Abstract
Objective
Patients with advanced hypopharyngeal squamous cell carcinomas (HSCCs) have poor prognoses. The use of surgical or non-surgical treatments for these patients remains a topic of debate. This study compared survival following surgical and non-surgical treatments of patients with advanced HSCC based on the Surveillance, Epidemiology and End Results (SEER) database.
Methods
Patients diagnosed with hypopharyngeal cancer from 2004 to 2018 were identified from the SEER database. Patients were divided into non-surgical group and surgical group, and patients in the surgical group were further divided into three groups: surgery-only, surgery with adjuvant radiation therapy and surgery with adjuvant chemoradiation therapy. The primary endpoint was overall survival (OS), and the secondary outcome was cancer-specific survival (CSS). Outcomes were analyzed using Kaplan–Meier analysis. A multivariate Cox regression analysis was also used to identify independent prognostic factors.
Results
The records of 1568 eligible patients with stage III or IV HSCC were examined. Receipt of surgery was associated with a longer OS [hazard ratio (HR) = 0.47, 95% confidence interval (CI): 0.4–0.56] and a longer CSS (HR = 0.47, 95% CI: 0.38–0.57) after adjusting for age, sex, race, tumor site, tumor size, tumor grade, TNM stage, AJCC stage, number of carcinomas, prior cancer, receipt of radiotherapy, and receipt of chemotherapy. The results for OS were similar in an exploratory analysis of different patient subgroups.
Conclusion
Among patients with advanced HSCC in the SEER database, treatment with surgery was associated with longer OS and CSS than treatment with a non-surgical modality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypopharyngeal squamous cell carcinoma (HSCC) accounts for only about 3 to 5% of all head and neck cancers, but it has the worst prognosis among the different types of these cancers [1]. The poor prognosis of patients with HSCC is mainly because these patients often have advanced-stage disease upon diagnosis. Early diagnosis of HSCC is difficult because insidious growth often occurs before the onset of symptoms, which first occur when there is a local invasion of the aerodigestive tract and nerves or when there are regional or distant metastases [2]. Neck node metastasis is common in HSCC because of the rich network of lymphatics in the hypopharyngeal region, and HSCC in all T stages commonly spreads via unilateral and bilateral regional metastatic cervical nodes, and to paratracheal and retropharyngeal nodes. Thus, most patients have stage III/IV HSCC upon diagnosis [3, 4].
The treatment to be used for locally advanced HSCC is controversial. The traditional approach for locally advanced HSCC is laryngopharyngectomy and pharyngeal reconstruction, although this causes the loss of natural speech [5]. Nonsurgical procedures, such as chemoradiation therapy (CRT) and primary radiotherapy, have become more common for patients with advanced HSCC, and these regimens provide similar cure and survival rates, and acceptable organ functional outcomes [6, 7]. Large clinical comparisons or randomized controlled studies are difficult to perform because these tumors are so rare. Thus, most publications that reported the surgery outcomes were case series. Only one randomized trial compared surgical with non-surgical approaches for the treatment of HSCC [8, 9]. Therefore, currently available data are insufficient to establish the optimal treatment for advanced HSCC.
This study compared the effects of surgical with non-surgical treatments for patients with advanced HSCC. We retrieved clinical data from the updated Surveillance, Epidemiology and End Results (SEER) database, including demographics, clinicopathological parameters, and treatment modalities, to compare the survival outcomes of HSCC patients who received surgical or non-surgical treatments.
Patients and methods
Patient selection
Data of patients who were initially diagnosed with HSCC from January 2004 to December 2018 were extracted from the SEER database. SEER*Stat version 8.3.9.2 (https://seer.cancer.gov/seerstat/download) was used to download these data. The presence of primary cancer in the hypopharynx was based on codes from the International Classification of Diseases for Oncology, Third Edition (ICD-O-3; C12.9, C13.0, C13.1, C13.2, C13.8, or C13.9).
The following patient variables were examined: age, year of diagnosis, sex, race, marital status, primary site, tumor size, American Joint Committee on Cancer (AJCC, 7th edition) stage, TNM stage, grade, and treatment. For statistical analyses, age at diagnosis was classified into four groups (< 60, 60–70,71–80, and > 80 years old), race was classified into four groups (White, Black, Asian or Pacific Islander, and American Indian/Alaska Native), the primary subsite was classified into four groups (pyriform sinus, postcricoid region, and posterior wall of hypopharynx, not otherwise specified), and tumor size was classified into four groups (< 2 cm, 2–4 cm, > 4 cm and unknown). Information on radiotherapy, chemotherapy and surgery was extracted from the following fields: radiation sequence with surgery, the reason for no cancer-directed surgery, radiation recode, chemotherapy recode and systemic therapy sequence with surgery.
