Abstract
Metastatic renal cell carcinoma (mRCC) encompasses a heterogeneous group of neoplasms with distinct clinical behavior and prognoses. As a result of the increasing number of therapeutic options in the metastatic setting, it is crucial to improve prognostic stratification ability. We aimed to evaluate the prognostic value of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and combination platelet count and neutrophil lymphocyte ratio (COP-NLR) in patients with mRCC. We evaluated a cohort of mRCC patients treated with first-line pazopanib or sunitinib. Levels of NLR, PLR and COP-NLR were measured prior to systemic treatment and evaluated as prognostic predictors. Primary endpoint was overall survival (OS). Data from 276 patients were included, of which 54.7% received first-line pazopanib and 45.3%, sunitinib. Memorial Sloan-Kettering Cancer Center risk classification was intermediate and poor in 50% and 42.6% of patients, respectively. High NLR (> 3.5) was associated with inferior OS (median 9.6 vs 17.8 months, P < 0.001). A high PLR (> 200) was associated with inferior OS (median 10.3 vs 17 months, P = 0.002). The median OS in the COP-NLR 1, 2 and 3 groups were 19.0 months (95% CI 15.3–26.0), 13.1 months (95% CI 9.8–17.0) and 7.4 months (95% CI 3.6–11.9), respectively (P < 0.001). In the multivariate analysis, high NLR and high COP-NLR were associated with inferior OS. Both high NLR and high COP-NLR were associated with poorer OS in our cohort of patients with mRCC treated with first-line pazopanib or sunitinib.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) represents 2–3% of all malignancies in adults, being responsible for 65,340 new cases and 14,970 deaths in 2018 in the United States [1]. Despite the increase in incidence over the past decades [2], five-year survival rates have increased from 34% in 1954 to 76% in 2009 [3], certainly because of developments in local and systemic therapies. The incorporation of VEGF targeted therapies and immune checkpoint inhibitors in the therapy of RCC has improved clinical outcomes [4] making treatment decisions even more complex in the metastatic setting. The increasing number of active therapies combined with the highly variable natural history of RCC emphasize the need to stratify patients according to genomic alterations, serum factors and disease characteristics that might be associated with better or worse outcomes.
In localized or locally advanced RCC, clinical, pathological and molecular factors are associated with outcome [5, 6]. The most consistent prognostic determinants are the anatomical extent of the disease [7], histopathological features (such as tumor grade) [8], and the presence of sarcomatoid or rhabdoid components [9].
In the metastatic setting, classic clinical models have been extensively used to estimate patients’ prognosis. The most commonly used prognostication systems are: (1) the Memorial Sloan-Kettering Cancer Center (MSKCC) system [5] which integrates five adverse factors: Karnofsky performance status (KPS); serum lactic dehydrogenase; serum calcium; hemoglobin concentration and the absence of prior nephrectomy; and (2) the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) system [6], which integrates clinical and serum factors: KPS; time from original diagnosis to systemic therapy; hemoglobin level, serum calcium, neutrophil count and platelet count.
More recently, data from The Cancer Genome Atlas (TCGA) have allowed the identification of prognostic signatures for RCC based on the tumor’s metabolic states. Analysis of different patterns of gene regulation, including those involved in fatty acid synthesis, acetylCoA carboxylase and fatty acid synthase, as well as the regulation of adenosine monophosphate activated kinase and multiple genes involved in the Krebs cycle and the mTOR pathway have allowed the stratification of patients into subgroups with different prognoses [10]. Although extremely elucidative of the RCC biology, a remarkably heterogeneous disease [11], these prognostic markers require complex molecular analyses. Thus, these prognostic molecular tools are not widely available for the vast majority of patients in clinical practice. With an increasing number of first-line treatment options, choosing the best therapeutic strategy is currently based largely on the prognostic classification of patients [12, 13]. Thus, it is essential to improve our stratification accuracy in an effort to better define which patients derive greater benefit from each possible therapeutic approach.
