Abstract
Radio-chemotherapy is a common treatment for locally advanced squamous cell head-and-neck cancers (LA-SCCHN). Cisplatin (100 mg/m2) every 3 weeks is very common but associated with considerable toxicity. Therefore, cisplatin programs with lower daily doses were introduced. There is a lack of studies comparing lower-dose programs. In this study, 85 patients receiving radio-chemotherapy with 20 mg/m2 cisplatin on 5 days every 4 weeks (group A) were retrospectively compared to 85 patients receiving radio-chemotherapy with 30–40 mg/m2 cisplatin weekly (group B). Groups were matched for nine factors including age, gender, performance score, tumor site, T-/N-category, surgery, hemoglobin before radio-chemotherapy, and radiation technique. One- and 3-year loco-regional control rates were 83 and 69 % in group A versus 74 and 63 % in group B (p = 0.12). One- and 3-year survival rates were 93 % and 73 % in group A versus 91 and 49 % in group B (p = 0.011). On multivariate analysis, survival was significantly better for group A (HR 1.17; p = 0.002). In groups A and B, 12 and 28 % of patients, respectively, did not receive a cumulative cisplatin dose ≥180 mg/m2 (p = 0.016). Toxicity rates were not significantly different. On subgroup analyses, group A patients had better loco-regional control (p = 0.040) and survival (p = 0.005) than group B patients after definitive radio-chemotherapy. In patients receiving adjuvant radio-chemotherapy, outcomes were not significantly different. Thus, 20 mg/m2 cisplatin on 5 days every 4 weeks resulted in better loco-regional control and survival in patients receiving definitive radio-chemotherapy and may be preferable for these patients. Confirmation of these results in a randomized trial is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radio-chemotherapy is a common modality for the treatment of locally advanced squamous cell carcinoma of the head-and-neck (LA-SCCHN). Randomized trials demonstrated that radio-chemotherapy resulted in significantly better survival than radiation therapy alone in patients who did not receive upfront surgery. In postoperative setting, randomized trials confirmed that patients with LA-SCCHN and specific risk factors (incomplete resection and/or extracapsular spread of lymph node metastasis) also benefit from the addition of chemotherapy to irradiation [1–6]. After publication of a large meta-analysis showing concurrent radio-chemotherapy to be superior to any other combinations of radiation therapy and chemotherapy, concurrent administration of radio-chemotherapy became the standard therapy [7]. Cisplatin, either alone or as part of combined chemotherapy regimens, is now the most frequently used agent for radio-chemotherapy of LA-SCCHN.
Several randomized trials confirmed that radio-chemotherapy including 100 mg/m2 of cisplatin alone given every 3 weeks was significantly superior to radiation therapy alone in both definitive and adjuvant settings. Consequently, three courses of 100 mg/m2 cisplatin alone became the most commonly used regimen for the radio-chemotherapy of LA-SCCHN [3–6]. However, this radio-chemotherapy has been associated with considerable acute toxicity and many radiation oncologists are hesitant to use three courses of 100 mg/m2 cisplatin and prefer regimens with lower cisplatin doses per administration (lower-dose programs) [8–10]. Other cisplatin monotherapy regimens include daily administration of 5–7 mg/m2, weekly administration of 30–40, and 20 mg/m2 on 5 days every 4 weeks [1, 2, 9–12]. However, there are only few studies comparing different lower-dose programs of cisplatin alone used in concurrent radio-chemotherapy protocols for LA-SCCHN. In the present study 20 mg/m2 of cisplatin alone given on 5 days every 4 weeks was compared to weekly administration of 30–40 mg/m2 of cisplatin alone for loco-regional control, survival, and toxicity.
