Abstract
Purpose
Fixed bulky nodal disease in patients with head and neck cancer of unknown primary (HNCUP) remains difficult to treat. This retrospective study evaluated the therapeutic efficacy of selective intra-arterial chemoradiotherapy with docetaxel and nedaplatin for fixed bulky nodal disease in HNCUP.
Methods
Data from seven consecutive patients with fixed bulky nodal disease in HNCUP who had undergone selective intra-arterial chemoradiotherapy were analyzed. Whole pharyngeal mucosa and all bilateral nodal areas were irradiated (total dose 50 Gy), and bulky nodal lesions were provided an additional 20 Gy. Intra-arterial chemotherapy used a combination of nedaplatin (80 mg/m2) and docetaxel (60 mg/m2). Outcome measures were local control, disease-free survival, overall survival, and adverse events. Statistical analyses were performed using the Kaplan–Meier method.
Results
Median follow-up period was 24 months (range 9–64). All patients had extracapsular extension (N3b) on imaging and clinical findings. Symptoms due to bulky disease were neck discomfort (100%), tumor bleeding (43%), tracheal obstruction (14%), and carotid sinus syndrome (28%). Median value for maximum diameter of cervical disease was 84 mm (range 70–107), and 3-year local control, disease-free survival, and overall survival rates were 100, 54, and 64%, respectively. Symptoms due to bulky disease disappeared in all patients after intra-arterial chemoradiotherapy. Grade 4 leukopenia occurred in two patients (28%) as an acute adverse event. No other serious acute adverse events were observed.
Conclusion
Selective intra-arterial chemoradiotherapy with docetaxel and nedaplatin can potentially achieve both favorable local control and survival in in HNCUP with fixed bulky nodal disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck cancer of unknown primary (HNCUP) represents about 2–7% of all head and neck squamous cell carcinomas [1, 2], and its optimal treatment is still controversial because no therapeutic strategy has been validated by prospective studies. The current National Comprehensive Cancer Network clinical practice guides [3] recommend management of stage IV (N2 or N3) HNCUP using a combination of neck lymph node dissection and subsequent postoperative radiotherapy with/without systemic chemotherapy. However, neck dissection is often difficult to perform in advanced fixed bulky nodal disease (e.g., invasion of the carotid artery or the trachea), which can also be refractory to systemic chemotherapy. Further, the ability of radiotherapy to eradicate tumors decreases as the tumor size increases, and local tumor control is dismal for bulky tumors (> 8 cm), even with dose escalation [4, 5]. Local symptoms of fixed bulky nodal disease include chronic oozing, bleeding and stench from ulcerative tumors infiltrating the skin, carotid sinus syndrome, and respiratory compromise due to tracheal obstruction by the tumor. Importantly, these symptoms cannot be adequately addressed by conservative and pharmacological therapy [6], and palliative radiotherapy for unresectable stage IV head and neck cancer is also not always satisfactory as reported symptom relief rates range from 47–59% [7]. Therefore, fixed bulky nodal disease in patients with HNCUP remains a challenge for surgeons, oncologists, and radiation oncologists.
Robbins et al. have demonstrated the efficacy of intra-arterial chemoradiotherapy using high-dose cisplatin (600 mg/m2; RADPLAT), a nonsurgical treatment option for advanced head and neck cancer [8, 9], which has led to its widespread use and the introduction of several other chemotherapeutic agents, i.e., in addition to cisplatin. Good outcomes have been reported with intra-arterial chemoradiotherapy using dual anticancer agents for recurrent head and neck cancer [10] and for advanced oral squamous cell cancers treated with intra-arterial chemoradiotherapy using docetaxel and nedaplatin, an analog of cisplatin with reduced nephrotoxicity [11, 12]. In contrast to RADPLAT, this regimen does not require hydration or the administration of cisplatin-neutralizing agents, and it is a less labor-intensive regimen. To achieve long-term local control of fixed bulky nodal disease in HNCUP, we administered intra-arterial chemoradiotherapy with docetaxel and nedaplatin and this study evaluated the efficacy of this treatment regimen.
