Abstract
Purpose
The prognostic utility of the prognostic nutritional index (PNI) in nasopharyngeal carcinoma (NPC) has never been systematically reviewed. Therefore, we performed this meta-analysis.
Methods
We performed comprehensive research via Embase, PubMed, Web of Science and the Cochrane Library. The pooled hazard ratios (HRs) and odds ratios (ORs) with 95% confidence intervals (CIs) were applied to explore the relationship between PNI and overall survival (OS), progression-free survival (PFS), locoregional failure-free survival (LRFFS), distant metastasis-free survival (DMFS) and clinical features. Both univariate analysis (UVA) and multivariate analysis (MVA) were used.
Results
A total of 8 eligible studies including 3631 patients were ultimately enrolled. A low PNI level was significantly associated with a shorter OS [(HR 2.06, P < 0.00001; UVA) and (HR 1.78, P < 0.00001; MVA)], PFS [(HR 2.27, P = 0.006; UVA) and (HR 1.45, P = 0.0003; MVA)] and DMFS [(HR 2.06, P < 0.00001; UVA) and (HR 2.04, P < 0.00001; MVA)]. However, only one study reported the LRFFS of NPC patients, and there was no significant difference [HR 1.68, P = 0.26]. Furthermore, female patients, higher tumor stage, a lower alanine transaminase (ALT) level and a lower white blood cell (WBC) level were associated with a lower PNI level.
Conclusion
Our meta‐analysis indicated that NPC patients with a low PNI level had worse OS, PFS and DMFS, and a low PNI level was associated with female patients, higher tumor stage, a lower ALT level and a lower WBC level. These findings indicate that PNI is a promising prognostic biomarker.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC), an epithelial malignancy of the nasopharynx, is characterized by an extremely uneven global distribution, occurring predominantly in east and Southeast Asia [1]. The incidence of NPC is higher in men than women and was reported to affect 129 thousand people worldwide in 2018 [2, 3]. Various aspects, including host genetics, Epstein-Barr virus (EBV) infection and environmental factors, have been identified as the significant risk factors of NPC [1]. Other potential danger agents are a family history of NPC, poor oral hygiene, and the regular use of tobacco, preserved foods and alcohol [4,5,6,7,8]. Despite advances in the treatment of NPC, the therapeutic effect remains unsatisfactory. Approximately 13.5–35.6% and 19.6–27.6% of patients will suffer from local recurrence and distant metastasis, respectively [9, 10]. NPC is classified based on the tumor-node-metastasis (TNM) staging system, which is employed for guiding treatment strategies, cancer control and predicting patient outcomes. However, the existing staging system has a limited ability for predicting survival or treatment effects, and the outcomes of patients with the same TNM stage are variable. Thus, identifying biomarkers associated with stratification of prognostic risk and therapeutic response and then optimizing treatment choices for the diverse subgroups in this population remains an essential theme for the next decade [1].
The prognostic nutritional index (PNI), as an effective tool to evaluate the nutritional and immunological status of cancer patients, is calculated based on two values: the serum albumin concentration and total lymphocyte count in the peripheral blood [11]. Recently, numerous studies have shown that PNI is a meaningful prognostic marker in patients with various types of malignant tumors such as esophageal cancer [12], colorectal cancer [13], and gynecological cancer [14]. Although emerging evidence has revealed that the pretreatment PNI is related to the long-term survival outcomes of NPC patients [15,16,17,18,19,20,21,22], its clinical prognostic utility has never been comprehensively and systematically reviewed. Therefore, we performed this meta-analysis to explore the prognostic role of the pretreatment PNI and to evaluate the relationship between PNI level and clinicopathological parameters in patients with NPC.
Methods and materials
Search strategy
We conducted this meta-analysis according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [23] and searched Embase, PubMed, Web of Science and the Cochrane Library for eligible studies up to April 2020. The literature was searched by a combination of medical subject heading (MeSH) terms and text words: “Nasopharyngeal Carcinoma”[Mesh], “PNI”, “prognostic nutritional index”. There were language restrictions and only articles published in English were included in the selection process. Furthermore, the search results were supplemented by systematically screening the reference lists of the eligible studies.
Inclusion and exclusion criteria
Inclusion criteria: (1) patients were diagnosed with NPC histopathologically; (2) the PNI was noted before clinical treatment; (3) the association of PNI with overall survival (OS), progression-free survival (PFS), locoregional failure-free survival (LRFFS) and/or distant metastasis-free survival (DMFS) were recorded; (4) available data with 95% confidence intervals (CIs) was provided.
