Abstract
Purpose
We aimed to explore whether the preoperative prognostic nutritional index (PNI) could be an indicator of prognostic outcomes in colorectal cancer (CRC) patients.
Methods
A systematic review and meta-analysis was conducted using the PubMed, Embase, and Web of Science databases. All original comparative studies published in English that were related to a high PNI versus a low PNI in CRC patients were included.
Results
A total of 10 studies involving 6372 patients were included in our meta-analysis. Our overall analysis indicated that the low-PNI group had a significantly reduced overall survival (OS) (HR = 1.87, 95% CI = 1.45–2.42, P < 0.01), cancer-specific survival (HR = 1.53, 95% CI = 1.07–2.19, P = 0.02), and disease-free survival (HR = 1.67, 95% CI = 1.23–2.26, P < 0.01) compared with the high-PNI group. Furthermore, our subgroup results indicated that a high PNI could be a significant indicator of improved OS in TNM stage II (HR = 1.93, 95% CI = 1.29–2.90, P < 0.01) and III (HR = 1.71, 95% CI = 1.25–2.34, P < 0.01), and a similar trend in TNM stage I or IV could also be observed though without statistical significance. Regarding postoperative complications, our pooled results indicated that the low-PNI group had a significantly increased incidence of total and severe postoperative complications.
Conclusions
Our findings indicated that CRC patients with a preoperative high PNI had a significantly improved OS. However, almost only Asian CRC patients were included based on current issue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies worldwide and is characterized by high morbidity and mortality [1]. Curative surgical resection remains the main method for resectable localized CRC based on the clinical guidelines [2]. However, the prognosis of patients differs even in patients with the same pathological stage. Moreover, approximately 30% of patients may still suffer from serious postoperative complications [3,4,5,6]. Hence, identifying the subgroup population that can benefit more from colorectal resection remains an urgent issue for further exploration. Novel biomarkers, especially preoperative host-related factors, are necessary to predict poor surgical and oncological outcomes of CRC.
The prognostic nutritional index (PNI) is calculated based on the serum albumin concentration and total lymphocyte count. The PNI was calculated according to the following formula from the report of Onodera et al.: 10 × albumin (g/dl) + 0.005 × total lymphocyte count (per mm3) [7]. Numerous studies have demonstrated that PNI is a significant indicator of postoperative complications and survival in various cancer patients (gastric cancer [8], breast cancer [9], CRC [10], lung cancer [11], esophageal cancer [12], and ovarian cancer [13]). Although past investigations have shown important findings related to PNI, its clinical significance has not yet been systematically discussed. In terms of PNI in CRC patients, studies have mainly followed a retrospective design with small sample sizes. In addition, the optimal cutoff value and prognosis in subgroup populations based on TNM stage need to be studied further. There is an urgent need to collect and update the current evidence on this issue for clinical application.
Based on the aforementioned findings, the aim of our study was to comprehensively explore the predictive value of PNI on postoperative and survival outcomes in CRC patients who underwent primary tumor resection through meta-analysis.
Methods
Search strategy
We conducted our systematic review and meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (http://www.prisma-statement.org/). Our search was restricted to the English language based on the following MeSH/main keywords: “prognostic nutritional index,” “PNI,” “colorectal,” “rectal,” “colonic,” “colon,” and “rectum” using datasets from PubMed, Embase, and the Web of Science (up to October 2018). The detailed search strategy for the PubMed dataset was as follows: (colorectal [All Fields] OR rectal [All Fields] OR colonic [All Fields] OR colon [All Fields] OR rectum [All Fields]) AND (cancer [All Fields] OR carcinoma [All Fields] OR neoplasms [All Fields]) AND (“PNI” [All Fields] OR “prognostic nutritional index” [All Fields]). To avoid redundant studies, we checked all authors and organizations and evaluated the recruitment period and population of patients enrolled in each study. In addition, the lists of references in the relevant studies were also screened for additional studies.
