Abstract
Purpose
Pediatric dystonia (PD) has a significant negative impact on the growth and development of the child. This study was done retrospectively to analyze functional outcomes in pediatric patients with dystonia who underwent deep brain stimulation.
Methods
In this retrospective analytical study, all the patients of age less than 18 years undergoing deep brain stimulation (DBS) for dystonia between 2012 and 2020 in a single center were analyzed and their functional outcomes were measured by the Burke–Fahn–Marsden-dystonia-rating-scale (BFMDRS).
Results
A total of 10 pediatric patients were included with a mean age of onset, duration of disease, and age at surgery being 5.75 years, 7.36 years, and 13.11 years, respectively, with a mean follow-up of 23.22 months. The mean pre-DBS motor score was 75.44 ± 23.53 which improved significantly at 6-month and 12-month follow-up to 57.27 (p value 0.004) and 50.38 (p value < 0.001), respectively. Limbs sub-scores improved significantly at both the scheduled intervals. There was a significant improvement in disability at 1-year follow-up with significant improvement in feeding, dressing, and walking components. There was a 27.34% and 36.64% improvement in dystonia with a 17.37% and 28.86% reduction in disability at 6 months and 12 months, respectively. There was a positive correlation between the absolute reduction of the motor score and improvement in disability of the patients at 6 months (rho = 0.865, p value 0.003).
Conclusions
DBS in PD has an enormous role in reducing disease burden and achieving a sustainable therapeutic goal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pediatric dystonia has a significant negative impact on the growth and development of the child, and their access to education and activity, causing significant disability, deformity, and rarely death [1, 2]. It is a spectrum of disorders that includes persistent debilitating clinical conditions with a significant effect on the patient and caretaker’s quality of life [3]. Dystonia is one of the most common types of movement disorders and is typically defined as the movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both [4]. In the pediatric age group, dystonia more often tends to be generalized as compared with adult-onset dystonia [5].
The basic pathophysiology of dystonia is poorly understood [6]. But rarely does one model fit all [7, 8]. The recently proposed network model comprehensively accommodates pathological and radiological evidence of dystonia being associated with abnormalities in multiple different brain regions. Various defects in sensorimotor integration, neural inhibitory processes, and maladaptive plasticity are proposed to be central elements in the occurrence of dystonia [9].
In line with revolutionary progress in medical science, the emergence of deep brain stimulation (DBS), as a treatment option for dystonia, has given hope and a chance for better disease control for the patients and families who are dissatisfied with medical management and the psychosocial impact of the disease itself [5, 10]. In contrast to traditional ablative procedures, DBS has various advantages which include far fewer adverse effects and complications, the fundamental scope of reversibility and programmability of the stimulation tailored to the need of the patients, and potential long-term sustainable and achievable therapeutic goals [11].
DBS was initially introduced by F. Mundinger in 1977 as a novel option for treating spasmodic torticollis [12]. After this breakthrough, the US Food and Drug Administrator (FDA) gave its approval for its use in tremors (1997) and Parkinson’s disease (2001) [13, 14]. In the year of 1996, an 8-year-old girl child became the first pediatric patient to receive bilateral Globus Pallidus Interna (GPi) DBS and she showed effective results twenty years after the surgery [15, 16]. Finally, in 2003, FDA gave humanitarian device exemption for drug-resistant dystonia in children of age seven or more. Since then DBS has been at the forefront in carefully selected patients of pediatric dystonia [17,18,19,20].
There is a scarcity of data in the published literature regarding outcomes of pediatric dystonia patients following DBS [19, 21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] (Table 1). In our study, we have retrospectively analyzed our results in pediatric patients with dystonia who underwent deep brain stimulation and discussed our findings in light of existing literature. To the best of our knowledge, this is the second-largest series in the world on pediatric dystonia.
Methods
This is a retrospective analytical study of pediatric patients who underwent deep brain stimulation for dystonia between 2012 and 2020 at a tertiary care hospital in India. All the patients of age less than 18 years with failed medical management undergoing DBS for dystonia were included in the study. Their medical records were accessed from the hospital medical records, and radiological images (MRI) were reviewed from hospital PACS.
Clinical evaluation and outcomes measures
After the failure of an adequate medical trial, patients were advised for DBS. After a multidisciplinary team discussion, only those patients without any contraindications were considered for DBS after obtaining written informed consent. All the patients included in the study underwent thorough neurological evaluation and video recording by two independent expert neurologists. Pre-op baseline functional status of the patients was assessed by Burke–Fahn–Marsden dystonia rating scale (BFMDRS) and their movement (M) and disability (D) sub-scores were calculated. The BFMDRS‑M score (range, 0–120) is the sum of 9 body region sub-scores, which were grouped into 4 anatomical areas: face (eyes and mouth), speech and swallowing (SS), axial (neck and trunk) segment, and limbs. The total BFMDRS‑D score (range, 0–30) is the sum of individual ratings for 7 activities: speech, handwriting, and the degree of dependence concerning hygiene, dressing, feeding, swallowing, and walking. Higher scores indicate worse motor impairment and disability. The same scoring was repeated at 6 months and 12 months post-op. In the postoperative period, all the scoring and evaluations were done on-state at the scheduled interval.
