Abstract
Background
Superselective intra-arterial infusion of cisplatin and concomitant radiotherapy (RADPLAT) is a very promising treatment modality for locally advanced head and neck squamous cell carcinoma. However, there are some concerns regarding its potential for the control of neck lymph node metastasis. The objective of this study was to investigate whether RADPLAT provided inferior regional control compared to intravenous chemoradiotherapy (IV-CRT).
Methods
A total of 172 patients with neck lymph node metastases, 66 of whom underwent RADPLAT and 106 IV-CRT, were enrolled in this study. We retrospectively compared regional control rates between RADPLAT and IV-CRT. Furthermore, to adjust for differences in factors related to patient background between the groups, we conducted inverse probability weighting (IPW) analysis using the propensity score.
Results
A comparison between the two groups revealed that the regional control rates were almost equal under unadjusted conditions; however, after adjustment by IPW analysis, the RADPLAT group had a relatively better regional control rate than did the IV-CRT group (1 year regional control rate: 86.6% vs. 79.4%). In addition, the analysis of relative risk factors for regional control in the RADPLAT group showed that the absence of intra-arterial cisplatin infusion into metastatic lymph nodes was the only independent risk factor (Hazard ratio: 4.23, p = 0.04).
Conclusion
This study showed that the regional control rate in patients treated with RADPLAT was noninferior to that for IV-CRT. Locally advanced head and neck cancers is a good indication for RADPLAT, even if the patients have neck lymph node metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Concurrent chemoradiotherapy (CCRT) is one of the standard treatments for advanced pharyngeal and laryngeal cancers and has the advantage of preserving swallowing and vocal functions. The most standard CCRT regimen is intravenous cisplatin. However, intravenous chemoradiotherapy (IV-CRT) is not effective in the cure of large primary tumors such as T4 disease.
Intra-arterial chemotherapy has been applied to treat localized malignant tumors in patients with head and neck cancer for over 70 years based on the fact that the head and neck region is particularly well suited to regional chemotherapy due to the blood supply being derived from the branches of the external carotid artery [1, 2]. In 1994, Robbins et al. developed a specific concomitant chemoradiotherapy protocol for head and neck cancer in which high-dose cisplatin was administered trans-arterially into selected tumor vessels, with simultaneously administered sodium thiosulfate to neutralize the cisplatin [3]. They reported excellent results with this regimen, consisting of the combination of radiotherapy and superselective intra-arterial infusion of cisplatin (hereafter RADPLAT) [3, 4], as did a subsequent randomized trial conducted in the Netherlands to compare RADPLAT with IV-CRT [5]. After a median follow-up of 33 months, no differences in locoregional control or overall survival were observed between the treatment arms. However, subgroup analysis in their study showed that the local control rates were significantly better in the RADPLAT group for tumors greater than 30 cc in volume and for tumors localized to one side. In addition, RADPLAT has been performed for patients with locally advanced sinonasal cancer in several institutions, and has been reported to result in a favorable survival rate [6,7,8]. We have also reported the efficacy of RADPLAT for locally advanced tumors in the maxillary sinus, oropharynx (base of tongue), larynx and hypopharynx [9,10,11,12].
Thus, RADPLAT has been shown to be a very promising treatment modality when performed in appropriately selected cases. However, there are some concerns regarding the control of neck lymph node metastasis due to the fact that, in RADPLAT, while high doses of cisplatin can be administered to the target tumor, cisplatin that has passed through the tumor is neutralized and rapidly discharged from the body. Maxillary sinus cancers, for which RADPLAT is best suited, rarely develop neck metastasis, but locally advanced cancers of the oropharynx, hypopharynx, and larynx are often associated with lymph node metastases.
Therefore, we retrospectively investigated whether RADPLAT provided inferior regional control compared to IV-CRT in patients with pharyngeal and laryngeal cancers with neck lymph node metastases. Furthermore, we examined the relative risk factors for regional control in patients treated with RADPLAT.