Patients and methods
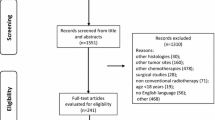
In this retrospective study, 170 patients were included who received concurrent radio-chemotherapy with cisplatin alone for LA-SCCHN. Criteria for inclusion in this study were locally advanced cancer of the oropharynx, hypopharynx, larynx or oral cavity requiring radio-chemotherapy as definitive or adjuvant treatment, no distant metastasis, age at least 18 years, no history of another type of cancer, an Eastern Cooperative Oncology Group (ECOG) performance score of ≤2, and no contraindications to receive cisplatin. Patients who did not meet these criteria were excluded from this study. Radiotherapy was delivered as three-dimensional (3D) conformal irradiation or as intensity-modulated radiotherapy (IMRT). IMRT included so-called classic IMRT and volumetric-modulated arc therapy (VMAT). The total radiation doses administered to the primary tumor and the involved lymph nodes were 66–70 Gy in case of definitive radio-chemotherapy or adjuvant radio-chemotherapy following macroscopically incomplete resection and 60–66 Gy in case of adjuvant radio-chemotherapy following macroscopically complete resection. Non-involved lymph node regions in the neck received 50–60 Gy. Regarding concurrent chemotherapy, two regimens with lower-dose cisplatin alone were compared. The regimens were given according to multidisciplinary protocols preferred at contributing institutions between 2003 and 2014, the timeframe that patients in this study were treated.
In group A (n = 85), chemotherapy included two courses of 20 mg/m2 cisplatin alone administered on 5 days every 4 weeks as bolus infusions on days 1–5 and 29–33. In group B (n = 85), chemotherapy also consisted of cisplatin alone, which in this group was administered once a week with doses of 30–40 mg/m2. Cisplatin was also given as bolus infusions, which were, in both groups, supplemented by prophylactic hydration with antiemetic drugs prior to and during its administration.
Both radio-chemotherapy groups were matched with respect to nine factors including age (≤57 vs. ≥58 years, median age 57 years), gender, ECOG performance score (0–1 vs. 2), tumor site (oropharynx vs. hypopharynx vs. larynx vs. oral cavity), T-category (T1–2 vs. T3–4), N-category (N0–2a vs. N2b–3), upfront surgery (no vs. yes), hemoglobin level prior to radio-chemotherapy (<12 vs. ≥12 g/dl), and radiation technique (3D conformal vs. IMRT) (Table 1). These factors were equally distributed in both radio-chemotherapy groups. The HPV (human papilloma virus)-status was not available in the majority of patients and, therefore, not included in the analysis. In those patients receiving upfront surgery, a microscopically complete resection was achieved in 20 of 25 patients (80 %) in group A and in 18 of 25 patients (72 %) in group B, respectively (p = 0.89; Chi-square test).
Chemotherapy dose groups, the nine factors used for matching the groups, and the cumulative cisplatin dose (<180 vs. ≥180 mg/m2) were evaluated for the endpoints loco-regional control and survival (i.e., death of any cause was considered as event). Both endpoints were calculated form the last day of radiation using the Kaplan–Meier method. The corresponding Kaplan–Meier curves of each investigated factor were compared with the log-rank test (univariate analyses) [13]. After Bonferroni correction, p values <0.0045 (11 tests) were considered significant, which represented an alpha-level of 0.05. Factors that achieved significance on log-rank test or showed a trend (p < 0.05) were evaluated for independence with the Cox proportional hazards model.
Toxicities were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [14]. The comparison of the two radio-chemotherapy groups for toxicities was performed with the Chi-square test. After Bonferroni correction for multiple tests (six investigated adverse events), p values <0.0083 represented an alpha-level of 0.05 and were considered significant.
Additional subgroup analyses with respect to loco-regional control and survival were performed for the 120 patients receiving definitive radio-chemotherapy and for those 50 patients receiving adjuvant radio-chemotherapy.
Results
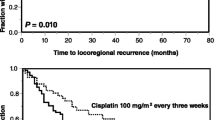
In the entire cohort, the 1- and 3-year loco-regional control rates were 83 and 69 % in group A, versus 74 and 63 % in group B (p = 0.12; Table 2). A trend toward improved loco-regional control was found for lower (T1–2) T-category (p = 0.030) and upfront surgery (p = 0.011) (Table 2). In the subsequent multivariate analysis, upfront surgery [hazard ratio (HR) 2.24; 95 % confidence interval (CI) 1.04–5.59; p = 0.037] achieved significance, whereas T-category (HR 1.42; 95 % CI 0.92–2.45; p = 0.12) was not significant.