Exclusion criteria: (1) case reports, letters, reviews, conference abstracts and articles published in only abstract form; (2) no adequate data; (3) repeated articles or data.
Quality assessment
In our meta-analysis, the Newcastle–Ottawa quality assessment Scale (NOS) was applied to assess the quality of the eligible reports [24]. In short, each study can have a maximum of 9 points: selection (4 points maximum), comparability (2 points maximum) and outcomes (3 points maximum). We defined articles with a score of 6 or higher as high-quality articles [25]. The quality was assessed by two objective investigators and any discrepancies were debated with a third reviewer to reach consensus.
Data extraction
Using a standardized data collection form, two authors extracted the data independently and any conflicts were resolved after discussion. The following items were extracted from each eligible study: first author, year of publication, region where the study was conducted, sample size, patient characteristics, sex, age (median, range), TNM stage, methods of PNI cut-off determination, PNI cut‐off value, EBV-DNA, EA/IgA titers, VCA/IgA titers, the numbers of low-PNI and high-PNI subjects and follow-up duration. In addition, direct extraction of hazard ratios (HRs) with 95% CIs concerning the prognostic value of PNI in terms of OS, PFS, LRFFS and DMFS; and the odds ratios (ORs) with 95% CIs concerning the association between PNI and clinicopathological features was also performed.
Statistical analysis
Revman 5.3.0 software was applied for the current pooled analysis. For the prognostic role of PNI and other risk factors of OS, PFS, LRFFS and DMFS, the pooled HRs along with 95% CIs were applied. The ORs with 95% CIs were used to estimate the relationship between the pretreatment PNI level and clinical features in NPC patients, such as tumor stage, node stage, TNM stage, sex, age, smoking habits, body mass index (BMI), alanine transaminase (ALT), aspartate transaminase (AST), lactate dehydrogenase (LDH), hemoglobin (HGB), white blood cell count (WBC). Both univariate analysis (UVA) and multivariate analysis (MVA) were used. Heterogeneity among the included trials was assessed by the Cochrane Q test and the I2 test. An I2 value greater than 50% and a P value less than 0.1 for the Q statistic indicated there was significant heterogeneity, so the random‐effects model was used for pooled analysis of the data. When heterogeneity was not evident (P value > 0.1 and I2 value < 50%), a fixed-effects model was applied to estimate the pooled HRs or ORs. Meanwhile, a sensitivity analysis was carried out to check the robustness of the pooled results. Using STATA software (version 15.1; Stata Corporation, College Station, TX, USA), we applied Begg’s test to evaluate the publication bias between the researches. A P value of less than 0.05 (two‐sided) was considered statistically significant.
Results
Retrieval of literature and study characteristics
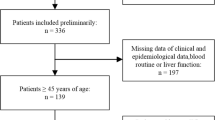
Originally, 110 citations were identified through the various databases. After adjusting for duplicates, 45 remained. Among these, 36 publications were eliminated for various reasons. After full text review of 9 studies, we excluded 1 paper inconsistent with the pre-established criteria. The process of literature selection and detailed identification of the relevant papers is depicted in Fig. 1. A total of eight studies [15,16,17,18,19,20,21,22] published between 2015 and 2020, including 3631 participants, were eventually included in our research after systematic selection. The NOS scores of the included studies were all above 6 points and the baseline characteristics of the eight included studies are presented in Table 1. The median follow-up time ranged from 13 to 109.5 months. For the PNI cut-off determination, six articles [16,17,18,19,20,21] used receiver-operating characteristic (ROC) curve analysis, and two articles [15, 22] defined the median value as the optimal cut-off value. Among the eight studies, seven studies [15, 16, 18,19,20,21,22] reported the prognostic value of PNI for OS, four [15, 19, 21, 22] for PFS, one [20] for LRFFS, and five [15, 17,18,19,20] for DMFS. Moreover, the cut-off value of PNI described in each article was different, ranging between 45.58 and 55, 45.45 and 55, and 45.58–55 for OS, PFS and DMFS, respectively.
PNI and OS
To evaluate the prognostic significance of the pretreatment PNI level in terms of OS, a total of five eligible studies [16, 19,20,21,22] provided relevant data allowing for pooling via UVA, and 6 articles [15, 16, 18,19,20,21] were able to be pooled via MVA. NPC patients with a low PNI experienced worse OS [(HR 2.06, 95% CI 1.61–2.64, P < 0.00001; I2 = 0%, P = 0.81; UVA; Fig. 2) and (HR 1.78, 95% CI 1.46–2.18, P < 0.00001; I2 = 0%, P = 0.45; MVA; Fig. 3)] compared with those with a high PNI. No significant heterogeneity was observed among the studies, supporting the validity of the results. Additionally, the results of the sensitivity analysis showed no individual report exerting a critical impact on the pooled data, also indicating the conclusion is reliable.