Materials and methods
Patients and eligibility
This single-center retrospective study was approved by the institutional ethics review board and was conducted in accordance with the principles of the Declaration of Helsinki. As this study was retrospective in nature, the requirement for written informed consent from the patients was waived. A review of the radiation therapy database identified 14 consecutive patients who had been treated for HNCUP between February 2016 and February 2020. All patients underwent computed tomography (CT) (Fig. 1a), magnetic resonance imaging (MRI), and positron emission tomography CT (PET-CT) (Fig. 1b) for disease staging and for excluding metastatic disease. Further, under anesthesia, they underwent direct laryngoscopy, esophagoscopy, biopsy of any suspicious regions, and directed mucosal biopsies of areas including the nasopharynx, base of the tongue, tonsil regions, and hypopharynx. Thus far, at our institution, when we were unable to prove the presence of tonsillar lesions using all available imaging examinations including PET and biopsy, our policy is to perform tonsillectomy in patients who select cervical dissection followed by radiotherapy; however, tonsillectomy is not performed in patients who select definitive chemoradiotherapy. Human papilloma virus (HPV) testing used immunohistochemical staining for the surrogate marker p16INK4A. Testing for Epstein–Barr virus (EBV) used in situ hybridization with an EBV-encoded small RNA oligonucleotide probe.
Illustrative treatment protocol: a 60-year-old woman who underwent intra-arterial chemoradiotherapy for fixed bulky node disease in head and neck cancer of unknown primary (Tx N3b M0). a Coronal contrast-enhanced computed tomography (CT) showing a bulky nodal lesion with a maximum diameter of 70 mm, extending from the left neck to the supraclavicular fossa. b Coronal positron emission tomography (PET) CT showing extensive fluorodeoxyglucose (FDG) uptake in the bulky nodal lesion in the left neck. c Angiography of the left subclavian artery showing a huge tumor blush in the left neck with feeding from the left transcervical artery (broken arrow), the thryrocervical artery (arrow), and a branch from the left vertebral artery (arrowhead). The left superior thyroid artery was also a feeding artery (no image). d Selective angiography of the left transcervical artery showing a tumor blush which corresponds to the bulky nodal lesion. e Localized intraoperative cone-beam CT of the left transcervical artery showing contrast enhancement corresponding to the medial and lateral portions of the lesion (arrows). As the contrast area was almost 30% of total tumor volume, 30% of the total dose of the anticancer agents was administered. f Coronal CT image showing the radiation field included as bilateral neck and whole pharyngeal mucosa. g Coronal contrast-enhanced CT at 5 years after treatment completion showing no local recurrence in the left neck. h Coronal PET-CT at 5 years after treatment completion showing no FDG uptake in the left neck
A multidisciplinary head and neck oncology team selected intra-arterial chemoradiotherapy for patients with inoperable fixed bulky nodal disease in HNCUP because it has been previously shown to potentially provide high rates of control of locally advanced head and neck cancers [8, 9]. For other patients with HNCUP, neck dissection was performed, followed by postoperative chemoradiotherapy or palliative radiotherapy. The inclusion criteria were as follows: (1) histologically proven squamous cell carcinoma of unknown primary without distant metastasis, except for bulky cervical nodal disease; (2) prognosis of ≥ 6 months can be expected if local neck control is achieved; (3) good performance status of 0 or 1; and (4) bone marrow, hepatic, and renal function within the normal range. Patients who underwent neck dissection followed by radiotherapy, with or without systemic chemotherapy, definitive radiotherapy with systemic chemotherapy, or palliative radiotherapy alone were excluded. Based on these eligibility criteria, seven consecutive patients were enrolled in the study (Fig. 2).