Emerging data suggest that systemic inflammatory response plays a role in the progression of many malignancies by promoting angiogenesis, tumor metastasis and cancer cell proliferation and survival [12], which could impact the prognosis and response to systemic therapies particularly in the era of checkpoint inhibitors, as they may potentially have predictive value. Indeed, the possibility of integrating broadly available clinical data to comprehensively explore the patterns of immune response to malignancy has been explored with increasing interest. Neutrophil-Lymphocyte ratio (NLR), Platelet-Lymphocyte ratio (PLR), the modified Glasgow Prognostic Score (mGPS) and the combination of a platelet count and the NLR (COP-NLR) are examples of accessible, reproducible, and inexpensive tools that have shown prognostic value in a variety of malignancies [14]. Accumulating data suggest that some of these tools also might be associated with outcomes in RCC [15, 16]. Studies involving mRCC, validation of these factors is scarce and their roles remain controversial. In this study, we aimed to investigate the association between pre-treatment NLR, PLR, COP-NLR and clinical outcomes in patients with metastatic RCC (mRCC) receiving first-line anti-VEGF therapy.
Material and Methods
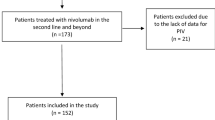
We performed a retrospective analysis of consecutive patients with mRCC who received treatment with first-line sunitinib or pazopanib between February 2009 and March 2017 at a single Brazilian cancer center (Instituto do Câncer do Estado de São Paulo). Patients receiving at least one dose of sunitinib or pazopanib were included. The tyrosine kinase inhibitor (TKI) offered depended on the period: sunitinib was available as first-line treatment in our institution from February 2009 to September 2013 and pazopanib was available from September 2013 until the present date. Medical records were reviewed to obtain clinical and demographic characteristics, laboratory tests and outcomes.
NLR was calculated by dividing the neutrophil count value by the number of lymphocytes and PLR was calculated by dividing the platelet count value by the number of lymphocytes. Laboratory results used were obtained prior to tyrosine kinase inhibitor (TKI) initiation. Patients with a NLR greater than 3.5 were classified as high NLR group and those with a NLR of 3.5 or less were considered as low NLR group. For the PLR, values greater than 200 were considered high PLR, while values of 200 or less were considered low PLR. The cut-off values were chosen based on previous evidence available [16]. The COP-NLR categories were defined as follows: patients with non-elevated platelets and low NLR were designated COP-NLR 0; patients with elevation of one of these parameters were denominated COP-NLR 1 and those with elevated platelets and high NLR were classified as COP-NLR 2. The cut-off value for the definition of platelet levels was defined as 310 × 109 / L, also in accordance with the available evidence [17].
Survival curves were estimated using the Kaplan-Meier method. The log-rank test was used to evaluate the difference between the curves. Overall survival (OS) was the time from TKI initiation until death from any cause. The correlation between NLR and PLR was evaluated by Spearman’s correlation test.
Prognostic factors were evaluated with univariate and multivariate analysis, using Cox proportional hazards model. The variables with a p value <0.1 in the univariate analysis were included in the multivariate analysis.
Stata software version 14 (StataCorp, Texa, USA) was used for the statistical analyses. P values <0.05 were considered statistically significant.
Results
Patients’ Characteristics
A cohort of 276 patients with metastatic RCC treated with first-line TKI was included in the analysis. Among them, 223 patients had histologic confirmation of clear cell RCC (ccRCC), 46 of nccRCC, and 7 of sarcomatoid component. One hundred and fifty-one patients (54.7%) received first-line pazopanib, while 125 (45.3%) received first-line sunitinib. Patients’ clinical and demographic data are summarized in Table 1.
NLR as a Prognostic Factor
A high NLR (>3.5) was observed in 124 patients (45.3%). During the median follow-up of 10.5 months, 101 (67.8%) patients have deceased in the low NLR group and 89 (70%), in the high NLR group.
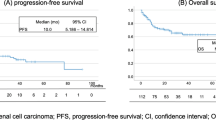
Median OS was 9.6 months in the high NLR group versus 17.8 months in the low NLR group (HR = 1.70, 95% CI 1.27–2.26, P < 0.001). One-year OS rates for the high and low NLR groups were 41.3% and 62.8%, respectively. The Kaplan-Meier survival curves according to NLR are presented in Fig. 1.
Among patients with nccRCC, the median OS was 15.6 months in the low NLR group, compared to 9.8 months in the high NLR group (HR 1.51, 95% CI 0.79–2.91, P = 0.206), while one-year OS rates were 63.3% and 43.4% for the low and high NLR groups, respectively. Kaplan-Meier curves of OS according to NLR in the nccRCC population are presented in Fig. 2.