Materials and methods
Patients
This is a retrospective study using the medical records of patients with head and neck cancers treated at Hokkaido University Hospital between January 2003 and December 2020. The inclusion criteria for this study were as follows: (1) previously untreated laryngeal, oropharyngeal or hypopharyngeal cancer, (2) histologically proven squamous cell carcinoma (SCC), (3) positive for neck lymph node metastasis at initial diagnosis, and (4) treatment with RADPLAT. The exclusion criteria were: induction chemotherapy or up-front neck dissection before RADPLAT, and previous radiotherapy to the neck or neck dissection for other cancers. As a control population, data for patients treated with IV-CRT were accumulated using the same criteria as for those treated with RADPLAT.
This study was approved by Hokkaido University Hospital Ethical Committee (Research number: 022-0031). In accordance with the “the ethical guidelines for medical and biological research involving human subjects”in Japan, we used an opt-out agreement without obtaining written informed consent from the patients [13].
Treatment
Radiation was administered in five x 2 Gy single daily fractions per week for a total dose of 65–74 Gy. In general, the prophylactic field was irradiated with a total of 44 Gy and the primary site and metastatic lymph nodes were irradiated with a total of 70 Gy.
For the RADPLAT group, cisplatin (100–120 mg/m2) was infused intra-arterially through a microcatheter placed angiographically to selectively encompass only the dominant blood supply of the targeted tumor. Cisplatin was injected mainly into the primary tumor; however, in some cases, it was also injected into the metastatic lymph nodes. The criteria for arterial injection into the metastatic lymph nodes were as follows: (1) metastatic lymph nodes were too large to control with radiation alone, (2) retropharyngeal lymph node metastasis, and (3) metastatic lymph nodes with a relatively small primary tumor. At the same time as the arterial infusion of cisplatin, sodium thiosulfate (20–24 g) was given intravenously to neutralize cisplatin toxicity. All arterial catheterizations were accomplished transcutaneously through the femoral artery, and the catheters were removed immediately after infusion. To encourage the rapid excretion of the cisplatin, 4 L of lactated Ringer’s solution was given over a 24 h period. A 5-HT3-receptor antagonist was given to all patients before arterial infusion to minimize nausea and vomiting. Intra-arterial infusion of cisplatin was given once a week for a total of 4–6 cycles.
For the IV-CRT group, cisplatin (40 mg/m2) was infused intravenously once a week for a total of 5–6 cycles.
The indications for RADPLAT at our institution are: anterior wall cancer of the oropharynx, lateral wall cancer of the oropharynx invading the anterior wall, and laryngeal and hypopharyngeal cancer localized to one side. In addition, hypopharyngeal carcinoma of greater than N2c was a relative contraindication due to the high risk of distant metastasis.
Data collection
We retrospectively collected patient clinical data including age, sex, primary site, TNM classification according to the UICC 7th edition, maximum diameter of the metastatic lymph node measured by computed tomography (CT) before treatment, treatment details, adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE) ver. 5.0 and treatment outcomes.
Statistical analysis
In this study, the primary endpoint was regional control, and the secondary endpoint was overall survival. The regional control period was defined as the first day of treatment as the start date of measurement, recurrent or residual metastatic lymph nodes identified on imaging or pathology after neck dissection as an event and any patient death as censoring. The overall survival period was defined as the first day of treatment as the start date of measurement, and any patient death as an event.
Patient characteristics between the RADPLAT and IV-CRT groups were compared using the chi-square test, Fisher’s exact test and Student’s t test. Comparison of the regional control rate between the two groups was performed using the Kaplan–Meier method with a log-rank test. Furthermore, to adjust for differences in background factors between the two groups, we conducted inverse probability weighting (IPW) analysis using the propensity score [14]. Subgroup analyses were conducted using univariate Cox proportional hazard regression analysis.
The relative risk factors for regional control in patients treated with RADPLAT were examined using multivariate Cox proportional hazards regression analysis.
A 2-tailed p value < 0.05 was considered statistically significant. Statistical analyses were performed using BellCurve for Excel (Social Survey Research Information Co., Ltd., Japan) and R software ver. 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.).