Statistical analysis
Categorical data were presented as numbers and percentages and compared using the χ2 test. Univariate Cox analysis and the log-rank test were used for the analysis of qualitative and quantitative variables to determine the significance of differences among groups. The adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) were calculated. Multivariate Cox regression analysis was used to assess the independent association of treatment with overall survival (OS) and cancer-specific survival (CSS). The multivariate models adjusted for demographic variables (Model I: age, sex and race); tumor-related variables (Model II: tumor site, tumor size, grade, TNM stage, AJCC stage, total cancer number and prior cancer); treatment variables (Model III: radiation and chemotherapy); and all of these variables (Model IV).
The primary endpoint was overall survival (OS), defined as the time from the initial diagnosis to death from any cause. The secondary endpoint was cancer-specific survival (CSS), defined as the interval from the initial diagnosis to death from HSCC. Kaplan–Meier plots and the log-rank test were used to compare the survival of the different groups. Subgroup analysis of demographic and clinical characteristics was presented in a forest plot.
All statistical tests were 2-sided, and a p-value below 0.05 was considered significant. All statistical analyses were performed using R software (http://www.R-project.org, The R Foundation) and Free Statistics Software version 1.2.
Results
Patient characteristics
The study cohort consisted of 1568 eligible patients who were diagnosed with advanced HSCC between 2004 and 2018 (Fig. 1). Patients were divided into non-surgical groups and surgical groups based on their medical history of whether or not initial surgery was performed. And patients underwent surgeries were further divided into four groups: surgery alone (OP alone), surgery with adjuvant radiation therapy (OP + aRT), surgery with adjuvant CRT (OP + aCRT) and radiation or chemotherapy before surgery (RT/chemotherapy + OP). The group of RT/chemotherapy + OP was deleted for statistics since the sample was too small (n = 5).
We compared the baseline characteristics of the different patient variables in groups that received various treatment modalities (Table 1). A total of 332 (21.2%) patients received surgery and 1236 (78.8%) received non-surgical treatment. Overall, the median age was 65 years (IQR: 57–73), 80.0% of the patients were men, and most patients were white (74.0%). The median follow-up time was 17 months (IQR: 6–42.2). A total of 58.9% of the patients had tumors in the pyriform sinus, 2.8% had tumors in the post-cricoid region, and 6.5% had tumors in the posterior wall of the pharynx. For statistical comparisons, we separately analyzed patients with tumors less than 20 mm (8.4%), 20 to 40 mm (33.9%), and more than 40 mm (29.8%). The four groups had significant differences in age, marital status, total number of carcinomas, prior cancer, receipt of radiotherapy, and receipt of chemotherapy (all P < 0.05).
Survival analyses
We then analyzed the relationship of OS and CSS with treatment modality (Table 2). In the unadjusted model, patients who received surgery had a longer OS (HR: 0.7, 95% CI: 0.60–0.82, P < 0.001) and a longer CSS (HR: 0.7, 95% CI: 0.58–0.84, P < 0.001). The significance of these relationships remained after multivariable analysis with adjustment for age, sex and race (model I), tumor site, tumor size, grade, TNM stage, AJCC stage, total cancer number, and prior cancer (model II), radiation and chemotherapy (model III), and all these variables (model IV). Both OS and CSS were also longer in three subgroups of the surgical group than in the non-surgical group.
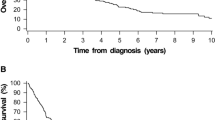
The 5-year OS rate was 37.8% (95% CI: 32.2–44.3%) in the surgery group and 28.4% (95% CI: 25.7–31.3%) in the non-surgical group. The 5-year CSS rate was 50.6% (95% CI: 44.6–57.5%) in the surgery group and 41.5% (95% CI: 38.4–44.8%) in the non-surgical group. Kaplan–Meier survival analysis and the results of the log-rank test indicated the surgery group had a longer OS (P < 0.01, Fig. 2A, 2B) and a longer CSS (P < 0.01 Fig. 2C, 2D).
Kaplan–Meier analysis of overall survival and cancer-specific survival. A Overall survival of patients who received surgical or non-surgical treatments. B Overall survival of patients who received no surgery or surgery alone or surgery with adjuvant radiation or surgery with adjuvant chemoradiation. C Cancer-specific survival of patients who received surgical or non-surgical treatments. D Cancer-specific survival of patients who received no surgery or surgery alone or surgery with adjuvant radiation or surgery with adjuvant chemoradiation
We also performed an exploratory analysis to assess the effect of treatment modality on the primary outcome in multiple subgroups (Fig. 3). The results of this stratified analysis indicated that the association of surgery with longer OS in the different subgroups was generally consistent with the results of the multivariable logistic regression analysis.