Treatment procedure
Chemotherapy
Intra-arterial chemotherapy regimen, a combination of nedaplatin and docetaxel, used a common femoral approach, was based on previous studies [11, 12], and was provided after consultation with the chemotherapy regimen committee, which primarily comprised oncologists. Intra-arterial chemotherapy was initially administered with nedaplatin (80 mg/m2) and docetaxel (60 mg/m2) at the start of radiotherapy and was given thrice at a frequency of once every 4 weeks (Fig. 3). Before arteriography, systemic heparin (700 units per 10 kg body weight) was injected to prevent clot formation. An additional 500 units were injected intravenously during the procedure, once every hour. A 5-Fr catheter, along with a coaxially placed 2.0-Fr microcatheter, was used to selectively catheterize the arteries supplying the bulky metastatic lymph nodes (Fig. 1c, d). Intraoperative cone-beam CT was performed to identify all tumor-feeding arteries and determine the anticancer agent doses for each vessel. The doses were determined according to the percentage of tumor volume supplied by the target artery, which was measured intraoperatively via angiography-assisted cone-beam CT to the total tumor volume (Fig. 1e). Before intra-arterial chemotherapy, the contrast medium was infused into the target artery using a syringe driver. The maximum flow rate at which there was no extra overflow of contrast medium from the target artery was confirmed fluoroscopically; this rate of contrast medium infusion was considered the optimal infusion rate of the anticancer agents.
Radiotherapy
Intensity-modulated radiotherapy using high-energy photons (6 MV) through a linear accelerator was provided with mask immobilization to secure the patient’s shoulders, head, and neck. The mucosal clinical target volume generally included the nasopharynx, oropharynx, hypopharynx, and larynx. The pharyngeal mucosa and all nodal areas (levels I–IV, bilaterally) were irradiated with a total dose of 50 Gy in 25 fractions over 5 weeks, followed by a boost of 20 Gy, in 10 fractions for bulky nodal lesions (Fig. 1f).
Evaluation
Treatment compliance was defined as completion of both radiotherapy and chemotherapy. Response to therapy was assessed by CT and MRI at four weeks after treatment completion (Fig. 1g) and by PET-CT from approximately 3 months after treatment completion to rule out false-positive results due to inflammation (Fig. 1h). Tumor response was evaluated using the Response Evaluation Criteria in Solid Tumors, ver. 1.1. The target lesion was evaluated by imaging (CT, MRI, and PET-CT) at the time of initial imaging evaluation by a board-certified radiologist with head and neck diagnostic radiology experience. Objective response rate was defined as the number of lesions with complete or partial response divided by the total number of treated lesions.
All treatment-related toxicities were evaluated according to the Common Terminology Criteria for Adverse Events, ver. 5.0. Follow-up frequency was weekly during treatment, every 6 weeks for the first year, every 3 months from the second to the fifth year, and every 6 months thereafter. Outcome measures were local control, disease-free survival, overall survival, and adverse events.
Statistical analysis
In this study, local control was defined as the control of bulky nodal disease, whereas regional control was defined as the control of regional lymph nodes. Local control was calculated from the time of treatment initiation until diagnosis of recurrence of bulky nodal disease, death, loss to follow-up, or end of follow-up, whichever came first. Disease-free survival was calculated from the time of treatment initiation until diagnosis of recurrence of bulky nodal disease, regional recurrence, distant metastasis, new tumor including primary lesion, death, loss to follow-up, or end of follow-up, whichever came first. Overall survival was calculated from the time of treatment initiation until death, loss to follow-up, or end of follow-up, whichever came first. Statistical analyses were performed using the Kaplan–Meier method to evaluate local control, disease-free survival, and overall survival rates. The level of statistical significance was set at P < 0.05. All statistical analyses were performed using R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients and disease characteristics
Detailed patient and tumor characteristics are listed in Table 1. The cohort comprised seven consecutive patients with a median age of 60 (range 45–71) years; six (86%) were men. All patients tested negative for HPV. Six of seven patients were EBV-negative and one patient was EBV-unclear status. The median value for maximum diameter of cervical lymph node metastasis was 84 (range 70–107) mm; in all patients, lymph node extracapsular extension was observed on imaging and clinical findings. Lymph nodes were confined to the neck in two patients (28%) but extended from the neck to the supraclavicular fossa in five patients (72%). Paralysis of lower cranial nerves due to bulky nodal disease was observed in two of seven patients; both of them had hoarseness associated with vocal cord palsy. Symptoms due to bulky lymph nodes were neck discomfort in all patients, bleeding from tumor ulceration in three of the seven patients (43%), dyspnea due to tracheal obstruction in one patient (14%), and cardiac arrest and syncope due to carotid sinus syndrome in two patients (28%); a pacemaker was inserted in these patients with carotid sinus syndrome before treatment initiation. Five patients had bulky nodal disease extending from the neck to the supraclavicular fossa, which was considered extremely difficult to resect completely. Although the lesion in two patients was confined to the neck, it was considered difficult to completely resect because the bulky nodal disease was accompanied by carotid invasion and extensive skin invasion. Three patients enrolled initially received induction chemotherapy with the TPF regimen comprising nedaplatin (60 mg/m2), docetaxel (60 mg/m2), and 5-fluorouracil (600 mg/m2); however, no patient responded to the therapy and all experienced further disease progression. No induction chemotherapy was administered to the latter half of the enrolled patients.
