Abstract
Purpose
The purpose of this study was to investigate the significance of the Prognostic Nutritional Index (PNI) in predicting prognoses and guiding treatment choices of nasopharyngeal carcinoma (NPC) patients receiving intensity-modulated radiotherapy (IMRT).
Methods
The 539 patients with newly diagnosed non-metastatic NPC were retrospectively analysed. The PNI was calculated as 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (per mm3). All patients were split randomly into a training set and a testing set. Receiver operating characteristic (ROC) curves were used to identify the cut-off value of PNI and test its prognostic validity. Survival curves were calculated by Kaplan–Meier method, and differences were compared with log-rank test.
Results
The median follow-up time was 109.5 months. The 5-year locoregional recurrence-free survival (LRRFS), distant metastasis-free survival (DMFS), disease-specific survival (DSS) and overall survival (OS) of the whole cohort were 90.6, 85.8, 85.3 and 82.7%, respectively. The PNI cut-off value was 52.0 in the training set, which was significant in predicting DMFS, DSS and OS in the testing set. According to the PNI cut-off value, 220 patients of II–IVb stage treated by concurrent chemoradiotherapy (CCRT) were classified into PNI ≤ 52.0 and >52.0 groups and the 5-year LRRFS, DMFS, DSS, and OS of PNI ≤ 52.0 group were significantly worse than the PNI > 52.0 group.
Conclusion
Our results suggest that the PNI is a reliable independent prognostic factor in NPC patients treated with IMRT. For stage II-IVb patients with PNI ≤ 52.0, CCRT alone does not achieve satisfactory outcomes, and further studies on treatment optimization are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common malignancies in south China, especially in the middle and western regions of Guangdong province (Wei et al. 2014). NPC is both a radiosensitive and chemosensitive tumour; hence, radiotherapy with or without chemotherapy is the standard treatment modality for non-disseminated NPC. However, the results of this approach were unsatisfactory in the era of two-dimensional conventional radiotherapy (2D-CRT). The 5-year overall survival (OS) is only 67–76%, and loco-regional recurrence and distant metastasis are the main causes of treatment failure, with an incidence of 13.5–35.6% and 19.6–27.6% (Yi et al. 2006; Peng et al. 2012; Zhang et al. 2015), respectively. In recent years, intensity-modulated radiotherapy (IMRT) has replaced 2D-CRT as the first choice for the treatment of NPC. A large number of studies (Lee et al. 2014, Su et al. 2011; Wang et al. 2014; Li et al. 2015) have shown that the 5-year loco-regional control rate has increased substantially to reach approximately 90% in NPC patients treated by IMRT with or without chemotherapy, but the distant metastasis rate remains high (14–26%) with no clear improvement in OS (77–84%). Thus, the treatment of NPC remains challenging, and it is of vital importance to identify factors that can predict the prognosis of NPC patients before IMRT to provide individual comprehensive therapy and improve treatment efficacy.
Recently, an increasing number of studies have focused on the influence of nutrition and immune status on the prognosis of cancer patients. The Prognostic Nutritional Index (PNI), which is calculated based on the serum albumin concentration and total lymphocyte count in the peripheral blood, is known to be an indicator of both the nutritional and immune status of cancer patients (Ikeya et al. 2015). In recent years, many reports have shown that the PNI could be used as a prognostic marker in patients with various malignancies, including advanced head and neck cancer (Goodwin and Torres 1984), small-cell lung cancer (Hong et al. 2015), renal cell carcinoma (Hofbauer et al. 2015), and digestive tract tumours (Ikeya et al. 2015; Jiang et al. 2014). However, the prognostic value of PNI has rarely been investigated in NPC patients. Furthermore, the TNM staging system is the most important tool for predicting prognosis and guiding the NPC treatment strategy, but the heterogeneity of patients with different risk factors in the same stage has limited the ability of this system to distinguish patients with different prognoses and make accurate treatment choices (Su et al. 2011; Li et al. 2015). Therefore, it is necessary to identify other prognostic factors that can help predict the prognosis and aid in the treatment of NPC patients. We conducted this retrospective study in NPC patients who received IMRT with or without chemotherapy to investigate the significance of PNI on predicting outcomes and guiding the selection of treatment strategies when combined with the TNM staging system.