PLR as a Prognostic Factor
A high PLR (> 200) was observed in 127 patients (46%). Ninety-nine (66%) deaths occurred during follow-up in the low PLR and 89 (71.7%) in the high PLR group.
The high PLR group had a median OS of 10.3 months in comparison with 17 months in the low PLR group (HR 1.57, 95% CI 1.17–2.10, P = 0.002). One-year OS rates were 33.2% in the high PLR group and 59.8% in the low PLR group. The Kaplan Meier OS curves according to PLR are presented in Fig. 3.
In the subgroup of patients with nccRCC, the median OS was 17 months in the low PLR group and 8.7 months in the high PLR group (HR 1.87, 95% CI 0.98–3.5, P = 0.052). One-year OS rates were 60.9% and 42.2% for the low and high PLR groups, respectively. OS curves of patients with nccRCC according to PLR are presented in Fig. 4.
Correlation between NLR and PLR
The Spearman’s correlation showed only a weak positive correlation between NLR and PLR (rs = 0.39), which was statistically significant (P < 0.001).
COP-NLR as a Prognostic Factor
Patients were classified as COP-NLR 1, 2 and 3 in the following proportions: 33%, 44.9% and 22.1%, respectively. The median OS in the COP-NLR 1, 2 and 3 groups was 19.0 months (95% CI 15.3–26.0), 13.1 months (95% CI 9.8–17.0) and 7.4 months (95% CI 3.6–11.9), respectively (P < 0.001). Overall survival curves according to COP-NLR groups are presented in Fig. 5.
For patients with nccRCC histologies, the median OS in the COP-NLR 1, 2 and 3 groups was 22.6 months (95% CI 1.9–49.3), 15.7 months (95% CI 8.5–38.4) and 5.9 months (95% CI 0.4–14.7), respectively (P = 0.08).
Univariate and Multivariate Analysis
The variables evaluated in the univariate analysis were gender, NLR, PLR, COP-NLR, age (≥ 60y vs < 60), histology, MSKCC risk group, number of metastatic sites, metastases in central nervous system, and TKI treatment.
The variables associated with OS in the univariate analysis were NLR, COP-NLR; histology (sarcomatoid differentiation), and MSKCC risk group. Since NLR, PLR and COP-NLR were associated with each other and all reached the univariate threshold of statistical significance for inclusion in the multivariate analysis, two multivariate analyzes were performed: Multivariate 1 included NLR and PLR; and Multivariate 2 included COP-NLR.
In the Multivariate 1 analysis, NLR, sarcomatoid differentiation and poor MSKCC risk had a statistically significant association with inferior OS. In the Multivariate 2 analysis, COP-NLR group 2, sarcomatoid differentiation and poor MSKCC risk had a statistically significant association with inferior OS. The results of the univariate and multivariate analyses are summarized in Table 2.
Discussion
Our study focused on the predictive value of systemic inflammatory biomarkers in the outcome of patients with metastatic RCC receiving sunitinib or pazopanib as first line therapy. Our study showed that a high NLR (> 3.5), a high PLR (> 200) or a high COP-NLR were associated with shorter median OS. In the multivariate analysis, validated prognostic factors, such as the presence of a sarcomatoid component and poor MSKCC risk group were associated with poorer prognosis. Additionally, emerging inflammatory biomarkers, such as a high pretreatment NLR (> 3.5) and a high COP-NLR were also associated with worse outcomes.
Several studies have demonstrated the role of inflammatory markers, including NLR and PLR, in the clinical evolution of RCC. Indeed, cumulative data suggest that elevations in NLR and PLR might be associated with unfavorable outcomes, either at baseline, prior to local treatments [18] or in advanced disease, prior to the initiation of systemic therapies [16]. The prognostic value of these inflammatory markers has been tested as an independent determinant [19], but also as a way to improve categorization of patient risk by incorporating information into the current prognostic assessment models, such as the IMDC [20, 21].