The feeding arteries in patients with bulky nodal disease who received intra-arterial chemotherapy are shown in Table 2. The most common feeding artery was the superior thyroid artery (six of seven patients), followed by the occipital artery (four of seven patients). The median number of feeding arteries in patients who received intra-arterial chemotherapy was three (range: 2–6). Six patients had feeding arteries only on the affected side, and the remaining one patient had feeding arteries on the bilateral side.
Treatment compliance
Two of seven patients were unable to receive the scheduled sessions of intra-arterial chemotherapy—one patient did not wish to undergo the third session of chemotherapy and the other was unable to receive the third session due to nonocclusive mesenteric ischemia. Radiotherapy for the patient with nonocclusive mesenteric ischemia was terminated at 60 Gy, whereas all otherpatients received the full dose of radiotherapy. Thus, overall treatment compliance rate in this study was 71% (5 of 7).
Adverse events and complications
Table 3 shows toxicity data for this study. Grade 4 leukopenia was the only observed severe acute adverse event and occurred in two patients (28%). However, in both patients, severe infectious disease was avoided by administering granulocyte colony stimulating factor. No grade 3 or higher acute adverse events, other than leukopenia, dermatitis, and pharyngeal mucositis, were observed. Acute toxicity was manageable in most patients, but all patients required morphine for oropharyngeal pain and none died due to treatment toxicity. During the treatment period, one patient (14%) required tube feeding. No intra-arterial chemotherapy-related otologic complications, hepatotoxicity, nephrotoxicity, or central nervous system disorders were observed and there were no severe late adverse events in this study.
Treatment outcome
Objective response was evaluated for all tumors using CT and MRI at the time of the initial imaging studies after completion of intra-arterial chemoradiotherapy, and complete and partial responses were observed in five (72%) and two (28%) of the seven patients, respectively. None of the patients presented with stable disease or progressive disease and the objective response was 100%. Imaging studies (CT, MRI, and PET-CT), performed from 6 months after completion of treatment, showed complete response in all tumors. Among the seven patients who were followed up for a median period of 24 months (range 9–64), local recurrence was observed in none but regional recurrence was seen in one patient. The patient with regional recurrence underwent lymph node resection; however, systemic chemotherapy was administered because multiple pulmonary metastases subsequently appeared. No patients had new primary cancer(s), but three showed distant metastases and two died from the progression of distant disease. One patient developed liver metastasis after 14 months and was lost follow-up after 23 months. The remaining four patients are alive and under outpatient follow-up with no recurrence.
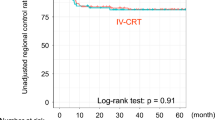
Symptoms due to fixed bulky cervical nodes disappeared in all patients after intra-arterial chemoradiotherapy, and none of the patients experienced recurrence of local cervical symptoms during follow-up, thereby achieving improvement and maintaining their quality of life. The 3-year local control, disease-free survival, and overall survival rates were 100, 54, and 64%, respectively (Fig. 4).
Discussion
This retrospective study reveals that intra-arterial chemoradiotherapy with a regimen of docetaxel and nedaplatin may provide favorable local control and survival rates in patients with fixed bulky nodal disease in head and neck cancer of unknown primary (HNCUP). Given the absence of prospective randomized trials due to the rarity of HNCUP, previous meta-analyses have reported a 5-year overall survival rate of 26.6% among HNCUP patients with T0N3 and/or extracapsular extension treated who had undergone surgery followed by (chemo)radiotherapy[13]. Another retrospective study reported local recurrence, 3-year disease-free, and overall survival rates of 33, 50, and 33%, respectively, after surgery followed by chemoradiotherapy for N3 HNCUP [14]. However, no studies have evaluated outcomes among patients with only extracapsular extension. In this study, the 3-year local control, disease-free, and overall survival rates after intra-arterial chemoradiotherapy for fixed bulky nodal disease (i.e., much more advanced extracapsular extension) were 100, 54, and 64%, respectively.