Materials and methods
Patient selection
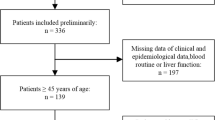
Between April 2001 and June 2010, 539 pathologically diagnosed, non-metastatic NPC patients were treated with radical IMRT at the Sun Yat-sen University Cancer Center (SYSUCC). All of them had complete laboratory and clinical data for this retrospective analysis.
Staging
As the patients were treated from April 2001 to June 2010 in this study, 54 patients treated before 2003 were staged according to the UICC/AJCC staging system 5th edition (Fleming et al. 1997), and the other patients were staged according to the UICC/AJCC staging system 6th edition (Sobin and Wittekind 2002). The staging of all patients was based on complete history and physical examinations, haematological and biochemical tests, nasopharyngoscopy, chest radiography, ultrasonography of the abdominal region, and computed tomography (CT) scans or magnetic resonance imaging (MRI) of the head and neck before treatment. For stage N3 patients, chest and abdominal CT scans, whole-body emission computed tomography (ECT) or [18F] fluorodeoxy-glucose positron emission tomography and computed tomography (PET/CT) were performed. In the current study, all patients were restaged according to the UICC/AJCC staging system 7th edition (Sobin et al. 2009). The demographic and clinical characteristics of the patients are shown in Supplementary 1.
Clinical data collection
The clinicopathological parameters of the patients, including age, gender, World Health Organization (WHO) histological type, TNM stage, gross tumour volume of the nasopharynx (GTVnx), treatment modality, smoking and alcohol consumption, and haematological and biochemical indexes, such as laboratory counts of neutrophils (NEU), lymphocytes (LYM), haemoglobin (HGB), platelets (PLT), total protein (TP), albumin (ALB), globulin (GLO), lactate dehydrogenase (LDH), glutamic-pyruvic transaminase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) concentrations before treatment, were retrieved from the medical records. PNI was calculated using the following formula: 10 × serum albumin value (g/dL) + 0.005 × total lymphocyte count in the peripheral blood (per mm3).
Treatments
All patients received radical IMRT, and all of them have finished radiotherapy as scheduled. IMRT was delivered with a dynamic multi-leaf intensity-modulating collimator (NOMOS Corporation, Sewickley, Pa) using a slice-by-slice arc rotation approach. The details of the IMRT technique and delineation of the target volumes, including the GTVnx, the positive neck lymph nodes (GTVnd), the high-risk sites of microscopic extension (CTV1), and the low-risk sites of microscopic extension (CTV2), have been previously described (Xiao et al. 2011). The prescribed dose was 66–68 Gy to GTVnx, 62–64 Gy to GTVnd, 60 Gy to CTV1 and 54 Gy to CTV2 in 30 fractions. In addition, the prescribed dose to the lower neck and the supraclavicular fossae for irradiation with the conventional RT technique was 50 Gy/25 fractions for prophylactic intent and 60–66 Gy/30–33 fractions for therapeutic intent.
Chemotherapy was administered to stage III/IVa–b patients, except those who were older than 65 years, had contraindications to chemotherapy or refused by themselves and part of stage II patients (UICC/AJCC staging system 6th edition). A total of 361 (67.0%) patients received chemotherapy. Of these 361 patients, 221 (61.2%) patients were given cisplatin-based concurrent chemo-radiotherapy (CCRT), 123 (34.1%) patients were given cisplatin-based neoadjuvant chemotherapy (NACT) followed by CCRT, 2 (0.6%) patients received CCRT with cisplatin-based adjuvant chemotherapy (ACT), and the rest [15 (4.2%) patients] accepted only 2 cycles of cisplatin-based NACT or ACT. All of them have completed the chemotherapy as planned.