PNI and PFS
PFS was mentioned in four reports [15, 19, 21, 22]. NPC patients with a low PNI experienced inferior PFS [(HR 2.27, 95% CI 1.27–4.05, P = 0.006; I2 = 68%, P = 0.05; UVA; Fig. 4) and (HR 1.45, 95% CI 1.18–1.78, P = 0.0003; I2 = 8%, P = 0.35; MVA; Fig. 5)] compared to those with a high PNI. According to the results of sensitivity analysis on MVA, no single trial was found to play a critical role in the results. Of note, we employed random-effects models to calculate the pooled HR because of significant heterogeneity existing for UVA (I2 = 68%, P = 0.05). According to the results of sensitivity analysis on UVA, the trial conducted by Oei et al. [19] was found to have a crucial effect on the result. After omitting the study, the pooled HR was still statistically significant between the low PNI and high PNI groups (HR 3.14, 95% CI 1.91–5.17, P < 0.0001; I2 = 0%, P = 1) and the results showed that a low PNI was likely to predict a shorter PFS.
PNI and DMFS
Five articles [15, 17,18,19,20] with a total of 2812 patients reported DMFS in NPC patients. Since no significant heterogeneity existing [(I2 = 0%, P = 0.71; UVA; Fig. 6) and (I2 = 0%, P = 0.45; MVA; Fig. 7), fixed-effects models were used for the analyses of DMFS. As displayed in Fig. 6 and Fig. 7, the pooled HRs were 2.06 (95% CI 1.60–2.67; P < 0.00001) and 2.04 (95% CI 1.66–2.50; P < 0.00001) on UVA and MVA, respectively. The pooled estimate for DMFS suggested that the pretreatment PNI was obviously related to DMFS and a reduced PNI was significantly associated with a poor DMFS. Furthermore, the results from the sensitivity analysis further certified the robustness of the combined results.
PNI and LRFFS
Only one study [20] with a total of 95 patients reported the LRFFS of NPC patients. No significant difference was found between the two groups [HR 1.68, 95% CI 0.70–4.26, P = 0.26].
PNI and clinical features
The pooled results demonstrated that a higher tumor stage (OR 1.25, 95% CI 1.01–1.54, P = 0.04; I2 = 0%, P = 0.85), a lower ALT level (OR 1.47, 95% CI 1.13–1.93, P = 0.005; I2 = 41%, P = 0.19) and a lower WBC level (OR 1.45, 95% CI 1.11–1.90, P = 0.007; I2 = 0%, P = 0.43) were associated with a lower PNI value. The pooled OR revealed that male NPC patients were associated with a higher PNI (OR 0.75, 95% CI 0.61–0.92, P = 0.007; I2 = 0%, P = 0.67) as well. There was no evidence of significant heterogeneity among the eligible studies. The association between PNI and the clinical features of the NPC patients are shown in Table 2.
However, no statistically significant differences were observed between PNI level and age (OR 1.77, 95% CI 1.00–3.12, P = 0.05; I2 = 79%, P = 0.002), node stage (OR 1.34, 95% CI 0.76–2.37, P = 0.31; I2 = 78%, P = 0.004), TNM stage (OR 1.24, 95% CI 0.64–2.38, P = 0.52; I2 = 58%, P = 0.09), smoking (OR 1.06, 95% CI 0.82–1.38, P = 0.65; I2 = 0%, P = 0.87), BMI (OR 1.29, 95% CI 0.52–3.20, P = 0.59; I2 = 82%, P = 0.02), AST (OR 1.12, 95% CI 0.86–1.46, P = 0.41; I2 = 0%, P = 0.72), LDH (OR 1.02, 95% CI 0.78–1.33, P = 0.90; I2 = 0%, P = 0.33) along with HGB (OR 1.93, 95% CI 0.72–5.20, P = 0.19; I2 = 92%, P < 0.00001). The sensitivity analysis demonstrated the trial conducted by Oei et al. [19] played a main role in the pooled result of age. When this outlier study was excluded, the pooled result was statistically significant (OR 1.46, 95% CI 1.12–1.90, P = 0.005; I2 = 0%, P = 0.49) and showed that advanced age was associated with a reduced pretreatment PNI level. With regard to node stage and TNM stage, the sensitivity analysis showed the report published by Oei et al. [19] exerted a significant impact on these results. When this outlier study was removed, the OR was statistically significant (OR 1.63 95% CI 1.23–2.16, P = 0.0007; I2 = 0%, P = 0.62) and still nonsignificant (OR 1.69, 95% CI 0.94–3.03, P = 0.08; I2 = 0%, P = 0.34), respectively. In the case of HGB, the sensitivity analysis showed that the article conducted by Wei et al. [16] played an important role in the result. After the article was excluded, the pooled result indicated that a lower HGB value was associated with a lower PNI value (OR 3.2, 95% CI 1.22–8.36, P = 0.02; I2 = 87%, P = 0.006).