Inclusion and exclusion criteria
According to the PICOS criteria (population, intervention, comparison, outcomes, and study design), studies were selected for our present meta-analysis according to the following eligibility criteria: (1) population: patients with CRC; (2) intervention: patients who underwent primary tumor resection (curative-intent resection); (3) comparison: low-PNI versus high-PNI CRC patients; (4) outcomes: primary outcome: overall survival (OS), secondary outcomes: disease-free survival (DFS), cancer-specific survival (CSS), and postoperative complications; OS was defined as death due to any cause, DFS was defined as disease recurrence or death, and CSS was defined as death due to cancer, and serious complications were defined based on the Clavien-Dindo classification [14]: grade II and higher were regarded as having complications, and grade III or IV was regarded as serious; and (5) study design: comparative studies (retrospective and prospective studies).
In addition, the exclusion criteria were (1) population: patients with unresectable metastatic CRC; (2) intervention: patients who did not undergo primary tumor surgical resection; (3) comparison: more than two groups; (4) outcomes: no data on the primary outcome of OS; and (5) study design: single-arm without comparison.
Data extraction and quality assessment of included studies
Two reviewers (Guangwei Sun and Yalun Li) reviewed and assessed each of the included studies independently, and the following information was collected: first author, country, year period, study population, number of patients enrolled, age, male/female percentage, cancer stage, and cutoff value of PNI. In addition, extraction of postoperative and survival outcome data was also performed by the two reviewers independently. Moreover, the Newcastle–Ottawa Scale (NOS) criteria were used to evaluate the quality of the studies included [15]. All disagreements in terms of the aforementioned studies were resolved by discussion between the two reviewers (Guangwei Sun and Yalun Li).
Statistical analysis
In our systematic review and meta-analysis, the most appropriate statistic for evaluating survival outcomes (time-to-event outcomes) was the hazard ratio (HR) derived from multivariate analyses of the included studies. If studies did not provide the HR directly, we obtained an estimated HR by the methods designed by Tierney [16]. In addition, we pooled the odds ratios (ORs) derived from the multivariate analyses of the postoperative complications. All analyses were performed using Stata software, version 12.0 (2011; Stata Corp., College Station, TX, USA). All the analyses in this study used a random-effects model because it provided more conservative estimates and was tailored to multicenter studies in which heterogeneity was usually present [17]. All statistical values were reported with the 95% confidence interval (CI), and a two-tailed P value less than 0.05 was defined as significant. Finally, publication bias was assessed using Begg’s and Egger’s tests based on the primary outcome of OS [18, 19].
Results
Selected studies
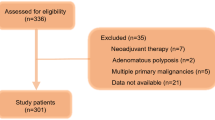
Based on our search strategy, a total of 850 published studies were identified. After removing the duplicates and screening the title and abstract, 15 studies concerning PNI in CRC patients who underwent primary tumor resection were eligible for our further evaluation. Among these 15 studies, four studies [20,21,22,23] enrolled an overlapped population that had been included in their other investigations based on the same center, and one study [24] divided patients into four groups without a single cutoff value for PNI. Hence, 10 studies involving 6372 patients that met our inclusion and exclusion criteria were included in our systematic review and meta-analysis [10, 25,26,27,28,29,30,31,32,33]. A flow chart of the search strategy, which includes the reasons for exclusion of studies, is illustrated in Fig. 1. Among our included studies, 9 studies [10, 26,27,28,29,30,31,32,33] were conducted in Asian countries (China, Korea, and Japan), and all the included studies followed a retrospective design. The cutoff value for PNIs ranged from 35 to 49.22; three studies [27, 28, 33] set 45 as the cutoff value and two studies used 45.5 [10, 30]. In addition, 9 studies had an NOS score ≥ 5 [10, 26,27,28,29,30,31,32,33]. The detailed information of our included studies is shown in Table 1.
Overall analyses of survival outcomes
All the included studies provided OS data, and our overall analysis indicated that the low-PNI group had a significantly shorter OS than the high-PNI group (HR = 1.87, 95% CI = 1.45–2.42, P < 0.01) (Fig. 2). In addition, Yang et al. [33] and Tokunaga et al. [10] provided data on CSS, and Park et al. [30] and Peng et al. [31] provided data on DFS. Our pooled analysis also indicated that patients with high PNI showed significantly improved CSS (HR = 1.53, 95% CI = 1.07–2.19, P = 0.02) and DFS (HR = 1.67, 95% CI = 1.23–2.26, P < 0.01) (Supplementary Fig. S1). Moreover, we did not observe publication bias in terms of OS by using Begg’s and Egger’s tests (Begg’s test, P = 0.25; Egger’s test, P = 0.08).