Follow-up
All patients were followed up every 3 months during the first 3 years, every 6 months during the 4–5th years and then annually thereafter. Each follow-up included a complete physical and fibreoptic nasopharyngoscopy or indirect nasopharyngeal speculum examinations. Biochemical profiles, chest X-ray, ultrasound of the liver and the abdomen and MRI of the head and neck were also routine elements of the assessment. Further investigations were arranged when clinically indicated. The last follow-up date was Dec 31, 2015.
Statistical analysis
All patients were randomly divided into a training set and a testing set. In the training set, receiver operating characteristic (ROC) curve analysis was used to evaluate the sensitivity and specificity of the PNI for predicting loco-regional recurrence, distant metastasis and death, and the Youden index was estimated to determine the optimal cut-off value of the PNI (Yin et al. 2016). The testing set was then stratified according to the optimum cut-off value. The ROC curve was also used to find the optimal cut-off value for GTVnx. The cut-off values for the haematological and biochemical parameters, such as laboratory NEU, HGB, and PLT counts, as well as TP, GLO, LDH, ALT, AST, and ALP concentrations, were determined by the upper limit of normal values.
Durations were calculated from the time of pathological diagnosis to last follow-up date or the date of event. Survival curves were calculated with the Kaplan–Meier method. Differences between curves were analysed with the log-rank test. Univariate and multivariate hazard ratios were calculated using the Cox proportional hazard model. All significant variables in the univariate analysis were entered into the multivariate analysis. All tests were two sided. P values <0.05 and a 95% confidence interval (CI) that did not include 1 were considered significant. Statistical analyses were performed using the SPSS software program (IBM SPSS Statistics 22.0).
Results
Treatment results and survival
The median follow-up time was 109.50 months (range 4.21–176.13 months). And the median of PNI was 55.90 (range 25.00–81.70). At the date of last follow-up, 40 (7.4%) patients had loco-regional recurrence alone, 70 (13.0%) patients had distant metastasis alone and 15 (2.8%) had both loco-regional recurrence and distant metastasis. A total of 128 (23.7%) patients died; among them, 98 (18.2%) patients died of NPC, 10 (1.9%) patients died of treatment toxicity and 20 (3.7%) patients died of non-tumour reasons. The 5-year locoregional recurrence-free survival (LRRFS), distant metastasis-free survival (DMFS), disease-specific survival (DSS) and OS of the whole cohort were 90.6, 85.8, 85.3 and 82.7%, respectively.
Identification of PNI cut-off points in the training set
The 539 patients were randomly divided into a training set (n = 269) and a testing set (n = 270). The median of PNI was 55.50 (range 25.00-81.70) in the training set, and 56.15 (range 31.20–71.70) in the testing set. According to the ROC analyses (Supplementary 2), a cut-off value of PNI for LRRFS was not identified in the training set [AUC (area under the ROC) = 0.570, P = 0.057]. For the DMFS, DSS and OS, the cut-off values of PNI were 56.25 (AUC = 0.599, P = 0.049), 52.05 (AUC = 0.607, P = 0.046) and 52.05 (AUC = 0.576, P = 0.043), respectively. We selected 52.0 as the optimum cut-off value to classify the testing set into PNI ≤ 52.0 and PNI > 52.0 groups for the DMFS, DSS and OS analyses. And the basic information of whole cohort between the PNI high and low subgroups was shown in Supplementary 1.
