Abstract
Background
We previously identified hypopharyngeal cancer as an independent risk factor for the incidence of newly diagnosed secondary cancers after the treatment of early-stage laryngeal, oropharyngeal, and hypopharyngeal cancers. We subsequently used a different patient cohort to validate the usefulness of this factor during the follow-up period in these patients.
Methods
Patients who underwent transoral surgery (TOS) as a definitive treatment between April 1, 2016, and September 30, 2020, were included. The incidence of secondary cancer was evaluated in hypopharyngeal and other cancers. Overall survival (OS), recurrence-free survival (RFS), and disease-free survival (DFS) outcomes were evaluated. Statistical analyses based on the risk factors were also performed.
Results
Incidence of new secondary cancer was 30% in hypopharyngeal cancer patients as compared to 11% in other cancer patients, and the risk was 3.60-fold (95% confidence interval 1.07–12.10) higher after definitive treatment for initial head and neck cancers. The 3-year OS, RFS, and DFS rates were 98%, 86%, and 67%, respectively.
Conclusions
Among patients with early-stage laryngeal, oropharyngeal, and hypopharyngeal squamous cell carcinoma, who were initially treated with TOS, hypopharyngeal cancer patients had a higher risk of newly diagnosed secondary cancers as observed during the follow-up period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of early-stage head and neck cancer patients has been increasing due to the advent of advanced diagnostic techniques such as positron emission tomography-computed tomography (PET-CT) [1] and narrow-band imaging (NBI) endoscopy [2], as well as increased awareness of head and neck cancer among doctors in other fields [3]. Most patients with head and neck cancer were diagnosed at an advanced stage, and the majority of the available prognosis data describe these advanced cases. Meanwhile, our previous study reported high curability [4,5,6] and a high incidence rate of newly diagnosed secondary cancers [7], in which the crude incidence was 10.6 per 100 person-years in early-stage laryngeal, oropharyngeal, and hypopharyngeal cancer patients treated with transoral surgery (TOS). We revealed that hypopharyngeal cancer was an independent risk factor for newly diagnosed secondary cancers during the follow-up period of these patients. Sub-analysis of the patients showed that the odds ratio was 3.96, 95% confidence interval (CI) was 1.07–14.6, and p value was 0.039. To validate the reliability of this risk factor, we prospectively continued the observation study for subsequent TOS performed in new patients.
Patients and methods
Enrollment
Patients who underwent TOS as a definitive treatment between April 1, 2016, and September 30, 2020, were enrolled.
Study setting
This is a single institute (academic hospital), parallel two-arm open-label, non-randomized observational study.
Endpoints
According to our previous study [7], hypopharyngeal cancer was a risk factor for newly diagnosed malignancies during the observation period after definitive treatment for primary head and neck cancer. The primary endpoint was the statistical difference in the incidence rate of newly diagnosed malignancy between hypopharyngeal cancer patients and other cancer patients. The secondary new cancer was defined as follow; pathologically diagnosed as malignant disease; the disease was appeared in other organs (e.g., the first cancer was hypopharyngeal cancer and the second was esophageal cancer), in other subsite of the same region of the head and neck (e.g., the first cancer was piriform sinus type and the second was postcricoid type of the hypopharynx), or the disease was found in the same subsite but the disease was separated from the first cancer mediating normal mucosa (e.g., both first and second cancers were appeared in the same side of the piriform sinus of the hypopharynx).
The secondary endpoint was the survival analysis between the risk factor-positive and -negative patients. The survival rates used were overall survival (OS): the event of survival and death; recurrence-free survival (RFS): events were locoregional recurrence, distant metastasis, and death; and disease-free survival (DFS): events were the uncontrollability of existing cancer characterized by locoregional remnant, locoregional recurrence, and/or distant metastasis, appearance of new primary cancer, and death.
Eligibility criteria
Head and neck cancer was classified according to the 7th edition (from 2016 to 2018) and 8th edition (since 2018) of the Tumor, Node, and Metastasis staging classification [8, 9]. The enrolled patients had clinical T1N0- and T2N0-stage laryngeal, oropharyngeal, and/or hypopharyngeal cancers.