The presence of extracapsular extension and supraclavicular involvement are associated with poor prognosis in HNCUP [14], and the incidence of distant metastasis also correlates with advanced nodal staging and extracapsular extension [15, 16]. Recently, it has been reported that tumor HPV status has a significant prognostic value in HNCUP and that it is similar to HPV-related oropharyngeal cancer [17]. Thus, in advanced N2/N3 HPV-negative HNCUP, there is a need to increase treatment intensity, such as surgery followed by chemoradiation [17]. However, patients with supraclavicular involvement and/or fixed bulky cervical disease often have carotid artery and tracheal involvement, which render both neck dissection and intensified treatment difficult.
Intra-arterial chemoradiotherapy using high-dose cisplatin was introduced by Robbins et al. as a nonsurgical treatment strategy for inoperable advanced head and neck cancer, and they report a complete response rate of 80% [9]. Additionally, this strategy was shown to be effective for controlling bulky nodal disease (N2–N3) with a complete response rate of 66% [8]. Intra-arterial chemotherapy has the advantage of achieving supra-dose concentrations of the agent at the tumor bed, i.e., it avoids the first pass effect encountered with conventional intravenous or oral delivery methods [18]. However, if the right amount of anticancer agent is not administered in the appropriate target artery, this benefit is not realized. Therefore, all patients treated with intra-arterial chemotherapy in this cohort underwent intraoperative cone-beam CT to determine the percentage contribution of each target artery to total tumor volume and anticancer agents were administered accordingly. Although the method of determining the dosage of agents and optimal infusion rate of anticancer agents in intra-arterial chemotherapy remains controversial, the favorable treatment results noted in this study may support the superiority of selective intra-arterial chemoradiotherapy, which calculates the amount of agent administered to each target artery using the intraoperative cone-beam CT and infuses the agents at a rate without overflow.
The incidence of grade 3 or higher leukopenia was 57%, with 29% experiencing grade 4 leukopenia. It is known that systemic chemotherapy with docetaxel for head and neck cancer causes severe myelosuppression [19], and the reported incidence of grade 3 or higher leukopenia is 53–67% [19, 20]. Our data concur with those from previous studies, suggesting that myelosuppression was indeed caused by docetaxel. However, because all patients with grade 4 leukopenia were able to avoid severe infectious disease upon granulocyte stimulating factor administration and no other grade 3 or higher hematologic events were reported, the safety profile of this chemotherapy regimen could be defined as adequate. Further, because reported incidence of ≥ grade 3 leukopenia due to intravenous chemoradiotherapy for head and neck cancer ranges from 41.6–95% [21, 22], hematologic toxicity can also be deemed acceptable. Additionally, this chemotherapy regimen may be more acceptable to primary doctors and patients because it does not require large amounts of hydration or cisplatin neutralizers, which are essential for RADPLAT.
The definition of target volume in radiotherapy for HNCUP remains controversial because of conflicting results from various studies that have compared selective and extensive regimens [23,24,25,26]. However, none of these studies considered the prognostic factors in HNCUP. Given the fact that a significant relationship has been shown between extracapsular extension and appearance of contralateral cervical disease [27], and that patients who develop primary tumors after initial treatment have a poorer prognosis than those who do not [28], bilateral and whole pharyngeal mucosa irradiation may be better for HNCUP with extracapsular extension. A previous study has reported that intensity-modulated radiotherapy for head and neck cancer, compared to three-dimensional conformal radiotherapy, improved dose delivery to the tumor and reduced the dose delivered to normal tissues, such as the spinal cord, brainstem, or salivary glands [29]. As the introduction of intensity-modulated radiotherapy for an extensive radiation field can reduce mucosal toxicity [30], irradiation with intensity-modulated radiotherapy should be actively performed. Since all patients in this study underwent intensity-modulated radiotherapy, it is likely that severe adverse mucosal toxicity was avoided.