Discussion
HSCC is associated with the worst survival rate among all head and neck cancers, and the 5-year survival rate of patients with stage III/IV HSCC is only 15 to 45% [3]. The major reason for the poor prognoses of these patients is that symptoms only appear in those with advanced cancer, thus delaying diagnosis and allowing early lymphatic spread [10]. For patients with advanced HSCC, the NCCN guidelines recommend surgery with partial or total laryngopharyngectomy, neck dissection and thyroidectomy, plus systemic therapy or radiotherapy, depending on the presence of certain adverse features. The alternatives are induction chemotherapy, concurrent systemic therapy, and enrollment in a clinical trial. The NCCN guidelines panel made a category 4 recommendation regarding this cancer, indicating major disagreement about the most appropriate treatment [2]. The objective of our study was to compare the effects of different treatments in patients with HSCC.
In an effort to ensure organ preservation, oncologists now consider nonsurgical protocols, such as CRT, as a treatment option for patients with resectable advanced cancers [3, 11, 12]. In particular, previous studies of HSCC using SEER data reported a transition toward radiation therapy (RT) and away from surgery since 1990, without a decline in 5-year survival [13]. A randomized controlled trial suggested there was no significant difference in the 5-year survival rate for patients with advanced HSCC who received radiotherapy/chemotherapy (30%) vs. surgery/radiotherapy (35%) [8]. Jang et al. studied 177 patients with advanced HSCC and reported similar oncological outcomes but different functional outcomes in groups that initially received surgical vs. non-surgical treatments [14].
Conversely, a recent retrospective study of advanced-stage HSCC by Tassler et al. suggested primary surgery had a survival benefit over organ preservation treatment [15]. Moreover, a 20-year population-based study in the Netherlands examined the overall survival of patients with T1–T4 HSCC and reported similar survival rates after total laryngectomy and (chemo)radiotherapy for those with T3 cancer, but a survival benefit for patients with T4 cancer who received primary surgery ± radiotherapy [10]. Our data confirmed equal oncological outcomes for the surgical and non-surgical groups, but we found that the surgical group had significantly longer OS and CSS after adjustment for major confounders. The same association of survival with treatment modality was evident in our subgroup analysis. But it should also be noted that because of our relatively small sample size, the power of detecting a moderate interaction was limited so that a negative finding would not necessarily confirm the absence of an interaction.
For treatment of laryngeal and hypopharyngeal cancer, the NCCN recommended that patients beyond UICC stage III should receive adjuvant radio(chemo) after primary surgery [16]. A total laryngopharyngectomy with adjuvant chemoradiation therapy (depending on pathology) is the most common treatment for patients with advanced HSCC [15, 17,18,19]. A population-based cohort study of 6647 patients with HSCC found that a combination of surgery and radiotherapy led to the best 5-year OS (48.5%) [13]. Kuo et al. reported that laryngopharyngectomy alone led to better survival than no surgery and that surgery with radiotherapy led to an even better outcome than surgery alone [11]. The present study found that patients in the surgical group had a better prognosis than those without surgeries, either with surgery alone or with adjuvant radiotherapy or radiochemotherapy after surgery.
Laryngectomy with partial or circumferential pharyngectomy and pharynx reconstruction is often favored for HSCC because it leads to better tumor control and improved post-operative recovery of swallowing function without aspiration [3, 20]. Total laryngectomy can cause total vocal dysfunction and lead to psychological distress, so many patients refuse this procedure. It is also important to consider that “organ preservation” and “function preservation” are not the same. In particular, the function may be better preserved after the removal of the larynx because it permits aspiration-free deglutition and a prosthetic voice, which is preferable to leaving an intact but functionless larynx [3]. Moreover, function-preserving strategies for curative treatment are not exclusively nonsurgical. In particular, for patients with advanced HSCC, there is often serious degradation of laryngeal and hypopharyngeal function, and surgery that provides reconstruction followed by chemoradiation may provide good functional and oncologic outcomes.
A limitation of this study is that it was a SEER-based population cohort study. Patients with missing data were excluded, and this may have led to bias. A limitation of the SEER registry is that it does not include information about surgical modalities, radiation dosing, type or dose of chemotherapy drugs, delays in treatment, or treatment dates. Therefore, we could not adjust for different surgical procedures, so our classification of the surgical and non-surgical groups was somewhat simplified.