Inclusion criteria
The eligibility criteria for enrollment in the trial included a pathologically proven squamous cell carcinoma and a primary tumor located in the larynx, oropharynx, or hypopharynx. Staging cT1 and cT2 tumors were based on visual and endoscopic examinations and imaging, such as computed tomography (CT) or magnetic resonance imaging. The primary site was assessed to determine whether it was resectable by TOS. The cN0M0 stage was evaluated using ultrasonic echo (US echo) or PET-CT. Synchronous malignant diseases were evaluated with PET-CT, and magnifying upper gastrointestinal endoscopy (UGIE) with NBI, especially to detect early-stage esophageal and gastric cancers. Performance status of 0–2 was determined in compliance with the Eastern Cooperative Oncology Group criteria. The patients had no contraindications for surgery under general anesthesia, were over 20 years of age (legally adult in Japan), and provided written informed consent.
Exclusion criteria
Exclusion criteria included incurable synchronous malignancies and a history of prior systemic illness. However, patients with previous malignancies were included if the malignancies were successfully cured or well-controlled (maintaining a complete response).
Treatment methods
Primary resection was performed using TOS. The mucosal lesion was confirmed by NBI endoscopy and stained with Lugol’s solution. The horizontal safety margin was set at a distance of 1–3 mm from the border of the lesion. Vertical resection was performed in the submucosal layer. After resection, a rapid pathological examination was performed on the margins of the horizontal and vertical sections. In cases with positive margins in the rapid pathological examination, additional resection was performed until a negative margin was confirmed. The resected specimen was stretched on a cork board to clarify the direction and was subsequently fixed with formalin for permanent pathological diagnosis. The wound in oropharyngeal and hypopharyngeal cancer patients was covered with a polyglycolic acid sheet.
Follow-up
Visual and laryngo-pharyngeal endoscopic (both normal light and NBI examination) observations of the primary site were performed every month for the first 2 years, and every 2–3 months from the third to the fifth year. Enhanced cervical CT or US of the primary site and regional lymph nodes was performed every 3–6 months for the first 2 years and every 6–12 months from the third to the fifth year. PET-CT was performed every year for the first 2 years, and enhanced whole-body CT was performed every year from the third to the fifth year for the evaluation of distant metastasis. Routine magnifying UGIE was performed by the decision of the gastroenterologist who performed the synchronous disease screening at the first head and neck cancer evaluation. The patient was referred to the appropriate department if a second primary cancer was diagnosed.
Statistical analyses
Patients’ age was analyzed and compared using the Mann–Whitney U test, while Fisher’s exact test was performed for other factors between hypopharyngeal cancer patients and other patients. Fisher’s exact test was used for the statistical analysis of the incidence rates between hypopharyngeal cancer patients and other patients. The Kaplan–Meier method was used to evaluate survival endpoints, and the log-rank test was used to analyze its statistical differences.
Results
Patient characteristics and examination results
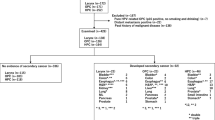
The patient characteristics are shown in Table 1, and the clinical courses are shown diagrammatically in Fig. 1. Seventy-seven patients were enrolled, and all patients were analyzed. Thirty patients were diagnosed with hypopharyngeal cancer, and 47 patients were diagnosed with other cancers (27 laryngeal cancer and 20 oropharyngeal cancer). Among the hypopharyngeal cancer patients, 21 patients indicated no secondary cancers, and nine patients had newly diagnosed secondary cancers during the follow-up period. The incidence rate of secondary cancer was 30% (9/21; Table 2), and the crude incidence of second primary cancer was 22.8 per 100 person-years. The primary sites of secondary cancer were head and neck (nine patients: six hypopharynx, three larynx), esophagus (four patients), prostate (two patients), and lung (one patient). Three patients developed new double cancers (larynx and hypopharynx, hypopharynx and esophagus, lung and prostate) and two patients developed new triple cancers (hypopharynx, hypopharynx, and larynx; hypopharynx, hypopharynx, and esophagus). On the other hand, among other cancer patients, 42 patients had no secondary cancers and five patients had newly diagnosed secondary cancers. The incidence rate of the secondary cancer was 11% (5/47), and the crude incidence of second primary cancers was 5.2 per 100 person-years. The primary sites of secondary cancer were head and neck (two oropharynx and one hypopharynx), lung, esophagus, and colon. One patient developed new double cancers, the primary sites of which were lung and colon. The difference in the incidence rate was statistically analyzed using Fisher’s exact test (p = 0.0391) and odds ratio (3.60 with a 95% CI of 1.07–12.10). Both tests indicate significant differences between these two groups. The time course of the secondary cancer incidence is shown in Fig. 2.