Other risk factors and OS/PFS/DMFS/LRFFS
We explored whether the OS was influenced by seven risk factors, the PFS by three and the DMFS by four. The pooled results of OS are summarized in Table 3. The pooled results of PFS are summarized in Table 4. The pooled results of DMFS are summarized in Table 5. The OS was notably affected by node stage (HR 1.39, 95% CI 1.02–1.90, P = 0.04; I2 = 28%, P = 0.24; UVA) and ALB (HR 2.65, 95% CI 1.35–5.21, P = 0.005; I2 = 0%, P = 0.34; UVA). The PFS was remarkably affected by tumor stage [(HR 1.53; 95% CI 1.14–2.04, P = 0.004; I2 = 0%, P = 0.92; UVA) and (HR 1.45; 95% CI 1.16–1.82, P = 0.001; I2 = 0%, P = 0.79; MVA)], age [(HR 1.72; 95% CI 1.28–2.32, P = 0.0004; I2 = 0%, P = 0.61; UVA) and (HR 1.62; 95% CI 1.19–2.21, P = 0.002; I2 = 0%, P = 0.57; MVA)]. Moreover, our results indicated that DMFS was associated with sex (HR 1.36, 95% CI 1.02–1.83, P = 0.04; I2 = 0%, P = 0.44; UVA), and tumor stage (HR 1.71, 95% CI 1.13–2.59, P = 0.01; I2 = 0%, P = 0.45; UVA). Additionally, only one study [20] reported the LRFFS for NPC patients, and thus, a pooled analysis of the relationship between risk factors and LRFFS could not be carried out.
Publication analysis
We accessed the publication bias among the studies by the Begg funnel plot, and the results showed that there was no significant publication bias among studies (Online Resource). Accordingly, this showed that the outcomes of our study were statistically robust.
Discussion
Owing to the limitations of the current TNM staging system, ascertaining predictive biomarkers of survival outcomes of NPC patients is an essential theme that remains to be further studied [1, 18]. The prognostic value of pretreatment PNI has been identified by a series of studies [12,13,14]. However, for NPC patients, the prognostic value has never been systematically investigated. To our knowledge, this report is the first one to shed light on the prognostic utility of PNI and to explore the association between PNI level and clinical characteristics in patients with NPC.
In our analysis, a conclusion could be safely reached that a low PNI level was significantly associated with a shorter OS [(HR 2.06, 95% CI 1.61–2.64, P < 0.00001; UVA) and (HR 1.78, 95% CI 1.46–2.18, P < 0.00001; MVA)], PFS [(HR 2.27, 95% CI 1.27–4.05, P = 0.006; UVA) and (HR 1.45, 95% CI 1.18–1.78, P = 0.0003; MVA)] and DMFS [(HR 2.06, 95% CI 1.60–2.67; P < 0.00001; UVA) and (HR 2.04, 95% CI 1.66–2.50; P < 0.00001; MVA)]. However, only one study [20] reported the LRFFS of NPC patients. No significant difference was found between the two groups [HR 1.68, 95% CI 0.70–4.26, P = 0.26].