The 1- and 3-year survival rates were 93 and 73 %, respectively, in group A, versus 91 and 49 %, respectively, in group B (p = 0.011; Table 3). On univariate analysis, improved survival was associated with a better (ECOG 0–1) performance status (p < 0.001) (Table 3). On multivariate analysis, survival was significantly associated with type of chemotherapy (HR 1.17; 95 % CI 1.06–1.30; p = 0.002) and ECOG performance score (HR 3.01; 95 % CI 1.75–5.05; p < 0.001). In groups A and B, 10 patients (12 %) and 24 patients (28 %), respectively, did not receive a cumulative cisplatin dose of ≥180 mg/m2 (p = 0.016). The rates of toxicities in terms of oral mucositis, dermatitis, hematotoxicity, nephrotoxicity, xerostomia, and subcutaneous fibrosis were not significantly different in both groups (Table 4).
The additional subgroup analyses revealed that the cisplatin regimen used in group A was significantly superior to the regimen in group B with respect to loco-regional control (p = 0.040) and survival (p = 0.005) in patients receiving definitive radio-chemotherapy (Table 5) but not in those patients receiving adjuvant radio-chemotherapy (Table 6).
Discussion
Cisplatin-based regimens are very often used for the radio-chemotherapy of LA-SCCHN, particularly regimens including cisplatin alone. Due to randomized trials demonstrating that 100 mg/m2 of cisplatin given every 3 weeks resulted in significantly better outcomes than radiation therapy alone, this regimen became the standard approach for LA-SCCHN in many centers. However, this radio-chemotherapy program was associated with high acute toxicity [9]. Thus, taking into account both efficacy and toxicity, the optimal radio-chemotherapy approach for LA-SCCHN requires further clarification.
In an attempt to minimize toxicity but retain the benefits of cisplatin, many centers use doses lower than 100 mg/m2 per administration. Several studies compared lower-dose programs to 100 mg/m2 of cisplatin every 3 weeks [9–11, 15–17]. However, there are few studies comparing exclusively different lower-dose of cisplatin programs. Therefore, this study was initiated to compare 20 mg/m2 of cisplatin on 5 days every 4 weeks to weekly administration of cisplatin with doses of 30-40 mg/m2. According to the results of this study, 20 mg/m2 of cisplatin on 5 days every 4 weeks resulted in significantly better outcomes than weekly administrations. In the entire cohort, the absolute differences of loco-regional control rates at 1 and 3 years were 9 and 6 % in favor of group A (Table 2), but these differences did not achieve significance (p = 0.12). However, in the subgroup analysis of patients receiving definitive radio-chemotherapy, the cisplatin regimen used in group A resulted in significantly better loco-regional control than weekly administration of cisplatin (group B). The absolute differences of loco-regional control rates at 1 and 3 years were 9 and 15 % in favor of group A (Table 5). In a retrospective study of 77 patients with locally advanced cancer of the uterine cervix, 20 mg/m2 of cisplatin alone on 5 days every 3 weeks (inpatient regimen) was compared to weekly administration of 40 mg/m2 of cisplatin (outpatient regimen) [18]. Progression-free survival at 3 years was significantly better in the 20 mg/m2 of cisplatin group than in the weekly cisplatin group (90 vs. 76 %, p = 0.01). In the multivariate analysis of that study, this finding maintained significance with an HR of 3.46 (96 % CI 1.25–9.58; p = 0.02). Acute toxicities were 3.43 times more common in the weekly cisplatin group (95 % CI 1.38–8.52; p = 0.02). The cumulative cisplatin doses were not stated. However, it appears quite likely that patients in the 20 mg/m2 of cisplatin group did receive higher cumulative doses, as it was the case in the present study.
In the present study, radio-chemotherapy with two courses of 20 mg/m2 cisplatin resulted in significantly better survival rates at 1 and 3 years than radio-chemotherapy with cisplatin administered once a week with doses of 30–40 mg/m2. However, according to the additional subgroup analyses, the superiority of the regimen used in group A was limited to patients receiving definitive radio-chemotherapy (Tables 5, 6). Therefore, the improved survival appears to be a consequence of the significantly improved loco-regional control in this particular subgroup.