Survival
The 3-year OS, RFS and DFS rates were similar between the hypopharyngeal cancer patients and other patients. The OS rates were 100% in hypopharyngeal cancer patients (observation period was 1–57 months, median 34.5) and 97% in other patients (observation period was 8–59 months, median 27 months) (p = 0.8502) (Fig. 3a). The RFS rates were 92% in hypopharyngeal cancer patients and 82% in other patients (p = 0.3718) (Fig. 3b). The DFS rates were 52% in hypopharyngeal cancer patients and 75% in other patients (p = 0.2091) (Fig. 3c). Moreover, log-rank test showed no significant difference in survival.
Discussion
At the time of diagnosis of head and neck cancers, approximately 60% of patients were classified as advanced-stage cancer patients. Thus, most of the data on the course of head and neck cancers have been collected from advanced-stage patients. These advanced-stage head and neck cancer patients have a high risk of treatment failure during the observation period after definitive treatment. Among these patients, 60–70% of the cases showed locoregional recurrences within 1 year after diagnosis, and 90–100% of the cases showed recurrences within 2 years. Furthermore, although these patients have a poor prognosis, the incidence of newly diagnosed secondary cancers in survivors exceeds the incidence of primary cancer treatment failures since four years after the diagnosis of initial head and neck cancers [10,11,12]. McDonald et al. [13] reported that among patients with laryngeal cancer of all stages with a median follow-up of 10 years, approximately 60% of the secondary cancers occurred in the aerodigestive tract, including 30% in the lungs, 20% in the head and neck, and 8% in the esophagus. The potential mechanisms of secondary carcinogenesis in advanced head and neck cancer patients include risk factors like smoking and alcohol consumption, in head and neck, esophageal, and lung cancers, or field cancerization theory. The incidence of secondary cancer development depends on the extent of evaluation, length of follow-up, and curability of the primary lesion. In addition, it depends on the patterns of tobacco and alcohol use [13, 14]. The authors emphasize that although the per-year risk of developing secondary cancers might not be affected by the primary head and neck cancer stage, the cumulative incidence and survival impact are most significant in early-stage patients [13, 14].
The advent of advanced diagnostic techniques such as PET-CT [1] and NBI endoscopy [2] as well as the increased awareness of head and neck cancers among doctors in other fields [3] has resulted in an increase in the number of early-stage head and neck cancer patients. However, there are a limited number of reports about second primary cancers in early-stage head and neck cancer, and we have limited knowledge about the follow-up course of these second primary early-stage cancers [15,16,17,18]. These reports mainly describe oral and glottic cancers, which are comparatively easy to diagnose at an early stage because the symptoms, such as pain and hoarseness, tend to appear in the early phase and the visual examination is relatively easy. Bhatia et al. [15] reported that 45 out of 176 early-stage oral cavity, oropharyngeal, hypopharyngeal, or laryngeal cancer patients developed secondary cancers within a 1.0–25.5 year follow-up period. The secondary cancer primary sites included 11 head and neck, 10 lung, six skin (not melanoma), three breast, colorectal, and prostate, two bladder, and one of each esophageal, anal, brain, B cell lymphoma, melanoma, endometrium, and gynecologic cancers. Ord et al. [17] reported that seven out of 112 stage I tongue cancer patients indicated secondary cancers during the 32–91 month follow-up period. Here, the breakdown of the secondary cancers were three gingival cancers, two oropharyngeal cancers, one buccal cancer, and one adenocarcinoma of lung. Thus, compared to advanced-stage patients with secondary cancers occurring in the head and neck, lung, or esophagus, early-stage head and neck cancer patients have varied sites of secondary cancer.