No complications related to intra-arterial chemotherapy for head and neck cancer occurred; however, other reports have described complications such as central nervous system disorders (e.g., neurological disorder, cerebral infarction and hemorrhage) [9, 31]. Because intra-arterial chemotherapy for head and neck cancer is not a safe treatment technique, great care should be taken when performing this technique in such patients.
There are a few limitations to this study. First, selection bias was not completely eliminated owing to the retrospective nature of the study. Second, because this is a single-center study with a small sample size owing to the rarity of advanced HNCUP, including a comparison group was not possible, which prevented conclusive analyses. Third, because of the retrospective nature of the study, adverse events could not be fully assessed, and it is likely that they have been underestimated. These limitations may preclude the generalizability of the obtained results to a broader population. However, in HNCUP with extracapsular extension, treatment intensification must be considered and selective intra-arterial chemoradiotherapy with docetaxel and nedaplatin might be a viable treatment option. Therefore, to adequately address these issues and validate the current results, future prospective randomized controlled trials on selective intra-arterial chemoradiotherapy in patients with HNCUP with extracapsular extension should be performed.
In conclusion, this study suggests that selective intra-arterial chemoradiotherapy with docetaxel and nedaplatin can potentially achieve favorable local control and yield higher survival rates in fixed bulky cervical disease in HNCUP.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Miller FR, Karnad AB, Eng T, Hussey DH, Stan McGuff H, Otto RA (2008) Management of the unknown primary carcinoma: long-term follow-up on a negative PET scan and negative panendoscopy. Head Neck 30:28–34. https://doi.org/10.1002/hed.20654
Waltonen JD, Ozer E, Hall NC, Schuller DE, Agrawal A (2009) Metastatic carcinoma of the neck of unknown primary origin: evolution and efficacy of the modern workup. Arch Otolaryngol Head Neck Surg 135:1024–1029. https://doi.org/10.1001/archoto.2009.145
National Comprehensive Cancer Network (NCCN) Clinical practice guidelines in oncology. Head and neck cancers, version 2.2020. http://www.nccn.org. Accessed 9 Nov 2020
Johnson CR, Khandelwal SR, Schmidt-Ullrich RK, RavaleseWazer JDE (1995) The influence of quantitative tumor volume measurements on local control in advanced head and neck cancer using concomitant boost accelerated superfractionated irradiation. Int J Radiat Oncol Biol Phys 32:635–641. https://doi.org/10.1016/0360-3016(95)00031-S
Dubben HH, Thames HD, Beck-Bornholdt HP (1998) Tumor volume: a basic and specific response predictor in radiotherapy. Radiother Oncol 47:167–174. https://doi.org/10.1016/s0167-8140(97)00215-6
Chan JY, To VS, Wong ST, Wei WI (2013) Quality of dying in head and neck cancer patients: the role of surgical palliation. Eur Arch Otorhinolaryngol 270:681–688. https://doi.org/10.1007/s00405-012-2059-7
Johnstone C, Lutz ST (2014) The role of hypofractionated radiation in the management of non-osseous metastatic or uncontrolled local cancer. Ann Palliat Med 3:291–303. https://doi.org/10.3978/j.issn.2224-5820.2014.10.01
Robbins KT, Wong FS, Kumar P, Hartsell WF, Vieira F, Mullins B, Niell HB (1999) Efficacy of targeted chemoradiation and planned selective neck dissection to control bulky nodal disease in advanced head and neck cancer. Arch Otolaryngol Head Neck Surg 125:670–675. https://doi.org/10.1001/archotol.125.6.670
Robbins KT, Kumar P, Wong FS, Hartsell WF, Flick P, Palmer R, Weir AB 3rd, Neill HB, Murry T, Ferguson R, Hanchett C, Vieira F, Bush A, Howell SB (2000) Targeted chemoradiation for advanced head and neck cancer: analysis of 231 patients. Head Neck 22:687–693. https://doi.org/10.1002/1097-0347(200010)22:7%3c687::aid-hed8%3e3.0.co;2-w
