy: a single-center experience. Korean J Intern Med. 2013;28:428–38.
Ma W, Jia J, Wang S, Bai W, Yi J, Bai M, et al. The prognostic value of 18F-FDG PET/CT for hepatocellular carcinoma treated with transarterial chemoembolization (TACE). Theranostics. 2014;4:736–44.
Song HJ, Cheng JY, Hu SL, Zhang GY, Fu Y, Zhang YJ. Value of 18F-FDG PET/CT in detecting viable tumour and predicting prognosis of hepatocellular carcinoma after TACE. Clin Radiol. 2015;70:128–37.
Kim SH, Won KS, Choi BW, Jo I, Zeon SK, Chung WJ, et al. Usefulness of F-18 FDG PET/CT in the evaluation of early treatment response after interventional therapy for hepatocellular carcinoma. Nucl Med Mol Imaging. 2012;46:102–10.
Song MJ, Bae SH, Lee SW, Song d S, Kim HY, Yoo lR, et al. 18F-fluorodeoxyglucose PET/CT predicts tumour progression after transarterial chemoembolization in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2013;40:865–73.
Lee JW, Yun M, Cho A, Han KH, Kim d Y, Lee SM, et al. The predictive value of metabolic tumor volume on FDG PET/CT for transarterial chemoembolization and transarterial chemotherapy infusion in hepatocellular carcinoma patients without extrahepatic metastasis. Ann Nucl Med. 2015;29:400–8.
Lin WY, Tsai SC, Hung GU. Value of delayed 18F-FDG-PET imaging in the detection of hepatocellular carcinoma. Nucl Med Commun. 2005;26:315–21.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This research was supported by the Korean Society of Nuclear Medicine Clinical Trial Network (KSNM CTN) working group funded by the Korean Society of Nuclear Medicine (KSNM-CTN-2014-02-1) and Korea University (grant no. K1422321).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
Written informed consent was waived.
Additional information
Seung Hyup Hyun and Jae Seon Eo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hyun, S.H., Eo, J.S., Lee, J.W. et al. Prognostic value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with Barcelona Clinic Liver Cancer stages 0 and A hepatocellular carcinomas: a multicenter retrospective cohort study. Eur J Nucl Med Mol Imaging 43, 1638–1645 (2016). https://doi.org/10.1007/s00259-016-3348-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3348-y