Subgroup analyses of OS based on pathological stage
Six included studies [10, 26, 27, 30, 31, 33] provided subgroup data of OS between two groups. Hence, we conducted subgroup analyses based on the pathological TNM stage (I = 602, II = 2244, III = 1474, IV = 259) to provide a comprehensive evaluation of the prognostic value of PNI. Our results indicated that high PNI could be a significant indicator of improved OS in CRC patients with TNM stage II (HR = 1.93, 95%CI = 1.29-2.90, P < 0.01) and III (HR = 1.71, 95% CI = 1.25–2.34, P < 0.01) cancer who had undergone surgical resection. For TNM stage I (HR = 1.88, 95% CI = 0.95–3.71, P = 0.07) and IV (HR = 1.19, 95% CI = 0.66–2.12, P = 0.57) patients, the high-PNI group also showed a similar trend of improved OS when compared with low-PNI patients, though this difference was not statistically significant. The detailed results of subgroup analyses are shown in Fig. 3.
Association of PNI and postoperative complications
Two studies [26, 28] provided data on total postoperative complications, and the pooled results showed that the low-PNI group had a significantly increased incidence of postoperative complications compared with the high-PNI group (OR = 1.94, 95% CI = 1.29–2.92, P < 0.01). In terms of severe postoperative complications, Mohri et al. [28] indicated that 41 patients (11.2%) had serious postoperative complications in their cohort. Serious complications included anastomotic leakage in 22 patients, severe infection in 19, bowel obstruction requiring further surgery in 7, severe cardiopulmonary failure in one, and pulmonary embolism in one. Furthermore, Cao et al. [26] demonstrated that 24 patients (10.5%) had serious postoperative complications, including bleeding in 2 patients, anastomotic leakage in 6 patients, serious infection in 13 patients, bowel obstruction in 1 patient, and pulmonary embolism in 1 patient. In addition, Tokunaga et al. [10] showed that 85 patients (15.3%) had serious postoperative complications but did not provide detailed information on the individuals. All three studies [10, 26, 28] demonstrated that PNI was an independent factor associated with the incidence of severe postoperative complications in multivariate analysis, and our pooled results also indicated that the low-PNI group had a significantly increased incidence of serious postoperative complications (OR = 2.27, 95% CI = 1.53–3.38, P < 0.01) (Fig. 4). Furthermore, in the subgroup analyses, Tokunaga et al. [10] indicated that the rate of serious postoperative complications in patients with stage II cancer was significantly higher in the low-PNI group (P < 0.01), but the same association was not seen in other stages of cancer. However, the subgroup analyses in study by Cao et al. [26] did not demonstrate a significant association of the incidence of serious postoperative complications in patients with stage I, II, and III cancers between the low-PNI and high-PNI groups.
Discussion
Surgical resection is still the main treatment method for resectable localized CRC; however, the subgroup population that would benefit most from surgery remains unclear. Hence, exploring preoperative predictive factors of postoperative outcomes is an important and urgent topic that remains to be further studied. The PNI, a simple and useful systemic inflammation-based prognostic score, is calculated based on laboratory assessments of total lymphocyte count and serum albumin level [7] and can reflect the pretreated host’s immunological and nutritional status [27]. However, regarding CRC, there is still no uniform consensus or comprehensive evidence concerning whether PNI could be a prognostic indicator in CRC patients. Hence, based on the aforementioned findings, we conducted this meta-analysis to explore whether preoperative PNI could be a predictive factor of postoperative and survival outcomes in CRC patients who underwent primary tumor resection.