There are several possible mechanisms to explain how a low PNI level is related to impaired OS, PFS and DMFS in NPC. As far as we know, PNI was originally proposed as a nutritional index and surgical risk indicator in 1980 [26], and then the value of PNI in predicting the surgical risk for patients with gastrointestinal cancer was revealed in 1984 [11]. PNI is calculated based on two values: 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count [11]. Hence, both hypoalbuminemia and/or lymphocytopenia can lead to a low PNI value. First, we need to analyze the aspects of albumin deficiency. On the one hand, nutritional problems are associated with worse survival outcomes and a reduced quality of life (QoL) of NPC patients has been confirmed by previous reports [18, 27,28,29]. The serum albumin level is one of the indicators used to evaluate the nutritional status of cancer patients [30] and it is positively correlated with nutritional status [22]. Therefore, a lower level of serum albumin directly reflects the malnutritional status of tumor patients. Increasing numbers of studies have demonstrated that malnutrition is a prevalent condition in tumor patients and the incidence ranges from 39 to 71% [31,32,33]. In patients with head and neck malignancies, undernutrition has been estimated in 30–50% [34]. In addition, concurrent chemoradiotherapy (CCRT) is considered the mainstay therapy in patients with locally advanced nasopharyngeal carcinoma (LA-NPC) [1], and malnutrition in patients with NPC is further worsened by unhealthy habits [35] and the toxic effects of chemoradiotherapy (CRT), such as fatigue, advanced mucositis, and gastrointestinal reactions, such as vomiting and nausea [36, 37]. In turn, poor nutritional status can increase the CRT adverse effects and decrease survival [38, 39]. According to numerous studies [40,41,42,43], malnutrition before and during treatment has been identified as a risk factor predicting worse outcomes in head and neck cancer and NPC patients due to the severity of acute toxicities, decreased chemotherapy dose intensity, treatment interruption, reduced radio-sensitivity and/or chemosensitivity of the tumor and compromised immunity. Although no delayed or interrupted radiotherapy or chemotherapy dose intensity decreases occurred, the researchers believe that in the patients with a low PNI level, by reducing patients radio-sensitivity and/or chemical sensitivity, and compromising their immunity, a deterioration of nutritional status during chemoradiation eventually affects the therapeutic efficacy and leads to a decline in the survival rate [18]. On the other hand, inflammation plays an important role in the occurrence and progression of malignant tumors [22], such as proliferation and survival of tumor cells, angiogenesis, tumor metastasis, and desensitization to anticancer drugs [44, 45]. Linked by the intrinsic and extrinsic pathways, the connection between inflammation and cancer can be explained well [45, 46]. Serum albumin is considered to be a marker of systemic inflammation [22] and it is associated with systemic inflammation along with high levels of pro-inflammatory cytokines [15]. Consequently, a low albumin level represents a worse prognosis [22, 38]. Second, we need to understand the phenomenon of low lymphocytes. Lymphocyte play a key role in the human immune system and tumor immune escape system. Previous studies have shown that infiltrating lymphocytes represent a tangible antitumor cellular immune response and a lymphocyte activated host immune response can help clear tumor cells or inhibit tumor cell growth [47]. What is more, lymphocytopenia has also been described as related to a decreased chemotherapeutic efficacy in cancer patients [48]. Thus, lymphocytopenia may predict an inferior prognosis or a higher mortality [38, 49, 50]. Taken together, PNI might predict the outcomes of NPC patients by quantifying the nutritional, systemic inflammatory response and immune condition of each patient.
Given its convenience, credibility and simplicity of acquisition before treatment in clinical practice and its significant value in predicting patient outcomes, PNI is a promising prognostic biomarker. For patients with a low PNI level, it may be vital to conduct early nutritional interventions and individualize proper treatment approaches.
Additionally, our pooled results indicated that female NPC patients, a higher tumor stage, a lower ALT level and a lower WBC level were associated with a lower PNI level. No statistically significant associations were observed between PNI level and age, node stage, TNM stage, smoking habits, BMI, AST, LDH or HGB. The sensitivity analysis demonstrated one trial [19] played a major role in the pooled result of age. When that study was excluded, the extensive consistency and aggregated results of the remaining reports clearly provided trustworthy evidence that advanced age was associated with a reduced PNI level. Considering the decline of their physical functions, Xue et al. [12] thought that elderly patients were more likely to suffer a low PNI during the development of malignancy. Thus, elderly patients with a low PNI may also be affected by this phenomenon. With regard to node stage, there was a tendency that a higher node stage was related to a lower PNI (OR 1.34, 95% CI 0.76–2.37, P = 0.31; I2 = 78%, P = 0.004), but there was no significant difference. According to the sensitivity analysis, we further found that the study of Oei et al. [19], was the source of statistical heterogeneity. When the study was removed, the OR was statistically significant (OR 1.63 95% CI 1.23–2.16, P = 0.0007; I2 = 0%, P = 0.62). We speculated the reason for this might be the balanced distribution of the number of patients with advanced node stage and lower node stage in the sets of low PNI and high PNI in this trial. Regarding HGB, the sensitivity analysis showed the negative result relied heavily on the study conducted by Wei et al. [16]. After omitting this trial, the pooled result was significantly different. We speculated the reason may be that the participants with a higher TNM stage in this trial [16] differed from the others, which might influence the final pooled outcome. Regarding sex, female NPC patients were associated with a lower PNI level. There be several reasons for this. First, a recent study has demonstrated that women are more likely to experience high weight loss (weight loss ≥ 10%) than male patients [15]. In addition, growing numbers of studies have revealed that weight loss is not just a marker reflecting a reduced intake or nutritional imbalance but also an indicator of a systemic inflammatory response [45, 51]. Finally, as mentioned earlier, PNI can predict the outcomes of patients with NPC by quantifying their nutritional, systemic inflammatory response and immune status. Therefore, the physical condition of patients, such as their poor nutrition, can be reflected not only by weight loss but also by PNI. Further, our meta‐analysis indicated that NPC patients with a reduced PNI level had inferior OS, PFS and DMFS, and a lower PNI level was associated with female patients. We hypothesized that female patients with NPC tended to suffer from anxiety before treatment, had no active healthy coping style, and experienced a reduced quality of life after radiotherapy [52], which may account for their poor survival outcome. Moreover, we also discovered tumor stage was notably associated with PFS and DMFS but not OS. PNI was associated with tumor‐infiltrating lymphocytes (TILs) status [53] and higher TILs are relevant to a lower tumor stage and a better outcome [54, 55]. As mentioned above, a lower PNI was a sign of malnutrition and dysfunction of the immune condition of the host. Based on the above evidence, this may explain why a higher tumor stage was associated with a low PNI and significantly affected the survival outcomes.