Prognostic analyses of the PNI in the testing set of NPC patients
In the testing set (n = 270), 59 (21.9%) patients had a PNI ≤ 52.0, and 211 (78.1%) patients had a PNI > 52.0. The 5-year DMFS, DSS and OS rates for patients with a PNI ≤ 52.0 vs. PNI > 52.0 were 78.7 vs. 86.5% (χ 2 = 5.528, P = 0.019), 72.1 vs. 88.5% (χ 2 = 10.305, P = 0.001) and 69.5 vs. 85.8% (χ 2 = 9.876, P < 0.001), respectively (Fig. 1). In the multivariate analysis, the PNI was a significant predictor of DMFS (HR = 0.454, 95% CI, 0.241–0.854; P = 0.014), DSS (HR = 0.338, 95% CI, 0.188–0.607; P < 0.001) and OS (HR = 0.369, 95% CI, 0.218–0.622; P < 0.001) (Table 1).
Prognostic analyses of the PNI in patients with loco-regionally intermediate and advanced stage disease
We sought to evaluate the significance of the PNI for predicting prognoses in patients with loco-regionally intermediate and advanced disease. Five hundred and nine patients with stage II–IVb disease were selected for the analysis. The results revealed a significant difference in the DMFS, DSS and OS rates between patients with a PNI ≤ 52.0 and patients with a PNI > 52.0 (76.7 vs. 87.6%, χ 2 = 10.952, P = 0.001; 72.4 vs. 88.4%, χ 2 = 23.110, P < 0.001; 68.8 vs. 86.2%, χ 2 = 21.445, P < 0.001). Multivariate analysis demonstrated that a PNI ≤ 52.0 was a predictor of poor DMFS (HR = 0.537, 95% CI, 0.338–0.853; P = 0.008), DSS (HR = 0.417, 95% CI, 0.274–0.635; P < 0.001) and OS (HR = 0.497, 95% CI, 0.337–0.734; P < 0.001) in patients with loco-regionally intermediate and advanced disease (Table 2).
The role of CCRT in the different PNI subclassifications of patients with loco-regionally intermediate and advanced stage disease
To study the role of CCRT in the different PNI subclassifications of patients with stage II–IVb disease, 289 of 509 patients with stage II–IVb disease were eliminated. Among those excluded, 149 patients were treated by IMRT alone, 123 patients received CCRT with NACT, 15 patients accepted NACT or ACT, and the remaining 2 patients were treated by CCRT with ACT. Thus, 220 patients with stage II-IVb disease treated with CCRT alone were analysed. The characteristics of these 220 patients are presented in Table 3. Of these 220 patients, 62 patients (28.2%) had a PNI ≤ 52.0, and 158 patients (71.8%) had a PNI > 52.0. The 5-year LRRFS, DMFS, DSS, and OS rates in patients with a PNI ≤ 52.0 were significantly worse than those with a PNI > 52.0 (85.6 vs. 94.6%, χ 2 = 4.038, P = 0.044; 76.5 vs. 86.3%, χ 2 = 3.859, P = 0.049; 72.2 vs. 87.7%, χ 2 = 6.231, P = 0.013; 69.4 vs. 83.5%, χ 2 = 4.934, P = 0.026) (Fig. 2).
Discussion
Identifying prognostic factors and selecting appropriate treatment strategies based on those prognostic factors is an effective way to improve treatment outcomes. For NPC, the TNM staging system, which reflects the extent of the tumour, has been the most widely used strategy for predicting prognosis and guiding treatment strategies for different risk groups (Chen et al. 2012a, b). However, because 2D-CRT was replaced by IMRT as the first-choice irradiation technique for NPC, the main pattern of treatment failure has been identified as distant metastasis, which may reduce the ability of the TNM staging system to accurately distinguish different risk groups (Lee et al. 2012). Therefore, it is important to identify other prognostic factors to improve the ability of the TNM staging system to predict prognosis. In recent years, there have been several published reports focusing on the correlation between the prognosis of NPC patients treated with IMRT and tumour- or host-related factors, such as the primary tumour volume (GTV-P) (Guo et al. 2012; Chen et al. 2011), the maximum standardized uptake value of the primary tumour (SUVmax-P), which is tested by 18F-FDG PET/CT (Xiao et al. 2015), weight loss during treatment (Qiu et al. 2011), baseline serum LDH levels (Li et al. 2015; Zhou et al. 2012), and the albumin-globulin ratio before treatment (pretreatment AGR) (Li et al. 2015). The results of those studies indicated that advanced large GTV-P (Guo et al. 2012; Chen et al. 2011), high SUVmax-P (Xiao et al. 2015), high weight loss during treatment (Qiu et al. 2011), high baseline serum LDH levels (Li et al. 2015; Zhou et al. 2012) and lower pretreatment AGR (Li et al. 2015) all are predictors of a poor prognosis and could be used to facilitate treatment options when combined with the TNM staging system.