Our study found that CRC patients with high preoperative PNI showed significantly improved OS compared to the low-PNI group. Meanwhile, in terms of subgroup analyses based on pathological TNM stage, CRC patients with TNM stage II and III cancers also showed significant differences in OS between the high- and low-PNI groups. In addition, preoperative low-PNI CRC patients demonstrated an increased incidence of total and serious postoperative complications compared to that of high-PNI patients. Our findings demonstrated that preoperative PNI could be a predictive factor of prognosis in CRC patients who underwent surgical resection, especially for locally advanced (pathological TNM stage II and III) cancers.
Regarding our overall analyses of OS, there are some potential explanations for the association between low PNI and impaired OS in CRC patients who underwent surgical resection. First, lymphocytes and serum albumin are significantly associated with the prognosis of CRC patients based on current evidence [34,35,36,37]. Hence, PNI calculated based on the lymphocytes and serum albumin could reflect the prognosis of CRC patients. In addition, our previous investigation also indicated that sarcopenia could be an indicator of prognosis in non-metastatic CRC patients [38]; therefore, PNI that reflects the nutritional and immune condition of patients might also influence the prognosis of patients. Moreover, previous investigations [10, 27, 33] indicated that low-PNI status was correlated with older ages and aggressive clinicopathological features, which led to a worse OS for CRC patients. In terms of the results of the subgroup analyses based on the pathological stage, we noted that the predictive value of PNI was significant in TNM stage II and III patients. Furthermore, a similar tendency was also observed in TNM stage I and IV patients, although without statistical significance. Previous evidence [39,40,41] also found that preoperative PNI could be a prognostic factor in unresectable metastatic CRC patients. Hence, the effect of PNI in TNM stage IV patients needs to be further studied based on large-scale individual data.
The present results suggested that postoperative complications occurred more frequently in the low-PNI group than in the high-PNI group. In addition, Tokunaga et al. [10] indicated that the rate of serious postoperative complications in patients with stage II cancer, but not other stages, was significantly higher in the low-PNI group (P < 0.01). This might be explained by the theory that the inflammatory response and malnutrition are important factors that contribute to postoperative complications, especially severe complications [26, 42]. Past reports have also shown that low albumin and lymphocyte levels are closely related to the development of an inflammatory response in CRC patients [43, 44]. Hence, PNI could be an indicator of postoperative complications, especially severe complications, for CRC patients who have undergone surgical resection.
To date, there is still no consensus on the uniform cutoff value for PNI for clinical applications. In our included studies, the cutoff value for PNIs ranged from 35 to 49.22; three studies [27, 28, 33] set 45 as the cutoff value, and two studies used 45.5 [10, 30]. In addition, the majority of the studies on this topic were conducted in Asian countries; hence, the reported cutoff values for PNI might be applicable in only Asian populations. Hence, we expect that more clinicians from European and American countries can focus on and further explore the significance of PNI in CRC patients. Based on the results of the current study, we could not determine the optimal cutoff value for PNI based on current evidence. Individual data and pooled analysis are needed to update our findings and determine the cutoff value for PNI for clinical practice.
There were some limitations in our current study. First, all the included studies were retrospective cohort studies. Hence, heterogeneities in patient selection might cause bias in our overall analyses. For example, Shibutani et al. [32] and Cao et al. [26] enrolled more elderly patients in the low-PNI groups. In addition, 9 of the 10 included studies were conducted in Asian countries; to the best of our knowledge, there is no related study of CRC published from European and American countries. Hence, our findings might be applicable only to Asian CRC patients. Furthermore, except of study by Akgul et al. [25] and Peng et al. [31] did not provide the number of tumor location (colon or rectal), other eight included studies had involved a total of 3234 colonic and 2676 rectal cancer patients. However, no included studies had provided the data of subgroup analyses based on the primary location of tumors (rectum or colon) between high-PNI and low-PNI groups. The prognosis was different based on the primary tumor location in CRC patients [45]; therefore, the mixed baseline information might restrict our further exploration of the significance of PNI in rectal and colon cancer.
Conclusion
Our findings indicated that CRC patients with preoperative high-PNI showed significantly improved OS and decreased postoperative complications when compared to the low-PNI group. Moreover, in terms of subgroup analyses based on pathological TNM stage, CRC patients with TNM stage II and III cancers also showed significant differences in OS between the high- and low-PNI groups. Hence, preoperative PNI could be a predictive factor of prognosis in CRC patients who underwent surgical resection, especially for patients with locally advanced cancers. However, individual data are needed to further explore the optimal cutoff value for PNI for clinical applications in CRC patients.