The crucial role of inflammation in the development and progression of RCC has been the object of intense debate in the past years. Translational studies have shown that inflammatory cytokines with pro-inflammatory, hematopoietic, and immunomodulatory effects may have significant prognostic implications in patients with RCC [22]. IL-6 is a multifunctional cytokine that has been consistently implicated in the pathogenesis of RCC [23]. In addition to pro-inflammatory effects, mechanisms leading to adverse effects of IL-6 include its function as an autocrine growth factor and as an inhibitor of dendritic cell differentiation in RCC models [22, 24]. A prospective French study evaluated serum levels of IL-6 in patients with RCC treated with IL-2 or IFN-α have demonstrated that elevated serum levels of IL-6 were independently associated with tumor progression and, consequently, shorter survival [23]. In this study, elevation in neutrophil count was also associated with a worse prognosis [23]. More importantly, in-depth knowledge of this and other immune response pathways translated into the development of increasingly effective immunotherapies for the treatment of patients with advanced RCC [25]. Thus, prognostic tools that intend to accurately stratify risk groups of mRCC should progressively evaluate the role of systemic parameters of inflammation.
Assuming that the antitumor inflammatory response involves a complex interaction between the innate and adaptive immune systems, it has been suggested that the evaluation of each individual’s systemic inflammatory status could be better evaluated – not only by the analysis of an isolated factor – but by a combined analysis of multiple biomarkers [13]. Preclinical data have suggested that the interaction between neutrophils and platelets represents a critical checkpoint in the early inflammatory processes [26]. Dynamic reorganization of neutrophil receptors allows simultaneous interactions with both the vascular wall and activated platelets, so that neutrophils that are recruited to injured vessels scan for activated platelets [26]. Thus, if the platelet-neutrophil interaction seems to be important for the continuation of the inflammatory process, it could be hypothesized that a categorization integrating both variables simultaneously could have clinical value. The categorization of patients according to preoperative platelet levels combined with NLR (COP-NLR) has shown to reliably predict the prognosis of patients with localized RCC [17]. To our knowledge, this is the first study to demonstrate the prognostic value of COP-NLR in patients with metastatic RCC.
Although the pathophysiology is somewhat similar in most cases of ccRCC, nccRCC represents a heterogeneous group composed of several histological variants, which are associated with distinct pathophysiological mechanisms, potentially determining different prognoses for different subtypes [27]. Accordingly, it is important that risk assessment tools that are intended to be applied to the nccRCC subgroup of patients are developed and validated specifically in this population. The PANORAMA study was an Italian multicenter retrospective analysis of 37 patients with metastatic nccRCC treated in the first line with pazopanib. Although in the univariate analysis a low NLR (NLR <3) was a favorable prognostic factor (P = 0.009), in the multivariate analysis only performance status and MSKCC score maintained an impact on PFS and OS [28]. Interestingly, our data also suggest a trend towards a negative prognostic value of high NLR and especially high PLR in patients with nccRCC.
Finally, while inflammatory biomarkers have demonstrated utility in patients with mRCC treated with VEGF targeted therapy, their role as a predictive factor continues to be challenged as the mRCC first-line treatment landscape evolves with the incorporation of immune checkpoint inhibitors alone or in combination with anti-VEGF TKIs. Preliminary data on RCC [29] and other malignancies [30], however, suggest that inflammatory biomarkers may retain their predictive capacity immune checkpoint era.
Our study has several limitations that should be considered when interpreting the results. First, this was a single-center retrospective analysis and the lack of molecular characterization of the patients may have influenced our results. While several provocative associations have been demonstrated between NLR, PLR and COP-NLR and prognosis, causal relationships are difficult to assess and the results may have been influenced by other clinical factors. Also, because this is a single-institution study, selection bias should be considered while interpreting our results. To this end, we tried to mitigate selection bias by including consecutive patients who received first-line sunitinib or pazopanib. In addition to these, because sunitinib and pazopanib were available in different periods of time in our institution (sunitinib, 2009–2013 and pazopanib 2013-present) differences between the two groups, regarding baseline characteristics and availability of subsequent lines of treatment might be considered when interpreting our results.
Conclusions
In summary, the results presented herein suggest that NLR and the integration between NLR and platelet values (COP-NLR) represent independent prognostic markers in patients with mRCC, especially in those with clear cell histology. Further studies should evaluate these biomarkers as predictors of responses to the different modalities of systemic therapies.