There are several potential shortcomings in our study. First, all of the included eligible reports were retrospective cohort studies and potential heterogeneity might cause bias in our analyses. Additional prospective clinical studies are required to support our conclusions. Second, in view of the included articles being mainly from Asian countries, and only one from Turkey, we expect more trials, especially from European and American countries, can further explore the prognostic utility of PNI in NPC patients. Third, only one study [20] mentioned LRFFS in our review, which might also generate some bias, and thus additional studies are needed. Fourth, given that various treatment regimens were used in the 8 included studies, and the limited number of eligible trials included in our study, subgroup analyses could not be conducted according to different treatment methods. RCTs and prospective trials with more participants are needed to confirm these results in the future. Fifthly, EBV is closely associated with NPC, but the relationship between EBV and PNI has not been explored and analyzed in a large number of studies. Therefore, we regret that we failed to conduct a meta-analysis on the correlation between EBV and PNI. Last but not least, although all of the studies were divided into two sets according to the cut-off pretreatment PNI level, the PNI cut-off value varied between 45.58 and 55, 45.45 and 55, and 45.58–55 for OS, PFS and DMFS, respectively. Based on the current evidence, which cut-off value is optimal remains unclear and comprehensive research, which can facilitate this biomarker being widely used in the clinic, is essential.
Conclusion
In summary, our meta‐analysis demonstrated that NPC patients with a lower PNI level had inferior OS, PFS and DMFS. In addition, we found that a lower PNI level was associated with female NPC patients, a higher tumor stage, a lower ALT level and a lower WBC level. Given the limitations of our study, further prospective studies are required to validate the clinical significance of PNI in NPC patients and to determine the best cut-off value and to consequently guide clinicians in predicting the outcomes of different risk subgroups and providing comprehensive individualized treatment approaches to improve survival outcomes.
References
Chen YP, Chan ATC, Le QT et al (2019) Nasopharyngeal carcinoma. Lancet (London, England) 394(10192):64–80
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Ferlay J, Colombet M, Soerjomataram I et al (2018) Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 103:356–387
Guo X, Johnson RC, Deng H et al (2009) Evaluation of nonviral risk factors for nasopharyngeal carcinoma in a high-risk population of Southern China. Int J Cancer 124(12):2942–2947
Tsao SW, Yip YL, Tsang CM et al (2014) Etiological factors of nasopharyngeal carcinoma. Oral Oncol 50(5):330–338
Liu Z, Chang ET, Liu Q et al (2016) Oral Hygiene and Risk of Nasopharyngeal Carcinoma-A Population-Based Case-Control Study in China. Cancer Epidemiol Biomarkers Prev 25(8):1201–1207
Chang ET, Liu Z, Hildesheim A et al (2017) Active and Passive Smoking and Risk of Nasopharyngeal Carcinoma: A Population-Based Case-Control Study in Southern China. Am J Epidemiol 185(12):1272–1280
Liu Z, Chang ET, Liu Q et al (2017) Quantification of familial risk of nasopharyngeal carcinoma in a high-incidence area. Cancer 123(14):2716–2725
Peng G, Wang T, Yang KY et al (2012) A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 104(3):286–293
Zhang MX, Li J, Shen GP et al (2015) Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: A 10-year experience with a large cohort and long follow-up. Eur J Cancer 51(17):2587–2595
Onodera T, Goseki N, Kosaki G (1984) Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai zasshi 85(9):1001–1005
Xue Y, Zhou X, Xue L et al (2019) The role of pretreatment prognostic nutritional index in esophageal cancer: A meta-analysis. J Cell Physiol 234(11):19655–19662
Sun G, Li Y, Peng Y et al (2019) Impact of the preoperative prognostic nutritional index on postoperative and survival outcomes in colorectal cancer patients who underwent primary tumor resection: a systematic review and meta-analysis. Int J Colorectal Dis 34(4):681–689