The PNI is determined by the serum albumin concentration and total lymphocyte count in the peripheral blood. The serum albumin concentration is regulated by the inflammatory response and nutritional status of the body. Meanwhile, lymphocytes are crucial components of the host’s cellular adaptive immune response against cancer cells. Therefore, the PNI is considered a good index that reflects both the nutritional and immune status of the host (Ikeya et al. 2015). Although the PNI was originally proposed by Onodera to assess postoperative complications of patients who underwent gastrointestinal surgery (Onodera et al. 1984), increasing evidence has shown that the PNI is closely related to long-term outcomes and represents an independent prognostic factor for the survival of patients with various cancers (Ikeya et al. 2015; Goodwin and Torres 1984; Hong et al. 2015; Hofbauer et al. 2015; Jiang et al. 2014; Du et al. 2015). In the current study, we conducted a retrospective study to investigate the significance of the PNI in predicting the prognosis of NPC patients. All 539 patients received radical IMRT. Although failure to find a cut-off value in LRRFS means that the PNI has no correlation with LRRFS, there was a significant difference in the 5-year DMFS, DSS and OS rates between patients with a PNI > 52.0 and those with a PNI ≤ 52.0 (all P < 0.05) in the testing set. And multivariate analysis also indicated that a PNI ≤ 52.0 was an independent predictor of worse DMFS, DSS and OS (all P < 0.05). These results demonstrate that the PNI may serve as an independent prognostic marker in NPC patients treated by IMRT with or without chemotherapy.
In the current study, all patients were randomly divided into a training set and a testing set. The cut-off value of the PNI was estimated in the training set (n = 269) using a ROC curve which could generate the best sensitivity and specificity than other methods (Zweig and Campbell 1993), and then the cut-off value was validated in the testing set of patients (n = 270). The optimal cut-off value of the PNI (52.0) was determined and confirmed to be an independent prognostic factor for DMFS, DSS, and OS in both univariate and multivariate analyses of the testing set. However, our study failed to identify a cut-off value for LRRFS, which may be explained by the high loco-regional control rate (>90%) in the era of IMRT. These results were similar to the previous study (Du et al. 2015).
Although radiotherapy combined with chemotherapy has been widely accepted as the standard treatment modality for advanced NPC, the optimal chemotherapy regimen for this disease has yet to be defined. Based on multiple phase III randomized studies, cisplatin-based CCRT plus ACT with cisplatin and fluorouracil is recommended as “category IIa” evidence for the treatment of loco-regionally intermediated and advanced NPC (stage II–IVb) by the NCCN Clinical Practice Guidelines in Oncology. However, recent results from randomized trials and clinical applications are discordant. For example, a phase III multicentre randomized controlled trial from Chen (Chen et al. 2012) indicated that the addition of three cycles of ACT with cisplatin and fluorouracil to CCRT did not significantly improve 2-year survival rates compared with CCRT alone in patients with non-metastatic stage III or IVb (excluding T3-T4N0, AJCC/UICC 6th edition) NPC. Early results from this trial suggest that ACT may not be beneficial in this group of patients. A recent retrospective study by Yi et al. (2014) found the outcomes of advanced NPC treated by cisplatin-based CCRT alone were poorer among patients treated by radical IMRT; the OS, disease-free survival (DFS) and DMFS rates were 71.7, 63.9 and 