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Benson AB III, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Gurski L, Freedman-Cass DA (2018) Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 16(7):874–901. https://doi.org/10.6004/jnccn.2018.0061
Dornfeld M, Lovely JK, Huebner M, Larson DW (2017) Surgical site infection in colorectal surgery: a study in antibiotic duration. Dis Colon Rectum 60(9):971–978. https://doi.org/10.1097/DCR.0000000000000807
Eto K, Urashima M, Kosuge M, Ohkuma M, Noaki R, Neki K, Ito D, Takeda Y, Sugano H, Yanaga K (2018) Standardization of surgical procedures to reduce risk of anastomotic leakage, reoperation, and surgical site infection in colorectal cancer surgery: a retrospective cohort study of 1189 patients. Int J Color Dis 33:755–762. https://doi.org/10.1007/s00384-018-3037-3
Takahashi H, Haraguchi N, Nishimura J, Hata T, Yamamoto H, Matsuda C, Mizushima T, Doki Y, Mori M (2018) The severity of anastomotic leakage may negatively impact the long-term prognosis of colorectal cancer. Anticancer Res 38(1):533–539. https://doi.org/10.21873/anticanres.12255
Chapman SJ, Pericleous A, Downey C, Jayne DG (2018) Postoperative ileus following major colorectal surgery. Br J Surg 105:797–810. https://doi.org/10.1002/bjs.10781
Onodera T, Goseki N, Kosaki G (1984) Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 85(9):1001–1005
Luo Z, Zhou L, Balde AI, Li Z, He L, ZhenWei C, Zou Z, Huang S, Han S, Wei Zhou M, Zhang GQ, Cai Z (2018) Prognostic impact of preoperative prognostic nutritional index in resected advanced gastric cancer: a multicenter propensity score analysis. Eur J Surg Oncol. https://doi.org/10.1016/j.ejso.2018.09.004
Mohri T, Mohri Y, Shigemori T, Takeuchi K, Itoh Y, Kato T (2016) Impact of prognostic nutritional index on long-term outcomes in patients with breast cancer. World J Surg Oncol 14(1):170. https://doi.org/10.1186/s12957-016-0920-7
Tokunaga R, Sakamoto Y, Nakagawa S, Miyamoto Y, Yoshida N, Oki E, Watanabe M, Baba H (2015) Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum 58(11):1048–1057. https://doi.org/10.1097/dcr.0000000000000458
Hu Y, Shen J, Liu R, Feng Z, Zhang C, Ling L, Chen L (2018) Prognostic value of pretreatment prognostic nutritional index in non-small cell lung cancer: a systematic review and meta-analysis. Int J Biol Markers 1724600818799876:372–378. https://doi.org/10.1177/1724600818799876
Migita K, Matsumoto S, Wakatsuki K, Ito M, Kunishige T, Nakade H, Sho M (2018) The prognostic significance of the geriatric nutritional risk index in patients with esophageal squamous cell carcinoma. Nutr Cancer 1–9. https://doi.org/10.1080/01635581.2018.1512640
Feng Z, Wen H, Ju X, Bi R, Chen X, Yang W, Wu X (2018) The preoperative prognostic nutritional index is a predictive and prognostic factor of high-grade serous ovarian cancer. BMC Cancer 18(1):883. https://doi.org/10.1186/s12885-018-4732-8
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16. https://doi.org/10.1186/1745-6215-8-16
Schmidt FL, Oh IS, Hayes TL (2009) Fixed- versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol 62(Pt 1):97–128. https://doi.org/10.1348/000711007X255327
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Iseki Y, Shibutani M, Maeda K, Nagahara H, Ohtani H, Sugano K, Ikeya T, Muguruma K, Tanaka H, Toyokawa T, Sakurai K, Hirakawa K (2015) Impact of the preoperative controlling nutritional status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS One 10(7):e0132488. https://doi.org/10.1371/journal.pone.0132488