Smoking increases the risk of at least 17 classes of cancer, such as lung (small cell carcinoma, squamous cell carcinoma, and adenocarcinoma), larynx, pharynx, oral cavity, esophagus (squamous cell carcinoma and adenocarcinoma), bladder, liver, stomach, acute myeloid leukemia, ovary, cervix, kidney, pancreas, and colorectal cancers [19]. Smoking induces DNA damage, which causes carcinogenesis. Tobacco carcinogens directly and indirectly induce DNA mutations in lung squamous cell carcinoma, lung adenocarcinoma, larynx, and liver cancers. Moreover, these carcinogens also indirectly induce DNA damage in bladder, cervix, kidney, pancreas, stomach, colorectal, and ovarian cancers and acute myeloid leukemia. In Japan, smoking is defined as a risk factor for nasal and paranasal sinuses, oral cavity, pharynx, larynx, esophagus, lung, liver, stomach, pancreas, uterine cervix, and bladder cancers. A study showed that 30% of male and 5% of female cancer patients suffer from smoking [20]. Alcohol is another risk factor for oral cavity, pharynx, esophagus, stomach, colon, liver, larynx, breast, cervix, and prostate cancers. The risk increases with the amount of alcohol consumed. Even if the amount is light to moderate, the incidence risk for esophagus, larynx, oral cavity, pharynx, colorectal, liver, stomach, breast, prostate and bladder cancers [21] increases. One of the mechanisms of alcohol-induced carcinogenesis is chemical mucositis by ethanol directly in the upper aerodigestive tract, which includes the oral cavity, pharynx, esophagus, and larynx. Another mechanism of carcinogenesis is by acetaldehyde, a degradation product of ethanol by acetaldehyde dehydrogenase (ALDH) 2, which indirectly affects the whole body. Forty-four percent of Japanese have the heterozygous genotype of normal and inactive ones (ND type) or the homozygous genotype of an inactive one (DD type) of ALDH2, and these people can be exposed to acetaldehyde whenever they drink. Furthermore, the incidence of all types of cancer was found to be increased with smoking in habitual alcohol drinkers [22]. In the non-smoking group, the incidence did not increase in accordance with an increase in alcohol consumption. Meanwhile, in the smoking group, the incidence increased in accordance with an increase in alcohol consumption, and people who had been drinking more than 81 g of alcohol every day with customary smoking indicated 2.3 times higher incidence of all cancers. Aging is another factor that is believed to increase the incidence of all cancers, and one mechanism of how aging affects the incidence has been reported [23]. Six-hundred and eighty-two micro-scale esophageal samples from physiologically normal esophageal epithelia were sequenced, and the progressive age-related expansion of clones carrying mutations in driver genes (predominantly NOTCH1) was observed. Driver-mutated clones emerged multifocally from early childhood and increased in number and size with aging, and ultimately replaced almost the entire esophageal epithelium in the extremely elderly. Compared with mutations in esophageal cancer, there was a marked overrepresentation of NOTCH1 and PPM1D mutations in physiologically normal esophageal epithelia. These mutations could be acquired before late adolescence and significantly increased in number with heavy smoking and drinking. Also, with regard to aging, the patients in this study were older (median 73 years old) than those in previous studies (median 61 years old [15] and 63 years old [16], and an average of 57.7 years old [17]). Smoking, alcohol consumption, and aging can be synergistic risk factors for all types of cancer.
We summed and analyzed 120 patients who were included in both the previous study and this study (Table 3), based on the eligibility criteria to undergo TOS. Considering the results, the odds ratio is almost the same in both analyses, the interval of 95% CI becomes narrower in the larger number analysis in the same treatment series, and the p value is smaller in the larger number analysis. Together, our result demonstrates that hypopharyngeal cancer is a risk factor for newly diagnosed secondary cancer incidence in early-stage laryngeal, oropharyngeal, and hypopharyngeal cancer patients during the follow-up period after definitive therapy. The early detection of secondary cancers contributes to high curability. Systemic examinations, such as PET-CT and esophagoscopy, to detect locoregional recurrence and distant metastasis, as well as secondary cancer, are necessary during the follow-up period. We also found that a preventive intervention to decrease the incidence of secondary cancer is an unmet medical need. Adequate management of early-stage primary head and neck cancer contributes to prolonged survival, which may impact the secondary cancer onset in elderly cancer survivors. This can explain a higher rate of the crude incidence of second cancer per 100 patients-years observed in our study (10.6 in our previous study, 11.9 in this study, and 11.3 in a total of 120 patients) than in the previous report, where the crude incidence of second cancer per 100 patients-years was 1.55 in all nine tumor types [18]. According to our results, even at an early stage, head and neck cancer patients, especially hypopharyngeal cancer patients have a high risk of secondary cancer, possibly due to smoking habits, alcohol consumption, and advanced age.