79.6%, respectively. No significant differences in survival between CCRT and radiotherapy alone in patients with stage III–IVb disease treated by radical IMRT have been observed. We attribute the inconsistent results among these randomized trials and retrospective studies to the heterogeneity of the risk factors of patients in the same disease stage, in addition to the different study designs (prospective vs. retrospective), changes in radiotherapy technology (2D-CRT to IMRT) and the recent progress in imaging (CT to MRI). In other words, it is very likely that ACT is not necessary in all stage III–IVb patients while CCRT alone also seems to be inadequate for all stage III–IVb patients. In this study, 220 patients with stage II–IVb treated by CCRT alone were included in a stratified analysis. The patients were divided into two groups according to their PNI. The results revealed that patients with a PNI > 52.0 showed better outcomes. The 5-year LRRFS, DMFS, DSS and OS rates in the patients with a PNI ≤ 52.0 were significantly poorer than those in patients with a PNI > 52.0 (LRRFS 85.6 vs. 94.6%, P = 0.044; DMFS 76.5 vs. 86.3%, P = 0.049; DSS 72.2 vs. 87.7%, P = 0.013; OS 69.4 vs. 83.5%, P = 0.026). These findings suggest that the PNI is a reliable host-related factor for further differentiation between heterogeneous patients with loco-regionally intermediate and advanced stage disease.
According to the previous reports (Qiu et al. 2011; Ravasco et al. 2005; Capuano et al. 2008; Shen et al. 2013), malnutrition before and during treatment has been identified as a risk factor predicted worse outcome in head and neck cancer or NPC patients due to severity of acute toxicities, decreasing chemotherapy dose intensity, interruption of treatment, reducing the radiosensitivity and/or chemosensitivity of tumour and compromised immunity. Although no delay or interruption of radiotherapy, or chemotherapy dose intensity reduction was observed in all patients in this study, we supposed that the nutritional status steadily deteriorated during CCRT in the patients with low PNI subgroup result in lower survival rates by reducing radiosensitivity and/or chemosensitivity, and compromised immunity. Therefore, individualized treatment strategy should be considered for those patients with low PNI, including early nutritional interventions and choosing appropriate comprehensive treatment strategies.
The principal limitation of this study is its retrospective nature. In addition, the PNI is a non-specific marker of tumour burden because other non-cancer conditions could be confounding, such as autoimmune and infectious diseases. A large, prospective multicentre study will be important to validate our findings.
In conclusion, the present study demonstrates that the PNI is a useful prognostic factor in NPC patients treated by IMRT. It may further enhance the accuracy of the TNM classification system for predicting the prognosis of different risk subgroups and should be included as a factor in the selection of treatment strategies for NPC patients, which may aid in the development of individualized treatment strategies to improve the treatment outcomes of NPC patients.
References
Capuano G, Grosso A, Gentile PC, Battista M, Bianciardi F, Di Palma A, Pavese I, Satta F, Tosti M, Palladino A, Coiro G, Di Palma M (2008) Influence of weight loss on outcomes in patients with head and neck cancer undergoing concurrent chemoradiotherapy. Head Neck 30(4):503–508. doi:10.1002/hed.20737
Chen C, Fei Z, Pan J, Bai P, Chen L (2011) Significance of primary tumor volume and T-stage on prognosis in nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Jpn J Clin Oncol 41:537–542. doi:10.1093/jjco/hyq242