In this study, significantly more patients in group B did not receive a cumulative cisplatin dose of 180 mg/m2, which represents weekly doses of 30 mg/m2 given over 6 weeks (concurrently with a total radiation dose of 60 Gy). This appears surprising, since acute toxicity rates were not significantly different between groups A and B. One may speculate about the reason for the difference regarding the cumulative cisplatin doses. Other acute toxicities not assessed in this study such as nausea/vomiting might have been more common in group B. The difference regarding the cumulative cisplatin doses can to a certain extent be explained by the fact that patients in group A received their cisplatin as inpatients, in contrast to group B patients, who received their weekly cisplatin as outpatients. The latter group likely had poorer compliance. It may be difficult to motivate patients with LA-SCCHN, who are often heavy smokers and alcohol consumers to come to an outpatient department or a private practice six to seven times during radiation course. Furthermore, supportive care is more easily provided for inpatients, where the infrastructure of a hospital and multiple disciplines are available “under one roof”.
The difference between groups A and B regarding the cumulative cisplatin doses is a source of potential bias [19]. Another source of a hidden bias may be a possible difference regarding the distribution of the human papilloma virus (HPV) status, which was not available in most patients of the present study. The HPV-status was reported to have a significant impact on the prognosis of patients with SCCHN [20, 21]. This must be considered a significant limitation of the present study. In general, retrospective studies like the present one always bear the risk of hidden selection biases. Furthermore, retrospective chart reviews can be affected by a recall bias and difficulties with the abstraction of the data from the charts. This applies particularly to toxicity data, although the CTCAE criteria are standardized [14]. Since patients of both groups were matched 1:1 also with respect to the tumor site, this factor likely did not have a relevant impact on the results of this study. In those patients receiving surgery, data regarding pathological aspects such as perineural invasion, lymphovascular invasion and extracapsular spread of lymph node metastases were not available, which may have led to a selection bias in this subgroup.
Therefore, the results of this study should ideally be confirmed in a prospective randomized trial. However, since institutions treating patients with LA-SCCHN generally prefer specific regimens, it may be difficult to perform such a randomized trial in an appropriately large cohort with a sufficient statistical power.
In conclusion, 20 mg/m2 cisplatin on 5 days every 4 weeks resulted in significantly better loco-regional control and survival than weekly administration of 30–40 mg/m2 of cisplatin in patients who received definitive radio-chemotherapy but not in patients receiving adjuvant treatment. The toxicity rates were not significantly different with both regimens. Taking into account the limitations and the retrospective design of this study, 20 mg/m2 cisplatin on 5 days every 4 weeks may be preferable to weekly administrations for definitive radio-chemotherapy. These results should be verified in a prospective randomized trial.
References
Jeremic B, Shibamoto Y, Stanisavljevic B, Milojevic L, Milicic B, Nikolic N (1997) Radiation therapy alone or with concurrent low-dose daily either cisplatin or carboplatin in locally advanced unresectable squamous cell carcinoma of the head and neck: a prospective randomized trial. Radiother Oncol 43:29–37
Jeremic B, Shibamoto Y, Milicic B, Nikolic N, Dagovic A, Aleksandrovic J, Vaskovic Z, Tadic L (2000) Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol 18:1458–1464
Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee DJ, Leaf A, Ensley J, Cooper J (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091–2098
Adelstein DJ, Li Y, Adams GL, Wagner H Jr, Kish JA, Ensley JF, Schuller DE, Forastiere AA (2003) An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21:92–98
Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, Iralt J, Maingon P, Rolland F, Bolla M, Cognetti F, Bourhis J, Kirkpatrick A, van Glabbeke M (2004) Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 350:1945–1952
Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, Machtay M, Ensley JF, Chao KS, Schultz CJ, Lee N, Fu KK (2004) Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350:1937–1944