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. https://doi.org/10.3322/caac.21442
Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ CK (2016) SEER Cancer statistics review, 1975–2013, National Cancer Institute. Bethesda, MD,. In: SEER web site. https://seer.cancer.gov/archive/csr/1975_2013/. Accessed 3 Apr 2018
Kidney and Renal Pelvis Cancer - Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/kidrp.html. Accessed 1 May 2019
Lalani A-KA, Xie W, Martini DJ et al (2018) Change in neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother cancer 6:5. https://doi.org/10.1186/s40425-018-0315-0
Motzer RJ, Mazumdar M, Bacik J et al (1999) Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17:2530–2530. https://doi.org/10.1200/JCO.1999.17.8.2530
Heng DYC, Xie W, Regan MM et al (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 27:5794–5799. https://doi.org/10.1200/JCO.2008.21.4809
Amin MB, Edge SB, American Joint Committee on Cancer (2017) AJCC cancer staging manual, 8th ed. Springer, Switzerland
Delahunt B, Cheville JC, Martignoni G et al (2013) The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 37:1490–1504. https://doi.org/10.1097/PAS.0b013e318299f0fb
Wei S, Al-Saleem T (2017) The pathology and molecular genetics of Sarcomatoid renal cell carcinoma: a mini-review. J Kidney Cancer VHL 4:19–23. https://doi.org/10.15586/jkcvhl.2017.70
Network TCGAR (2013) Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499:43–49. https://doi.org/10.1038/nature12222
Gerlinger M, Rowan AJ, Horswell S et al (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366:883–892. https://doi.org/10.1056/NEJMoa1113205
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444. https://doi.org/10.1038/nature07205
Watt DG, Proctor MJ, Park JH, et al (2015) The neutrophil-platelet score (NPS) predicts survival in primary operable colorectal Cancer and a variety of common cancers. https://doi.org/10.1371/journal.pone.0142159
Tsujino T, Komura K, Matsunaga T et al (2017) Preoperative measurement of the modified Glasgow prognostic score predicts patient survival in non-metastatic renal cell carcinoma prior to nephrectomy. Ann Surg Oncol 24:2787–2793. https://doi.org/10.1245/s10434-017-5948-6
Boissier R, Campagna J, Branger N et al (2017) The prognostic value of the neutrophil-lymphocyte ratio in renal oncology: a review. Urol Oncol Semin Orig Investig 35:135–141. https://doi.org/10.1016/J.UROLONC.2017.01.016
Semeniuk-Wojtaś A, Lubas A, Stec R et al (2018) Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and C-reactive protein as new and simple prognostic factors in patients with metastatic renal cell Cancer treated with tyrosine kinase inhibitors: a systemic review and meta-analysis. Clin Genitourin Cancer. https://doi.org/10.1016/J.CLGC.2018.01.010
Tsujino T, Komura K, Ichihashi A et al (2017) The combination of preoperative platelet count and neutrophil lymphocyte ratio as a prognostic indicator in localized renal cell carcinoma. Oncotarget 8:110311–110325. https://doi.org/10.18632/oncotarget.22688
Hu H, Yao X, Xie X et al (2017) Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol 35:261–270. https://doi.org/10.1007/s00345-016-1864-9
Bilen MA, Dutcher GMA, Liu Y et al (2018) Association between pretreatment neutrophil-to-lymphocyte ratio and outcome of patients with metastatic renal cell carcinoma treated with nivolumab. Clin Genitourin Cancer. https://doi.org/10.1016/J.CLGC.2017.12.015
Tanaka N, Mizuno R, Yasumizu Y et al (2017) Prognostic value of neutrophil-to-lymphocyte ratio in patients with metastatic renal cell carcinoma treated with first-line and subsequent second-line targeted therapy: a proposal of the modified-IMDC risk model. Urol Oncol Semin Orig Investig 35:39.e19–39.e28. https://doi.org/10.1016/J.UROLONC.2016.10.001
Chrom P, Stec R, Bodnar L, Szczylik C (2018) Incorporating neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in place of neutrophil count and platelet count improves prognostic accuracy of the international metastatic renal cell carcinoma database consortium model. Cancer Res Treat 50:103–110. https://doi.org/10.4143/crt.2017.033
Koo AS, Armstrong C, Bochner B et al (1992) Interleukin-6 and renal cell cancer: production, regulation, and growth effects. Cancer Immunol Immunother 35:97–105