Conclusion
Our results suggest that patients with advanced HSCC may have longer OS and CSS following surgery rather than non-surgical treatment. Further well-designed prospective studies are needed to establish a definitive benefit of surgery for these patients.
References
Cooper JS, Porter K, Mallin K, Hoffman HT, Weber RS, Ang KK, Gay EG, Langer CJ. National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck. 2009;31(6):748–58.
Kwon DI, Miles BA, Education Committee of the American H, Neck S. Hypopharyngeal carcinoma: do you know your guidelines? Head Neck 2019, 41(3):569-576
Takes RP, Strojan P, Silver CE, Bradley PJ, Haigentz M Jr, Wolf GT, Shaha AR, Hartl DM, Olofsson J, Langendijk JA, et al. Current trends in initial management of hypopharyngeal cancer: the declining use of open surgery. Head Neck. 2012;34(2):270–81.
Bradley PJ. Symptoms and signs, staging and co-morbidity of hypopharyngeal cancer. Adv Otorhinolaryngol. 2019;83:15–26.
Bova R, Goh R, Poulson M, Coman WB. Total pharyngolaryngectomy for squamous cell carcinoma of the hypopharynx: a review. Laryngoscope. 2005;115(5):864–9.
Habib A. Management of advanced hypopharyngeal carcinoma: systematic review of survival following surgical and non-surgical treatments. J Laryngol Otol. 2018;132(5):385–400.
Bradley PT, Bradley PJ. Treatment of hypopharyngeal carcinoma with primary chemoradiotherapy: functional morbidity. Curr Opin Otolaryngol Head Neck Surg. 2012;20(2):89–96.
Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88(13):890–9.
Lefebvre JL, Andry G, Chevalier D, Luboinski B, Collette L, Traissac L, de Raucourt D, Langendijk JA, Head E, Neck Cancer G. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol. 2012;23(10):2708–14.
Petersen JF, Timmermans AJ, van Dijk BAC, Overbeek LIH, Smit LA, Hilgers FJM, Stuiver MM, van den Brekel MWM. Trends in treatment, incidence and survival of hypopharynx cancer: a 20-year population-based study in the Netherlands. Eur Arch Otorhinolaryngol. 2018;275(1):181–9.
Kuo P, Chen MM, Decker RH, Yarbrough WG, Judson BL. Hypopharyngeal cancer incidence, treatment, and survival: temporal trends in the United States. Laryngoscope. 2014;124(9):2064–9.
Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–8.
Newman JR, Connolly TM, Illing EA, Kilgore ML, Locher JL, Carroll WR. Survival trends in hypopharyngeal cancer: a population-based review. Laryngoscope. 2015;125(3):624–9.
Jang JY, Kim EH, Cho J, Jung JH, Oh D, Ahn YC, Son YI, Jeong HS. Comparison of oncological and functional outcomes between initial surgical versus non-surgical treatments for hypopharyngeal cancer. Ann Surg Oncol. 2016;23(6):2054–61.
Tassler AB, Gooding WE, Ferris RL. Hypopharyngeal cancer treatment: does initial surgery confer survival benefit? Head Neck. 2019;41(7):2167–73.
Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(7):873–98.
Harris BN, Biron VL, Donald P, Farwell DG, Luu QC, Bewley AF, Chen AM, Daly ME. Primary surgery vs chemoradiation treatment of advanced-stage hypopharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(7):636–40.
Tian S, Li Q, Li R, Chen X, Tao Z, Gong H, Wang X, Hu X. Development and validation of a prognostic nomogram for hypopharyngeal carcinoma. Front Oncol. 2021;11: 696952.
Hochfelder CG, McGinn AP, Mehta V, Castellucci E, Kabarriti R, Ow TJ. Treatment sequence and survival in locoregionally advanced hypopharyngeal cancer: a surveillance, epidemiology, and end results-based study. Laryngoscope. 2020;130(11):2611–21.
Wei WI, Chan JYW. Surgical treatment of advanced staged hypopharyngeal cancer. Adv Otorhinolaryngol. 2019;83:66–75.
Funding
This research was funded by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS), grant number 2021-I2M-1-023 and 2020-I2M-2-009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflict of interest.
Ethics approval
Ethics approval and consent to participate Informed consent was waived for this study because patient data from the SEER database were de-identified and openly available.Conflicts of Interest: The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, Y., Li, S., Xia, X. et al. Oncological outcomes from surgical vs. non-surgical treatments for advanced hypopharyngeal squamous cell carcinoma: a surveillance, epidemiology, and end results-based study. Clin Transl Oncol 24, 2379–2387 (2022). https://doi.org/10.1007/s12094-022-02890-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02890-z