Wang X, Wang Y (2019) The prognostic nutritional index is prognostic factor of gynecological. cancer: A systematic review and meta-analysis. Int J Surg 67:79–86
Du XJ, Tang LL, Mao YP et al (2015) Value of the prognostic nutritional index and weight loss in predicting metastasis and long-term mortality in nasopharyngeal carcinoma. J Transl Med 13:364
Wei GB, Lu YY, Liao RW et al (2016) Prognostic nutritional index predicts prognosis in patients. with metastatic nasopharyngeal carcinoma. Onco Targets Ther 9:5955–5961
Yang L, Xia L, Wang Y et al (2016) Low Prognostic Nutritional Index (PNI) Predicts Unfavorable. Distant Metastasis-Free Survival in Nasopharyngeal Carcinoma: A Propensity Score-Matched Analysis. PLoS ONE 11(7):e0158853
Miao J, Xiao W, Wang L et al (2017) The value of the Prognostic Nutritional Index (PNI) in predicting outcomes and guiding the treatment strategy of nasopharyngeal carcinoma (NPC) patients receiving intensity-modulated radiotherapy (IMRT) with or without chemotherapy. J Cancer Res Clin Oncol 143(7):1263–1273
Oei RW, Ye L, Kong F et al (2018) (2018) Prognostic value of inflammation-based prognostic index in patients with nasopharyngeal carcinoma: a propensity score matching study. Cancer Manag Res 10:2785–2797
Gundog M, Basaran H (2019) Pretreatment low prognostic nutritional index and low albumin-globulin ratio are predictive for overall survival in nasopharyngeal cancer. Eur Arch Otorhinolaryngol 276(11):3221–3230
He Q, Huang YC, Wan G et al (2019) A novel prognostic marker based on risk stratification with prognostic nutritional index and age for nasopharyngeal carcinoma patients who received neoadjuvant chemotherapy. Biomark Med 13(12):1013–1023
Zeng X, Liu G, Pan Y et al (2020) Prognostic Value of Clinical Biochemistry-Based Indexes in. Nasopharyngeal Carcinoma Front Oncol 10:146
Downing PA, Griffin AS, Cornwallis CK et al (2020) The Benefits of Help in Cooperative Birds: Nonexistent or Difficult to Detect? Am Nat 195(6):1085–1091
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Yang Y, Gao P, Song Y et al (2016) The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur J Surg Oncol 42(8):1176–1182
Buzby GP, Mullen JL, Matthews DC et al (1980) Prognostic nutritional index in gastrointestinal surgery. Am J Surg 139(1):160–167
Li G, Gao J, Liu ZG et al (2014) Influence of pretreatment ideal body weight percentile and albumin on prognosis of nasopharyngeal carcinoma: Long-term outcomes of 512 patients from a single institution. Head Neck 36(5):660–666
Hong JS, Hua YJL, Su L et al (2017) Modified-Nutrition Index is a Significant Prognostic Factor for the Overall Survival of the Nasopharyngeal Carcinoma Patients who Undergo Intensity-modulated Radiotherapy. Nutr Cancer 69(7):1011–1018
He Y, Chen L, Chen L et al (2017) Relationship Between the Comprehensive Nutritional Index and the EORTC QLQ-H&N35 in Nasopharyngeal Carcinoma Patients Treated with Intensity-Modulated Radiation Therapy. Nutr Cancer 69(3):436–443
Von Meyenfeldt M (2005) Cancer-associated malnutrition: an introduction. Eur J Oncol Nurs 9:S35–S38
Silva de Melo FR, de Oliveira MGOA, Souza ASR et al (2015) Factors associated with malnutrition in hospitalized cancer patients: a croos-sectional study. Nutr J 14:123
Arends J (2017) Nutrition in Cancer: Effective in Prevention and Treatment? Dtsch Med Wochenschr 142(12):889–895
Gyan E, Raynard B, Durand JP et al (2018) Malnutrition in Patients With Cancer: Comparison of Perceptions by Patients, Relatives, and Physicians-Results of the NutriCancer2012 Study. JPEN J Parenter Enteral Nutr 42(1):255–260
Kono T, Sakamoto K, Shinden S et al (2017) Pre-therapeutic nutritional assessment for predicting severe adverse events in patients with head and neck cancer treated by radiotherapy. Clin Nutr 36:1681–1685
Nam JM, McLaughlin JK, Blot WJ et al (1992) Cigarette smoking, alcohol, and nasopharyngeal carcinoma: a case-control study among US whites. J Natl Cancer Inst 84(8):619–622
Bossola M (2015) Nutritional interventions in head and neck cancer patients undergoing chemoradiotherapy: a narrative review. Nutrients 7(1):265–276