Yabuuchi H, Kuroiwa T, Tajima T, Tomita K, Ochiai N, Kawamoto K (2003) Efficacy of intra-arterial infusion therapy using a combination of cisplatin and docetaxel for recurrent head and neck cancers compared with cisplatin alone. Clin Oncol 15:467–472. https://doi.org/10.1016/j.clon.2003.07.003
Maeda A, Toh S, Higaki Y, Tomita K (2003) Super-selective intra-arterial infusion chemotherapy for oral cancer. Stomato Pharyngol 15:345–351. https://doi.org/10.14821/stomatopharyngology1989.15.345
Kobayashi W, Teh BG, Sakaki H, Sato H, Kimura H, Kakehata S, Nagahata M (2010) Superselective intra-arterial chemoradiotherapy with docetaxel-nedaplatin for advanced oral cancer. Oral Oncol 46:860–863. https://doi.org/10.1016/j.oraloncology.2010.10.001
Balaker AE, Abemayor E, Elashoff D, St John MA (2012) Cancer of unknown primary: does treatment modality make a difference? Laryngoscope 122:1279–1282. https://doi.org/10.1002/lary.22424
Argiris A, Smith SM, Stenson K, Mittal BB, Pelzer HJ, Kies MS, Haraf DJ, Vokes EE (2003) Concurrent chemoradiotherapy for N2 or N3 squamous cell carcinoma of the head and neck from an occult primary. Ann Oncol 14:1306–1311. https://doi.org/10.1093/annonc/mdg330
Nieder C, Gregoire V, Ang KK (2001) Cervical lymph node metastases from occult squamous cell carcinoma: cut down a tree to get an apple? Int J Radiat Oncol Biol Phys 50:727–733. https://doi.org/10.1016/s0360-3016(01)01462-6
Jereczek-Fossa BA, Jassem J, Orecchia R (2004) Cervical lymph node metastases of squamous cell carcinoma of unknown primary. Cancer Treat Rev 30:153–164. https://doi.org/10.1016/j.ctrv.2003.10.001
Cheraghlou S, Torabi SJ, Husain ZA, Otremba MD, Osborn HA, Mehra S, Yarbrough WG, Burtness BA, Judson BL (2019) HPV status in unknown primary head and neck cancer: prognosis and treatment outcomes. Laryngosope 129:684–691. https://doi.org/10.1002/lary.27475
Spring PM, Valentino J, Arnold SM, Sloan D, Kenady D, Kudrimoti M, Haydon RC, Lee C, Given C, Mohiuddin M, Regine WF (2005) Long-term results of hyperfractionated radiation and high-dose intraarterial cisplatin for unresectable oropharyngeal carcinoma. Cancer 104:1765–1771. https://doi.org/10.1002/cncr.21368
Dreyfuss A, Clark J, Norris C, Rossi RM, Lucarini JW, Busse PM, Poulin MD, Thornhill L, Costello R, Posner MR (1996) Docetaxel: an active drug for squamous cell carcinoma of the head and neck. J Clin Oncol 14:1672–1678. https://doi.org/10.1200/JCO.1996.14.5.1672
Schoffski P, Catimel G, Planting AS, Droz JP, Verweij J, Schrijvers D, Gras L, Schrijvers A, Wanders J, Hanauske AR (1999) Docetaxel and cisplatin: an active regimen in patients with locally advanced, recurrent or metastatic squamous cell carcinoma of head and neck. Results of a phase II study of the EORTC Early Clinical Studies Group. Ann Oncol 10:119–122
Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss JH, van den Weyngaert D, Awada A, Cupissol D, Kienzer HR, Rey A, Desaunois I, Bernier J, Lefebvre JE, EORTC 24971/TAX 323 Study Group (2007) Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 357:1695–1704. https://doi.org/10.1056/NEJMoa071028
Posner MR, Glisson B, Frenette G, Al-Sarraf M, Colevas AD, Norris CM, Seroskie JD, Shin DM, Olivares R, Garay CA (2001) Multicenter phase I-IItrialI–IItrial of docetaxel, cisplatin, and fluorouracil induction chemotherapy for patients with locally advanced squamous cell cancer of the head and neck. J Clin Oncol 19:1096–1104. https://doi.org/10.1200/JCO.2001.19.4.1096
Harper CS, Mendenhall WM, Parsons JT, Stringer SP, Cassisi NJ, Million RR (1990) Cancer in neck nodes with unknown primary site: role of mucosal radiotherapy. Head Neck 12:463–469. https://doi.org/10.1002/hed.2880120603