Results
Patient characteristics and treatment contents
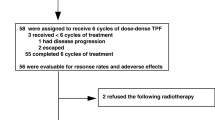
Of the patients who met the criteria for inclusion in this study, 66 were in the RADPLAT group and 106 in IV-CRT group. Table 1 shows the characteristics of patients in the RADPLAT and IV-CRT groups. Patient characteristics with statistically significant differences between the two groups were primary site and T classification. A comparison of the primary sites revealed that the number of patients with p16-negative oropharyngeal cancer was high (31.8%) in the RADPLAT group and low (12.3%) in the IV-CRT group. With regard to T classification, more advanced tumors were observed in the RADPLAT group than in the IV-CRT group. Other factors including age, sex, N classification, clinical stage and maximum diameter of metastatic lymph node showed no significant intergroup differences.
In terms of treatment contents, the total radiation dose was almost equal between the two groups. The median number of cycles of chemotherapy was four in the RADPLAT group and six in the IV-CRT group. The median total cisplatin dose was 400 mg/m2 in the RADPLAT group and 240 mg/m2 in the IV-CRT group. Intra-arterial infusion to metastatic lymph nodes was performed in 66.7% of patients in the RADPLAT group. Planned or salvage neck dissection was performed in 31.8% of patients in the RADPLAT group and 28.3% of those in the IV-CRT group (Table 2). There were no significant differences between the two groups regarding early adverse events during the treatment period (Table 3). No patient had a cerebrovascular accident or neurologic problem attributable to catheterization in the RADPLAT group.
Comparison of the regional control rate between RADPLAT and IV-CRT
To compare the regional control between the RADPLAT and IV-CRT groups, we drew the regional control curves using the Kaplan–Meier method. As shown in Fig. 1, the curves for the two groups almost overlapped. The 1 year regional control rate was 84.6% in the RADPLAT group and 83.8% in the IV-CRT group. However, since there were significant differences between the two groups in terms of primary sites and T classification, we conducted IPW analysis to adjust for potential confounding factors. An excellent balance between the two groups was achieved for all covariates (Fig. 2A). As a result, the IPW-adjusted regional control curves showed that the RADPLAT group had a relatively better regional control rate than did the IV-CRT group (p = 0.47, Fig. 2B), with a 1-year regional control rate of 86.6% in the RADPLAT group and 79.4% in the IV-CRT group.
To examine the relation between RADPLAT and IV-CRT in different patient subsets, subgroup analyses were conducted, with results summarized in Fig. 3. However, no subgroup was observed to demonstrate significantly better regional control among the RADPLAT patients.
Comparison of the overall survival rate between RADPLAT and IV-CRT
Next, we compared overall survival between RADPLAT and IV-CRT. A comparison of the unadjusted populations revealed that the IV-CRT group showed a better survival rate than did the RADPLAT group, although the difference was not significant (Fig. 4A). On the other hand, a comparison of the adjusted populations by IPW analysis showed that the RADPLAT group had a relatively better survival rate than did the IV-CRT group, although the difference was again not significant (Fig. 4B).
Risk factors for regional control in patients treated with RADPLAT
Finally, we examined the relative risk factors for regional control in the RADPLAT group using multivariate Cox proportional hazards regression analysis. We selected the following variables as explanatory variables: age, sex, primary sites, N classification, maximum diameter of metastatic lymph node, total cisplatin dose and intra-arterial infusion to metastatic lymph nodes. The results showed that the absence of intra-arterial infusion to metastatic lymph nodes was the only independent risk factor (Table 4).
Discussion
This is the first study comparing the regional control rate between RADPLAT and IV-CRT for patients with head and neck squamous cell carcinoma. We found that the regional control obtained using RADPLAT was nearly equivalent to that using IV-CRT in pharyngeal and laryngeal cancer (Fig. 1). Furthermore, after adjusting for patient background, that obtained using RADPLAT was unexpectedly comparable or better than that using IV-CRT (Fig. 2B).
One of the possible reasons for this result is that cisplatin intra-arterially injected to the primary tumor may have passed into the lymph nodes via lymphatic flow. Previously, we investigated cisplatin distribution to regional lymph nodes in patients with tongue cancer treated with preoperative intra-arterial cisplatin chemotherapy. The results showed that the platinum concentration was significantly higher in the sentinel nodes than in the non-sentinel nodes [15].