Negrier S, Perol D, Menetrier-Caux C et al (2004) Interleukin-6, interleukin-10, and vascular endothelial growth factor in metastatic renal cell carcinoma: prognostic value of interleukin-6 - from the Groupe Français d’Immunothérapie. J Clin Oncol 22:2371–2378. https://doi.org/10.1200/JCO.2004.06.121
Menetrier-Caux C, Montmain G, Dieu MC et al (1998) Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood 92:4778–4791
Motzer RJ, Tannir NM, McDermott DF et al (2018) Nivolumab plus Ipilimumab versus Sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277–1290. https://doi.org/10.1056/NEJMoa1712126
Sreeramkumar V, Adrover JM, Ballesteros I, et al (2014) Neutrophils scan for activated platelets to initiate inflammation. Science (80- ) 346:1234–1238. https://doi.org/10.1126/science.1256478
Tsimafeyeu I (2017) Management of non–clear cell renal cell carcinoma: current approaches. Urol Oncol Semin Orig Investig 35:5–13. https://doi.org/10.1016/j.urolonc.2016.07.011
Buti S, Bersanelli M, Maines F et al (2017) First-line PAzopanib in NOn–clear-cell renal cArcinoMA: the Italian retrospective multicenter PANORAMA study. Clin Genitourin Cancer 15:e609–e614. https://doi.org/10.1016/j.clgc.2016.12.024
De Giorgi U, Procopio G, Giannarelli D et al (2019) Association of Systemic Inflammation Index and Body Mass Index with survival in patients with renal cell Cancer treated with Nivolumab. Clin Cancer Res 25:3839–3846. https://doi.org/10.1158/1078-0432.CCR-18-3661
Bilen MA, Martini DJ, Liu Y et al (2019) The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer 125:127–134. https://doi.org/10.1002/cncr.31778
Availability of Data and Material
Medical records and laboratory data are available and stored in institutional databases.
Author information
Authors and Affiliations
Contributions
Guilherme Nader Marta: study conception and design, data collection, data interpretation and analysis, article drafting, critical revision of content.
Pedro Isaacsson Velho: study conception and design, data collection, critical revision of content.
Renata R. C. Colombo Bonadio: data collection, data interpretation and analysis.
Mirella Nardo: study conception and design, data collection, critical revision of content.
Sheila F. Faraj: data collection, data interpretation and analysis, critical revision of content.
Manoel Carlos L. de Azevedo Souza: data collection, critical revision of content.
David Q. B. Muniz: study conception and design, data interpretation and analysis, critical revision of content.
Diogo Assed Bastos: study conception and design, data interpretation and analysis, critical revision of content.
Carlos Dzik: study conception and design, data interpretation and analysis, critical revision of content.
Corresponding author
Ethics declarations
Conflict of Interest
Guilherme Nader Marta has received travel/accommodations grants from Bayer Schering Pharma and Roche.
Pedro Isaacsson Velho has received research funding to his institution from Bristol Myers-Squibb and honoraria/consulting fee from Roche, AstraZeneca Bristol Myers-Squibb and Pfizer.
Renata R. C. Colombo Bonadio has received travel grants from Roche.
Mirella Nardo has no conflict of interest to declare.
Sheila F. Faraj has no conflict of interest to declare.
Manoel Carlos L. de Azevedo Souza has received speakers bureau’s grants from Novartis, MSD, Bristol Myers-Squibb and Amgen and has received travel/accommodations grants from Astellas and Zodiac.
David Q. B. Muniz: has received research funding to his institution from Pfizer, travel/accommodations grants from Janssen and has received speakers bureau’s grants from Pfizer and Janssen.
Diogo Assed Bastos has received research funding to his institution from Janssen, Astellas, Pfizer and honoraria/consulting fee from Roche, Janssen, MSD.
Carlos Dzik has received consulting or advisory grants from Janssen-Cilag, Ipsen, Novartis; speakers bureau’s grants Janssen Oncology and travel/accommodations from Astellas Pharma, Janssen Oncology.
Ethics Approval
This study was approved by the institutional research center (NP 716/14).
Consent to Participate
In view of the retrospective nature of this study, waiver of consent was requested.
Code Availability
not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nader Marta, G., Isaacsson Velho, P., Bonadio, R.R.C. et al. Prognostic Value of Systemic Inflammatory Biomarkers in Patients with Metastatic Renal Cell Carcinoma. Pathol. Oncol. Res. 26, 2489–2497 (2020). https://doi.org/10.1007/s12253-020-00840-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-020-00840-0