Okugawa Y, Toiyama Y, Oki S, Ide S, Yamamoto A, Ichikawa T, Kitajima T, Fujikawa H, Yasuda H, Saigusa S, Hiro J, Yoshiyama S, Kobayashi M, Araki T, Kusunoki M (2018) Feasibility of assessing prognostic nutrition index in patients with rectal cancer who receive preoperative chemoradiotherapy. J Parenter Enter Nutr 42(6):998–1007. https://doi.org/10.1002/jpen.1041
Song Y, Yang Y, Gao P, Chen X, Yu D, Xu Y, Zhao J, Wang Z (2017) The preoperative neutrophil to lymphocyte ratio is a superior indicator of prognosis compared with other inflammatory biomarkers in resectable colorectal cancer. BMC Cancer 17(1):744. https://doi.org/10.1272/jnms.84.22410.1186/s12885-017-3752-0
Tokunaga R, Sakamoto Y, Nakagawa S, Izumi D, Kosumi K, Taki K, Higashi T, Miyata T, Miyamoto Y, Yoshida N, Baba H (2017) Comparison of systemic inflammatory and nutritional scores in colorectal cancer patients who underwent potentially curative resection. Int J Clin Oncol 22(4):740–748. https://doi.org/10.1007/s10147-017-1102-5
Noh GT, Han J, Cho MS, Hur H, Min BS, Lee KY, Kim NK (2017) Impact of the prognostic nutritional index on the recovery and long-term oncologic outcome of patients with colorectal cancer. J Cancer Res Clin Oncol 143(7):1235–1242. https://doi.org/10.1007/s00432-017-2366-x
Akgul O, Cetinkaya E, Yalaza M, Ozden S, Tez M (2017) Prognostic efficacy of inflammation-based markers in patients with curative colorectal cancer resection. World J Gastrointest Oncol 9(7):300–307. https://doi.org/10.1016/j.disamonth.2017.07.00110.4251/wjgo.v9.i7.300
Cao X, Zhao G, Yu T, An Q, Yang H, Xiao G (2017) Preoperative prognostic nutritional index correlates with severe complications and poor survival in patients with colorectal cancer undergoing curative laparoscopic surgery: a retrospective study in a single Chinese institution. Nutr Cancer 69(3):454–463. https://doi.org/10.1080/01635581.2017.1285038
Jian-Hui C, Iskandar EA, Cai Sh I, Chen CQ, Wu H, Xu JB, He YL (2016) Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol 37(3):3277–3283. https://doi.org/10.1007/s13277-015-4008-8
Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M (2013) Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg 37(11):2688–2692. https://doi.org/10.1007/s00268-013-2156-9
Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, Matsukuma A, Ezaki T (2012) The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today 42(6):532–535. https://doi.org/10.1007/s00595-011-0061-0
Park BK, Park JW, Han EC, Ryoo SB, Han SW, Kim TY, Chie EK, Jeong SY, Park KJ (2016) Systemic inflammatory markers as prognostic factors in stage IIA colorectal cancer. J Surg Oncol 114(2):216–221. https://doi.org/10.1002/jso.24299
Peng J, Zhang R, Zhao Y, Wu X, Chen G, Wan D, Lu Z, Pan Z (2017) Prognostic value of preoperative prognostic nutritional index and its associations with systemic inflammatory response markers in patients with stage III colon cancer. Chin J Cancer 36(1):96. https://doi.org/10.1186/s40880-017-0260-1
Shibutani M, Maeda K, Nagahara H, Ohtani H, Iseki Y, Ikeya T, Sugano K, Hirakawa K (2015) The prognostic significance of the postoperative prognostic nutritional index in patients with colorectal cancer. BMC Cancer 15:521. https://doi.org/10.1186/s12885-015-1537-x
Yang Y, Gao P, Chen X, Song Y, Shi J, Zhao J, Sun J, Xu Y, Wang Z (2016) Prognostic significance of preoperative prognostic nutritional index in colorectal cancer: results from a retrospective cohort study and a meta-analysis. Oncotarget 7(36):58543–58552. https://doi.org/10.18632/oncotarget.10148
Yamamoto M, Saito H, Uejima C, Tanio A, Takaya S, Ashida K, Fujiwara Y (2018) Combined pre- and postoperative lymphocyte count accurately predicts outcomes of patients with colorectal cancer. Dig Surg 1–8. https://doi.org/10.1159/000492340