Colletier PJ, Garden AS, Morrison WH, Goepfert H, Geara F, Ang KK (1998) Postoperative radiation for squamous cell carcinoma metastatic to cervical lymph nodes from an unknown primary site: outcomes and patterns of failure. Head Neck 20:674–681. https://doi.org/10.1002/(sici)1097-0347(199812)20:8%3c674::aid-hed3%3e3.0.co;2-h
Strojan P, Anicin A (1998) Combined surgery and postoperative radiotherapy for cervical lymph node metastases from an unknown primary tumor. Radiother Oncol 49:33–40. https://doi.org/10.1016/s0167-8140(98)00082-6
Grau C, Johansen LV, Jakobsen J, Geertsen P, Andersen E, Jensen BB (2000) Cervical lymph node metastases from unknown primary tumors: results from a national survey by the Danish society for head and neck oncology. Radiother Oncol 55:121–129. https://doi.org/10.1016/s0167-8140(00)00172-9
Suoglu Y, Erdamar B, Katircioglu OS, Karatay MC, Sunay T (2002) Extracapsular spread in ipsilateral neck and contralateral neck metastases in laryngeal cancer. Ann Otol Rhinol Laryngol 111:447–454. https://doi.org/10.1177/000348940211100510
Issing WJ, Taleban B, Tauber S (2003) Diagnosis and management of carcinoma of unknown primary in the head and neck. Eur Arch Otorhinolaryngol 260:436–443. https://doi.org/10.1007/s00405-003-0585-z
Eisbruch A, Harris J, Garden AS, Chao CK, Straube W, Harari PM, Sanguineti G, Jones CU, Bosch WR, Ang KK (2010) Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00–22). Int J Radiat Oncol Biol Phys 76:1333–1338. https://doi.org/10.1016/j.ijrobp.2009.04.011
Maghami E, Ismaila N, Alvarez A, Chernock R, Duvvuri U, Geiger J, Gross N, Haughey B, Paul D, Rodriguez C, Sher D, Stambuk HE, Waldron J, Witek M, Caudell J (2020) Diagnosis and management of squamous cell carcinoma of unknown primary in the head and neck: ASCO guideline. J Clin Oncol 38:2570–2596. https://doi.org/10.1200/JCO.20.00275
Heianna J, Yamashita Y, Iraha Y, Murayama S (2020) A rare case of cerebral hemorrhage associated with intra-arterial infusion chemotherapy for advanced sphenoid sinus cancer. J Can Res Ther 16:686–689. https://doi.org/10.4103/jcrt.JCRT_1444_16
Acknowledgements
We would like to thank Keiichi Nishimaki, Chief of Neurosurgery, Akita Red Cross Hospital, and Satoshi Takahashi, Associate Professor of Radiology, Akita University School of Medicine, for useful discourse on neuro-interventional radiology.
Funding
No funding from any source was received in association with this work.
Author information
Authors and Affiliations
Contributions
JH, WM, KI, and ST were responsible for the conceptualization. JH, WM, HM, and TA designed the study. HH and SA were responsible for use of the patient-reported outcomes following initial treatment. JH and HT were responsible for data curation and formal analysis. JH was responsible for ethics approval and writing the original draft. SM was responsible for supervision. All authors contributed to patient inclusion and manuscript writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no relationships with any companies whose products or services may be related with the subject matter of the article.
Ethical approval
This single-center retrospective study was approved by the ethics committee of the University of Ryukyus for Medical and Health Research Involving Human Subjects (reference number: 1443) and was conducted in accordance with the principles of the Declaration of Helsinki.
Consent to participate
This study was retrospective in nature, the requirement for written informed consent from the patients was waived.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heianna, J., Makino, W., Hirakawa, H. et al. Therapeutic efficacy of selective intra-arterial chemoradiotherapy with docetaxel and nedaplatin for fixed bulky nodal disease in head and neck cancer of unknown primary. Eur Arch Otorhinolaryngol 279, 3105–3113 (2022). https://doi.org/10.1007/s00405-021-07121-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-07121-9