Furthermore, the patient composition may change in the future, as the number of human papilloma virus (HPV)-positive oropharyngeal cancer patients has been increasing. The HPV-positive rate of oropharyngeal cancer is 30–50% in Japan [24, 25], similar to the results of this study (35%, 7/20). It is predicted that the incidence rate of HPV-positive oropharyngeal cancer will increase, and the number of older HPV-negative oropharyngeal cancer patients with smoking and drinking history will decrease. HPV-positive oropharyngeal cancer tends to occur in younger patients; the incidence rate increases from the age of 40 and a good prognosis is expected. In these long-term survivors, we have to pay attention to HPV-related secondary cancers, such as cervical, vaginal, vulvar, anal, and penile cancers, until the HPV vaccine is effective in preventing these cancers.
A limitation of the present study is that it was conducted at a single institution with a limited sample size.
Conclusions
We analyzed patients with early-stage laryngeal, oropharyngeal, and hypopharyngeal cancers. The independent predictive factor in newly diagnosed secondary cancers was hypopharyngeal cancer. One possible reason may be common risk factors such as smoking and alcohol consumption, especially for aerodigestive tract cancers. Additionally, for all types of malignancies, advanced age at the time of diagnosis of the first head and neck cancer is another risk factor. These risk factors may synergistically affect each other.
Availability of data and material
The datasets used in the present study are available from the corresponding author upon request. All data generated or analyzed in this study are included in this published article.
References
Dammann F, Horger M, Mueller-Berg M et al (2005) Rational diagnosis of squamous cell carcinoma of the head and neck region: comparative evaluation of CT, MRI, and 18FDG PET. AJR Am J Roentgenol 184:1326–1331
Watanabe A, Taniguchi M, Tsujie H et al (2008) The value of narrow band imaging endoscope for early head and neck cancers. Otolaryngol Head Neck Surg 138:446–451
Morita M, Saeki H, Ito S et al (2014) Surgical strategies for esophageal cancer associated with head and neck cancer. Surg Today 44:1603–1610
Nishimura G, Sano D, Yabuki K et al (2017) The second-look procedure for transoral videolaryngoscopic surgery for T1 and T2 laryngeal, oropharyngeal, and hypopharyngeal cancer patients: protocol for a nonrandomized clinical trial. JMIR Res Protoc 6:e235
Nishimura G, Sano D, Arai Y et al (2019) A prospective clinical trial of the second-look procedure for transoral surgery in patients with T1 and T2 laryngeal, oropharyngeal, and hypopharyngeal cancer. Cancer Med 8:7197–7206
Nishimura G, Sano D, Arai Y et al (2021) Validation of the risk factors for primary control of early T-stage laryngeal, oropharyngeal, and hypopharyngeal squamous cell carcinoma by transoral surgery: a prospective observational study. Int J Clin Oncol 26:1995
Nishimura G, Sano D, Arai Y et al (2021) The incidence of newly diagnosed secondary cancer; Sub-analysis the prospective study of the second-look procedure for transoral surgery in patients with T1 and T2 head and neck cancer. Int J Clin Oncol 26:59–65
Sobin L, Wittekind C (2009) TNM classification of malignant tumours, 7th edn. Wiley-Liss, New York
Brierley JD, Gospodarowicz MK, Wittekind C (2017) TNM classification of malignant tumours, 8th edn. Wiley-Liss, New York
Vikram B (1984) Changing patterns of failure in advanced head and neck cancer. Arch Otolaryngol 110:564–565
Vokes EE, Kies M, Haraf DJ et al (1995) Induction chemotherapy followed by concomitant chemoradiotherapy for advanced head and neck cancer: impact on the natural history of the disease. J Clin Oncol 13:876–883