Chen L, Mao Y-P, Xie FY, Liu LZ, Sun Y, Tian L, Tang LL, Lin AH, Li L, Ma J (2012a) The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiother Oncol 104:331–337. doi:10.1016/j.radonc.2011.10.009
Chen L, Hu CS, Chen X-Z, Hu GQ, Cheng ZB, Sun Y, Li WX, Chen YY, Xie FY, Liang SB, Chen Y, Xu TT, Li B, Long GX, Wang SY, Zheng BM, Guo Y, Sun Y, Mao YP, Tang LL (2012b) Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 13:163–171. doi:10.1016/S1470-2045(11)70320-5
Du X-J, Tang L-L, Mao YP, Guo R, Sun Y, Lin AH, Ma J (2015) Value of the prognostic nutritional index and weight loss in predicting metastasis and long-term mortality in nasopharyngeal carcinoma. J Transl Med 13:364. doi:10.1186/s12967-015-0729-0
Fleming ID, Cooper JS, Henson DE (1997) AJCC cancer staging manual. 5th edn. Lippincott-Raven, Philadelphia
Goodwin WJ Jr, Torres J (1984) The value of the prognostic nutritional index in the management of patients with advanced carcinoma of the head and neck. Head Neck Surg 6:932–937. doi:10.1002/hed.2890060507
Guo R, Sun Y, Yu X-L, Yin WJ, Li WF, Chen YY, Mao YP, Liu LZ, Li L, Lin AH, Ma J (2012) Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother Oncol 104:294–299. doi:10.1016/j.radonc.2012.09.001
Hofbauer SL, Pantuck AJ, de Martino M, Lucca I, Haitel A, Shariat SF, Belldegrun AS, Klatte T (2015) The preoperative prognostic nutritional index is an independent predictor of survival in patients with renal cell carcinoma. Urol Oncol 33:68.e1–68.e7. doi:10.1016/j.urolonc.2014.08.005
Hong S, Zhou T, Fang W, Xue C, Hu Z, Qin T, Tang Y, Chen Y, Ma Y, Yang Y, Hou X, Huang Y, Zhao H, Zhao Y, Zhang L (2015) The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol 36:3389–3397. doi:10.1007/s13277-014-2973-y
Ikeya T, Shibutani M, Maeda K, Sugano K, Nagahara H, Ohtani H, Hirakawa K (2015) Maintenance of the nutritional prognostic index predicts survival in patients with unresectable metastatic colorectal cancer. J Cancer Res Clin Oncol 141:307–313. doi:10.1007/s00432-014-1799-8
Jiang N, Deng JY, Ding XW, Ke B, Liu N, Zhang RP, Liang H (2014) Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol 20:10537–10544. doi:10.3748/wjg.v20.i30.10537
Lee AW, Ng WT, Chan LK, Chan OS, Hung WM, Chan CC, Cheng PT, Sze H, Lam TS, Yau TK (2012) The strength/weakness of the AJCC/UICC staging system (7th edition) for nasopharyngeal cancer and suggestions for future improvement. Oral Oncol 48:1007–1013. doi:10.1016/j.oraloncology.2012.03.022
Lee AW, Ng WT, Chan LL, Hung WM, Chan CC, Sze HC, Chan OS, Chang AT, Yeung RM (2014) Evolution of treatment for nasopharyngeal cancer—success and setback in the intensity-modulated radiotherapy era. Radiother Oncol 110:377–384. doi:10.1016/j.radonc.2014.02.003
Li AC, Xiao W-W, Shen GZ, Wang L, Xu AA, Cao YQ, Huang SM, Lin CG, Han F, Deng XW, Zhao C (2015) Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of IMRT: long-term results and benefits of chemotherapy. Oncotarget 6:24511–24521. doi:10.18632/oncotarget.4312
Onodera T, Goseki N, Kosaki G (1984) Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 85:1001–1005
Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, Han J, Wu G (2012) A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 104:286–293. doi:10.1016/j.radonc.2012.08.013
Qiu C, Yang N, Tian G, Liu H (2011) Weight loss during radiotherapy for nasopharyngeal carcinoma: a prospective study from Northern China. Nutr Cancer 63:873–879. doi:10.1080/01635581.2011.582223
Ravasco P, Grillo IM, Vidal PM, Camilo ME (2005) Impact of nutrition on outcome: a prospective randomised controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 27:659–668. doi:10.1002/hed.20221