Another possible cause is the effect of the direct intra-arterial injection of cisplatin into metastatic lymph nodes. Multivariate Cox proportional hazards regression analysis showed that the absence of intra-arterial infusion to metastatic lymph nodes was the only independent risk factor (Table 4). Murono et al. similarly reported that intra-arterial cisplatin infusion into metastatic lymph nodes was effective for regional control [16]. They also noted that the staining pattern of metastatic lymph nodes by angio-CT and extranodal extension could be factors for predicting unfavorable regional control. As with the primary tumor, we also performed angio-CT prior to injecting cisplatin into metastatic lymph nodes to accurately identify the inflow vessels and exclude dangerous anastomotic vessels. When the staining pattern of the metastatic lymph nodes was examined for the 13 cases for which angio-CT images could be retrospectively evaluated, the neck lymph node was controlled in all 8 cases in which the lymph nodes were entirely stained, whereas regional recurrence occurred in 1 of the 5 cases in which they were partially stained. Ikushima et al. intra-arterially injected the same dose of cisplatin via the facial artery or the external carotid artery to metastatic lymph nodes in the Ib region in patients with oral cancers and compared the histological changes in the metastatic nodes [17]. The results showed that the superselective group had a better response to treatment than did the non-superselective group.
Our criteria for arterial injection into metastatic lymph nodes were the presence of large metastatic lymph nodes, retropharyngeal lymph node metastasis, and metastatic lymph nodes with a relatively small primary tumor. The cure of large metastatic lymph nodes requires high-dose cisplatin; hence, direct infusion of cisplatin is considered effective. However, metastatic lymph nodes that are too large may not be controlled, even with intra-arterial injection into lymph nodes. In this study, of the 9 patients with ≥ 30 mm metastatic lymph nodes into which direct injection was performed, 4 (49%) failed to achieve control of the lymph node metastasis. On the other hand, only 2 (6%) of 35 patients with < 30 mm metastatic lymph nodes into which direct injection was performed failed to achieve regional control.
The retropharyngeal lymph node, which is sometimes metastasized in advanced head and neck cancers, is difficult to resect in salvage surgery after CRT for anatomical reasons. Therefore, this lymph node must be controlled by CRT and is considered a good indication for RADPLAT. Previously, we reported the effectiveness of RADPLAT targeting retropharyngeal lymph node metastasis. The results showed that there was no recurrence of retropharyngeal lymph node metastasis in nine patients treated with RADPLAT [18].
The cisplatin dose per cycle in RADPLAT is determined by patient body surface area (100–120 mg/m2). The dose of cisplatin injected into the metastatic lymph nodes is determined on a case-by-case basis based on the localization and size of the primary and metastatic lymph nodes. If the primary tumor is relatively small, the tumor blood vessels flowing into the primary may be small or limited in number. Therefore, high-dose cisplatin may cause vasculitis and occlusion of the tumor vessels early in the treatment period. In such cases, the reduced dose of cisplatin for the primary tumor is allocated to the metastatic lymph nodes. In this study, RADPLAT was found to be superior to IV-CRT in terms of overall survival after adjustment for confounding factors (Fig. 4B). However, this result does not mean that RADPLAT is more useful than IV-CRT for all patients with neck metastases. Subgroup analysis of regional control also could not find any population for which RADPLAT was particularly useful (Fig. 3). RADPLAT is a highly curative treatment in which high-dose cisplatin is selectively infused into the target tumors in locally advanced cancer, and its indication should be determined by the site of the local tumor (primary site, tumor volume, unilaterality). RADPLAT is not always cost-effective and is both a time-consuming and labor-intensive procedure, so it must be performed for patients carefully selected on the basis of the indications. However, the results of this study indicated that the presence of neck metastases should not deter patients from undergoing RADPLAT.
The limitations of this study are the small number of cases and its retrospective design. A prospective study comparing regional control between RADPLAT and IV-CRT is necessary to confirm the results of this study. However, we could conduct a retrospective study adjusted for confounding factors using the IPW method. We consider that this is a meaningful analysis method for such a rare cancer.