Yilmaz M, Atilla FD, Uluer H et al (2018) Effect of malnutrition on mortality with new espen criteria in patients with hematological malignancies. Clin Nutr 37(Supl. 1):S197–S198
Gupta D, Lis CG (2010) Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 9:69
Mantzorou M, Koutelidakis A, Theocharis S et al (2017) Clinical Value of Nutritional Status in Cancer: What is its Impact and how it Affects Disease Progression and Prognosis? Nutr Cancer 69(8):1151–1176
Qiu C, Yang N, Tian G et al (2011) Weight loss during radiotherapy for nasopharyngeal carcinoma: a prospective study from Northern China. Nutr Cancer 63:873–879
Ravasco P, Grillo IM, Vidal PM et al (2005) Impact of nutrition on outcome: a prospective. randomised controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 27:659–668
Capuano G, Grosso A, Gentile PC et al (2008) Influence of weight loss on outcomes in patients with head and neck cancer undergoing concurrent chemoradiotherapy. Head Neck 30(4):503–508
Shen LJ, Chen C, Li BF et al (2013) High weight loss during radiation treatment changes the prognosis in under-/ normal weight nasopharyngeal carcinoma patients for the worse: a retrospective analysis of 2433 cases. PLoS ONE 8(7):e68660
Diakos CI, Charles KA, McMillan DC et al (2014) Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15(11):e493–e503
Mantovani A, Allavena P, Sica A et al (2008) Cancer-related inflammation. Nature 454(7203):436–444
Candido J, Hagemann T (2013) Cancer-related inflammation. J Clin Immunol 33(Suppl 1):S79–S84
Do H, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Ceze N, Thibault G, Goujon G et al (2011) Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol 68(5):1305–1313
Ray-Coquard I, Cropet C, Van GM et al (2009) Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 69(13):5383–5391
He JR, Shen GP, Ren ZF et al (2012) Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head Neck 34(12):1769–1776
Muliawati Y, Haroen H, Rotty LW (2012) Cancer anorexia-cachexia syndrome. Acta Med Indones 44(2):154–162
Hong J, Tian J, Zhang W et al (2013) Patient characteristics as indicators for poor quality of life after radiotherapy in advanced nasopharyngeal cancer. Head Neck Oncol 5(2):17
Okadome K, Baba Y, Baba H (2019) Response to Comment on "Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients With Esophageal Cancer". Ann Surg 270(6):e105–e106
Gooden MJM, de Bock GH, Leffers N et al (2011) The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 105(1):93–103
De Ruiter EJ, Ooft ML, Devriese LA et al (2017) The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology 6(11):e1356148
Funding
Not applied.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by, Min Tang, Zhongxiong Jia and Ju Zhang. The first draft of the manuscript was written by Min Tang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethics approval
Not applied.
Consent to participate
Not applied.
Consent for publication
Not applied.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (TIF 60 kb)
Funnel plot of the hazard ratio of overall survival (OS) (UVA) (Beggʼs test, P = 0.221)
Supplementary file2 (TIF 60 kb)
Funnel plot of the hazard ratio of overall survival (OS) (MVA) (Beggʼs test, P = 0.060)
Supplementary file3 (TIF 62 kb)
Funnel plot of the hazard ratio of progression-free survival (PFS) (UVA) (Beggʼs test, P = 0.602)
Supplementary file4 (TIF 62 kb)
Funnel plot of the hazard ratio of progression-free survival (PFS) (MVA) (Beggʼs test, P = 0.734)
Supplementary file5 (TIF 59 kb)
Funnel plot of the hazard ratio of distant metastasis-free survival (DMFS) (UVA) (Beggʼs test, P = 1.000)
Supplementary file6 (TIF 59 kb)
Funnel plot of the hazard ratio of distant metastasis-free survival (DMFS) (MVA) (Beggʼs test, P =1.000)
About this article
Cite this article
Tang, M., Jia, Z. & Zhang, J. The prognostic role of prognostic nutritional index in nasopharyngeal carcinoma: A systematic review and meta-analysis. Int J Clin Oncol 26, 66–77 (2021). https://doi.org/10.1007/s10147-020-01791-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01791-x