Shen LJ, Chen C, Li BF, Gao J, Xia YF (2013) High weight loss during radiation treatment changes the prognosis in under-/normal weight nasopharyngeal carcinoma patients for the worse: a retrospective analysis of 2433 cases. PLOS One 8(7):e68660. doi:10.1371/journal.pone.0068660
Sobin LH, Wittekind CH (2002) International Union Against Cancer (UICC): TNM classification of malignant tumors. Wiley-Liss, New York
Sobin LH, Gospodarowicz MK, Wittekind C (eds) (2009) TNM Classification of Malignant Tumours, 7th edn. Wiley-Blackwell, Oxford
Su SF, Han F, Zhao C, Huang Y, Chen CY, Xiao WW, Li JX, Lu TX (2011) Treatment outcomes for different subgroups of nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Chin J Cancer 30:565–573. doi:10.5732/cjc.010.10547
Wang W, Feng M, Fan Z, Li J, Lang J (2014) Clinical outcomes and prognostic factors of 695 nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. BioMed Res Int 2014:814948. doi:10.1155/2014/814948
Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou ZX, Chen WQ (2014) Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer 33:381–387. doi:10.5732/cjc.014.10086
Xiao WW, Huang SM, Han F, Wu SX, Lu LX, Lin CG, Deng XW, Lu TX, Cui NJ, Zhao C (2011) Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer 117:1874–1883. doi:10.1002/cncr.25754
Xiao W, Xu A, Fei Han, Lin X, Lu L, Shen G, Huang S, Fan W, Deng X, Zhao C (2015) Positron emission tomography-computed tomography before treatment is highly prognostic of distant metastasis in nasopharyngeal carcinoma patients after intensity-modulated radiotherapy treatment: a prospective study with long-term follow-up. Oral Oncol 51:363–369. doi:10.1016/j.oraloncology.2015.01.009
Yi J-L, Gao L, Huang X-D, Li SY, Luo JW, Cai WM, Xiao JP, Xu GZ (2006) Nasopharyngeal carcinoma treated by radical radiotherapy alone: ten-year experience of a single institution. Int J Radiat Oncol Biol Phys 65:161–168. doi:10.1016/j.ijrobp.2005.12.003
Yi J, Huang X, Gao L, Luo J, Zhang S, Wang K, Qu Y, Xiao J, Xu G (2014) Intensity-modulated radiotherapy with simultaneous integrated boost for locoregionally advanced nasopharyngeal carcinoma. Radiat Oncol 9:56. doi:10.1186/1748-717X-9-56
Yin J, Samawi H, Linder D (2016) Improved nonparametric estimation of the optimal diagnostic cut-off point associated with the Youden index under different sampling schemes. Biom J. doi:10.1002/bimj.201500036
Zhang MX, Li J, Shen GP, Zou X, Xu JJ, Jiang R, You R, Hua YJ, Sun Y, Ma J, Hong MH, Chen MY (2015) Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer 51:2587–2595. doi:10.1016/j.ejca.2015.08.006
Zhou GQ, Tang L-L, Mao YP, Chen L, Li WF, Sun Y, Liu LZ, Li L, Lin AH, Ma J (2012) Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys 82:e359–e365. doi:10.1016/j.ijrobp.2011.06.1967
Zweig MH, Campbell G (1993) Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39:561–577
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that he/she has no conflict of interest.
Funding
This study was funded by the Science and Technology Project of Guangdong Province (Grant Number 2014A020212433), the Doctoral Fund of Ministry of Education (Grant Number 20130171120111); and the National Natural Science Foundation of China (CN) (Grant Number 8140111403).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
J. Miao, W. Xiao authors contribute to this work equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miao, J., Xiao, W., Wang, L. et al. The value of the Prognostic Nutritional Index (PNI) in predicting outcomes and guiding the treatment strategy of nasopharyngeal carcinoma (NPC) patients receiving intensity-modulated radiotherapy (IMRT) with or without chemotherapy. J Cancer Res Clin Oncol 143, 1263–1273 (2017). https://doi.org/10.1007/s00432-017-2360-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-017-2360-3