Conclusion
This study showed that regional control rate in patients treated with RADPLAT was noninferior to that for IV-CRT. Locally advanced head and neck squamous cell carcinoma is a good indication for treatment with RADPLAT, even if the patients have neck lymph node metastases.
References
Klopp CT, Smith DF, Alford TC (1961) Palliation achieved in carcinoma of the head and neck with intra-arterial chemotherapy. Am J Surg 102:830–834
Sullivan RD (1962) Continuous intra-arterial infusion chemotherapy of head and neck cancer. Trans Am Acad Ophthalmol Otolaryngol 66:111–117
Robbins KT, Vicario D, Seagren S et al (1994) A targeted supradose cisplatin chemoradiation protocol for advanced head and neck cancer. Am J Surg 168:419–422
Robbins KT, Kumar P, Regine WF et al (1997) Efficacy of targeted supradose cisplatin and concomitant radiation therapy for advanced head and neck cancer: the Memphis experience. Int J Radiat Oncol Biol Phys 38:263–271
Rasch CR, Hauptmann M, Schornagel J et al (2010) Intra-arterial versus intravenous chemoradiation for advanced head and neck cancer: Results of a randomized phase 3 trial. Cancer 116:2159–2165
Samant S, Robbins KT, Vang M et al (2004) Intra-arterial cisplatin and concomitant radiation therapy followed by surgery for advanced paranasal sinus cancer. Arch Otolaryngol Head Neck Surg 130:948–955
Shiga K, Yokoyama J, Hashimoto S et al (2007) Combined therapy after superselective arterial cisplatin infusion to treat maxillary squamous cell carcinoma. Otolaryngol Head Neck Surg 136:1003–1009
Kanoto M, Oda A, Hosoya T et al (2010) Impact of superselective transarterial infusion therapy of high-dose cisplatin on maxillary cancer with orbital invasion. AJNR Am J Neuroradiol 31:1390–1394
Homma A, Oridate N, Suzuki F et al (2009) Superselective high-dose cisplatin infusion with concomitant radiotherapy in patients with advanced cancer of the nasal cavity and paranasal sinuses: a single institution experience. Cancer 115:4705–4714
Kano S, Homma A, Oridate N et al (2011) Superselective arterial cisplatin infusion with concomitant radiation therapy for base of tongue cancer. Oral Oncol 47:665–670
Taki S, Homma A, Suzuki F et al (2012) Combined modality therapy for laryngeal cancer with superselective intra-arterial cisplatin infusion and concomitant radiotherapy. Int J Clin Oncol 17:441–446
Furusawa J, Homma A, Onimaru R et al (2015) Indications for superselective intra-arterial cisplatin infusion and concomitant radiotherapy in cases of hypopharyngeal cancer. Auris Nasus Larynx 42:443–448
Eba J, Nakamura K (2022) Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn J Clin Oncol 52:539–544
Austin PC, Stuart EA (2015) Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34:3661–3679
Sakashita T, Homma A, Oridate N et al (2012) Platinum concentration in sentinel lymph nodes after preoperative intra-arterial cisplatin chemotherapy targeting primary tongue cancer. Acta Otolaryngol 132:1121–1125
Murono S, Komori T, Endo K et al (2021) Intra-arterial chemotherapy targeting metastatic cervical lymph nodes in head and neck cancer. Acta Otolaryngol 141:1063–1069
Ikushima I, Korogi Y, Ishii A et al (2007) Superselective arterial infusion chemotherapy for squamous cell carcinomas of the oral cavity: histopathologic effects on metastatic neck lymph nodes. Eur Arch Otorhinolaryngol 264:269–275
Suzuki T, Sakashita T, Homma A et al (2016) Effectiveness of superselective intra-arterial chemoradiotherapy targeting retropharyngeal lymph node metastasis. Eur Arch Otorhinolaryngol 273:3331–3336
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kano, S., Suzuki, T., Yoshida, D. et al. The superselective intra-arterial infusion of cisplatin and concomitant radiotherapy (RADPLAT) is effective for metastatic lymph nodes in head and neck squamous cell carcinoma. Int J Clin Oncol 28, 1121–1128 (2023). https://doi.org/10.1007/s10147-023-02363-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02363-5