Shinji S, Ueda Y, Yamada T, Koizumi M, Yokoyama Y, Takahashi G, Hotta M, Iwai T, Hara K, Takeda K, Okusa M, Kan H, Uchida E (2018) Combined use of preoperative lymphocyte counts and the post/preoperative lymphocyte count ratio as a prognostic marker of recurrence after curative resection of stage II colon cancer. Oncotarget 9(2):2553–2564. https://doi.org/10.18632/oncotarget.23510
Ghuman S, Van Hemelrijck M, Garmo H, Holmberg L, Malmstrom H, Lambe M, Hammar N, Walldius G, Jungner I, Wulaningsih W (2017) Serum inflammatory markers and colorectal cancer risk and survival. Br J Cancer 116(10):1358–1365. https://doi.org/10.1038/bjc.2017.96
Chiang JM, Chang CJ, Jiang SF, Yeh CY, You JF, Hsieh PS, Huang HY (2017) Pre-operative serum albumin level substantially predicts post-operative morbidity and mortality among patients with colorectal cancer who undergo elective colectomy. Eur J Cancer Care 26(2). https://doi.org/10.1111/ecc.12403
Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X, Zhang Q, Li Z (2018) Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Color Dis 33:1419–1427. https://doi.org/10.1007/s00384-018-3128-1
Ihara K, Yamaguchi S, Shida Y, Ogata H, Domeki Y, Okamoto K, Nakajima M, Sasaki K, Tsuchioka T, Kato H (2015) Poor nutritional status before and during chemotherapy leads to worse prognosis in unresectable advanced or recurrent colorectal cancer. Int Surg. https://doi.org/10.9738/intsurg-d-15-00079.1
Ikeguchi M, Urushibara S, Shimoda R, Yamamoto M, Maeta Y, Ashida K (2014) Inflammation-based prognostic scores and nutritional prognostic index in patients with locally-advanced unresectable colorectal cancer. World J Surg Oncol 12:210. https://doi.org/10.1186/1477-7819-12-210
Ikeya T, Shibutani M, Maeda K, Sugano K, Nagahara H, Ohtani H, Hirakawa K (2015) Maintenance of the nutritional prognostic index predicts survival in patients with unresectable metastatic colorectal cancer. J Cancer Res Clin Oncol 141(2):307–313. https://doi.org/10.1007/s00432-014-1799-8
Daniele A, Divella R, Abbate I, Casamassima A, Garrisi VM, Savino E, Casamassima P, Ruggieri E, DEL R (2017) Assessment of nutritional and inflammatory status to determine the prevalence of malnutrition in patients undergoing surgery for colorectal carcinoma. Anticancer Res 37(3):1281–1287. https://doi.org/10.21873/anticanres.11445
Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF, McArdle CS (2005) The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer 92(4):651–654. https://doi.org/10.1038/sj.bjc.6602419
McSorley ST, Watt DG, Horgan PG, McMillan DC (2016) Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol 23(9):2832–2840. https://doi.org/10.1245/s10434-016-5204-5
Qiu MZ, Pan WT, Lin JZ, Wang ZX, Pan ZZ, Wang FH, Yang DJ, Xu RH (2018) Comparison of survival between right-sided and left-sided colon cancer in different situations. Cancer Med 7:1141–1150. https://doi.org/10.1002/cam4.1401
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent.
Disclosure statement
The authors have nothing to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(TIF 0.98 mb)
Rights and permissions
About this article
Cite this article
Sun, G., Li, Y., Peng, Y. et al. Impact of the preoperative prognostic nutritional index on postoperative and survival outcomes in colorectal cancer patients who underwent primary tumor resection: a systematic review and meta-analysis. Int J Colorectal Dis 34, 681–689 (2019). https://doi.org/10.1007/s00384-019-03241-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-019-03241-1