Khuri FR, Lippman SM, Spitz MR et al (1997) Molecular epidemiology and retinoid chemoprevention of head and neck cancer. J Natl Cancer Inst 89:199–211
McDonald S, Haie C, Rubin P et al (1989) Second malignant tumors in patients with laryngeal carcinoma: diagnosis, treatment, and prevention. Int J Radiat Oncol Biol Phys 17:457–465
Cooper JS, Pajak TF, Rubin P et al (1989) Second malignancies in patients who have head and neck cancer: incidence, effect on survival and implications based on the RTOG experience. Int J Radiat Oncol Biol Phys 17:449–456
Bhatia AK, Lee JW, Pinto HA et al (2017) Double-blind, randomized phase 3 trial of low-dose 13-cis retinoic acid in the prevention of second primaries in head and neck cancer: long-term follow-up of a trial of the eastern cooperative oncology group-ACRIN cancer research group (C0590). Cancer 123:4653–4662
Rogers SN, Swain A, Carroll C et al (2019) Incidence, timing, presentation, treatment, and outcomes of second primary head and neck squamous cell carcinoma after oral cancer. Br J Oral Maxillofac Surg 57:1074–1080
Ord RA, Isaiah A, Dyalram D et al (2018) Is long-term follow-up mandatory for stage I oral tongue cancer? J Oral Maxillofac Surg 76:2676–2683
Xiang M, Chang DT, Pollom EL (2020) Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer 126:3560–3568
Alexandrov LB, Ju YS, Haase K et al (2016) Mutational signatures associated with tobacco smoking in human cancer. Science 354:618–622
Cancer Registry and Statistics. National Cancer Center, Japan: Cancer Information Service. https://ganjoho.jp/public/pre_scr/cause_prevention/smoking/index.html. Accessed 16 Nov 2021 (in Japanese)
Zaitsu M, Takeuchi T, Kobayashi Y et al (2020) Light to moderate amount of lifetime alcohol consumption and risk of cancer in Japan. Cancer 126:1031–1040
Inoue M, Tsugane S, JPHC Study Group (2005) Impact of alcohol drinking on total cancer risk: data from a large-scale population-based cohort study in Japan. Br J Cancer 92:182–187
Yokoyama A, Kakiuchi N, Yoshizato T et al (2019) Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature 565:312–317
Hama T, Tokumaru Y, Fujii M et al (2014) Prevalence of human papillomavirus in oropharyngeal cancer: a multicenter study in Japan. Oncology 87:173–182
Toman J, Von Larson S, Umeno H et al (2017) HPV-positive oropharyngeal cancer via p16 immunohistochemistry in Japan. Ann Otol Rhinol Laryngol 126:152–158
Acknowledgements
Not applicable
Funding
This work was partly supported by a Grant from the 2019 to 2020 Strategic Research Promotion (No. SK2803) of Yokohama City University and the Grants-in-Aid for Scientific Research (C)—KAKENHI—(No. 21K09636) by the Ministry of Education, Culture, Sports, and Technology (MEXT).
Author information
Authors and Affiliations
Contributions
GN and NO conceived, performed statistical analyses and edited the manuscript. GN, NO, DS, and YA designed the study. GN, HT, TH, YK, KT, TW, and YH acquired, analyzed, and interpreted the data. GN, DS, and YA evaluated the quality control of the data and algorithms. GN prepared this manuscript, and all authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
Ethical approval for the study was obtained from the Yokohama City University Institutional Review Board (#B210400057).
Consent to participate
Written informed consent was obtained from the participants to publish their data.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nishimura, G., Sano, D., Arai, Y. et al. A risk factor for newly diagnosed secondary cancer in patients with early-stage laryngeal, oropharyngeal, or hypopharyngeal cancer: sub-analysis of a prospective observation study. Int J Clin Oncol 27, 488–494 (2022). https://doi.org/10.1007/s10147-021-02080-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-02080-x