Pignon JP, Le Maître A, Maillard E, Bourhis J, MACH-NC Collaborative Group (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92:4–14
de Castro G, Jr Snitcovsky IM, Gebrim EM, Leitão GM, Nadalin W, Ferraz AR, Federico MH (2007) High-dose cisplatin concurrent to conventionally delivered radiotherapy is associated with unacceptable toxicity in unresectable, non-metastatic stage IV head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 264:1475–1482
Ho KF, Swindell R, Brammer CV (2008) Dose intensity comparison between weekly and 3-weekly Cisplatin delivered concurrently with radical radiotherapy for head and neck cancer: a retrospective comparison from New Cross Hospital, Wolverhampton, UK. Acta Oncol 47:1513–1518
Fayette J, Molin Y, Lavergne E, Montbarbon X, Racadot S, Poupart M, Ramade A, Zrounba P, Ceruse P, Pommier P (2015) Radiotherapy potentiation with weekly cisplatin compared to standard every 3 weeks cisplatin chemotherapy for locoregionally advanced head and neck squamous cell carcinoma. Drug Des Dev Ther 9:6203–6210
Rades D, Kronemann S, Meyners T, Bohlen G, Tribius S, Kazic N, Schroeder U, Hakim SG, Schild SE, Dunst J (2011) Comparison of four cisplatin-based radiochemotherapy regimens for nonmetastatic stage III/IV squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 80:1037–1044
Huguenin P, Beer KT, Allal A, Rufibach K, Friedli C, Davis JB, Pestalozzi B, Schmid S, Thöni A, Ozsahin M, Bernier J, Töpfer M, Kann R, Meier UR, Thum P, Bieri S, Notter M, Lombriser N, Glanzmann C (2004) Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol 22:4665–4673
Kaplan EL, Meier P (1958) Non parametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
National Institutes of Health/National Cancer Institute (2009) Common terminology criteria for adverse events (CTCAE) version 4.0. http://www.evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
Geiger JL, Lazim AF, Walsh FJ, Foote RL, Moore EJ, Okuno SH, Olsen KD, Kasperbauer JL, Price DL, Garces YI, Ma DJ, Neben-Wittich MA, Molina JR, Garcia JJ, Price KA (2014) Adjuvant chemoradiation therapy with high-dose versus weekly cisplatin for resected, locally-advanced HPV/p16-positive and negative head and neck squamous cell carcinoma. Oral Oncol 50:311–318
Espeli V, Zucca E, Ghielmini M, Ghielmini M, Giannini O, Salatino A, Richetti A (2012) Weekly and 3-weekly cisplatin concurrent with intensity-modulated radiotherapy in locally advanced head and neck squamous cell cancer. Oral Oncol 48:266–271
Tsan DL, Lin CY, Kang CJ, Huang SF, Fan KH, Liao CT, Chen IH, Lee LY, Wang HM, Chang JT (2012) The comparison between weekly and three-weekly cisplatin delivered concurrently with radiotherapy for patients with postoperative high-risk squamous cell carcinoma of the oral cavity. Radiat Oncol 7:215
Einstein MH, Novetsky AP, Garg M, Hailpern SM, Huang GS, Glueck A, Fields AL, Kalnicki S, Goldberg GL (2007) Survival and toxicity differences between 5-day and weekly cisplatin in patients with locally advanced cervical cancer. Cancer 109:48–53
Strojan P, Vermorken JB, Beitler JJ, Saba NF, Haigentz M Jr, Bossi P, Worden FP, Langendijk JA, Eisbruch A, Mendenhall WM, Lee AW, Harrison LB, Bradford CR, Smee R, Silver CE, Rinaldo A, Ferlito A (2016) Cumulative cisplatin dose in concurrent chemoradiotherapy for head and neck cancer: a systematic review. Head Neck 38(Suppl 1):E2151–E2158
Rades D, Seibold ND, Hoffmann A, Gebhard MP, Noack F, Thorns C, Schild SE (2013) Impact of the HPV-positivity definition on the prognostic value of HPV status in patients with locally advanced squamous cell carcinoma of the head and neck. Strahlenther Onkol 189:856–860
Strojan P, Zadnik V, Šifrer R, Lanišnik B, Didanović V, Jereb S, Poljak M, Kocjan BJ, Gale N (2015) Incidence trends in head and neck squamous cell carcinoma in Slovenia, 1983–2009: role of human papillomavirus infection. Eur Arch Otorhinolaryngol 272:3805–3814
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not funded.
Conflict of interest
D. R. has received research grants and speaker honoraria from Merck Serono. Otherwise, the authors declare that they have no conflicts of interest related to this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was not required, since it was a retrospective study.
Rights and permissions
About this article
Cite this article
Rades, D., Seidl, D., Janssen, S. et al. Comparing two lower-dose cisplatin programs for radio-chemotherapy of locally advanced head-and-neck cancers. Eur Arch Otorhinolaryngol 274, 1021–1027 (2017). https://doi.org/10.1007/s00405-016-4326-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4326-5