Neurosurgical procedure and deep brain stimulation programming
All the cases included in our study underwent DBS by a single surgeon (DS). All patients underwent frame-based MRI and microelectrode‑guided stereotactic implantation of the leads (model 3389; Medtronic Inc, Minneapolis, MN). A preoperative MRI was done for all patients to locate the targets (GPi, STN, and VOP). After obtaining written informed consent for surgery, they underwent implantation of a quadripolar electrode (Medtronic, Minneapolis) in the targets under local/general anesthesia. The intraoperative targets were refined and tailored by neurophysiological monitoring. A Multitrack pentapolar micro‑drive recording system (FHC, Medtronic) was used for this purpose. A standard assessment of three microelectrode tracks (anterior, posterior, and medial) in the sagittal plane was used for all cases. Postoperative imaging (CT/MRI) was performed to confirm the accurate placement of electrodes. All these patients underwent immediate postoperative brain imaging (CT/MRI), which was fused with the preoperative images to confirm the accuracy of electrode placement (Fig. 1A–F). Standard protocols were followed while doing the initial programming. All the contacts were tested in a monopolar fashion. Once the best contact was identified (which is usually having the highest threshold for side effects and the lowest threshold for the target clinical response), a fine augmentation of voltage could be achieved up to 4 V. The ventral‑most contacts were preferred for therapy in most of the cases. When the response was unsatisfactory, we gradually increased the pulse width to the sub‑threshold of adverse effects level. After all these adjustments, if the responses were still suboptimal, the next contiguous contact (usually more dorsal) was used. The same sequential methods were followed for them as well. During scheduled follow‑ups, all the patients were examined for hardware-related issues [39,40,41].
Imaging of planning the target on the planning system. A Navigation protocol MRI (T1-weighted post-contrast 1.5 T) with the frame on the day of the surgery. This is used as the registration series. B The fusion between the 2 MRI sequences (left — T1-weighted post-contrast 3 T and right T1-weighted post-contrast 1.5 T). The 3 T sequences are used for direct planning as the anatomy is better defined. 1.5 T images used as registration image due to it being more accurate due to lesser distortion. C, D The virtual trajectory planning on the MRI images. E and F The post-operative CT scan fused with the preoperative images to check the accuracy of the lead placement
Statistical analysis
The retrospective data were retrieved and tabulated in an Excel sheet. The collected data were analyzed in SPSS V28.0.0.0. Descriptive statistics of the quantitative data were described as mean, standard deviation, and range, and those of the nominal data were described as frequency and percentage. Analysis of longitudinal data was done using repeated-measures ANOVA; sphericity assumption was tested using Mauchly’s sphericity test. The pair-wise correlation was done using Bonferroni correction. The effect size of each test was tabulated as partial eta-squared. Correlation testing was conducted using Spearman’s rank correlation. A p value less than 0.05 was considered statistically significant.
Data sharing
Data that support the findings of this study are available upon request to the corresponding author.
Results
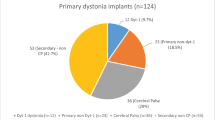
A total of 10 pediatric patients (8 males, 2 females) (Table 2) underwent DBS for the above-mentioned pathology and all were included in the study. Among them, the majority of the patient underwent bilateral GPi-DBS (#Case 1, #Case 2, #Case 4, #Case 6, #Case 7, #Case 8, #Case 9, #Case 10), one patient underwent B/L STN and GPi DBS (#Case 3), and one patient underwent left STN and VOP DBS (#Case 5). Patients were decided for surgical management only after failure of a trial of adequate medical management, including anticholinergics, benzodiazepines, neuroleptics, baclofen, and botulinum toxin injections. All patients included in the study had a severe disability with normal cognitive functioning and psychiatric profile as assessed by neurologists and neuro-psychiatrists of the hospital. All the targets (GPi, STN, and VOP) were found suitable based on magnetic resonance imaging (MRI).
The mean age of onset, presentation, and surgery in our study population was 5.75 ± 3.91 years (range: 0.50–12 years), 10.05 ± 2.51 years (range: 4.50–13.0 years), and 13.11 ± 3.48 years (range: 6–18 years) respectively. The mean duration of disease before surgical intervention was 7.36 ± 5.21 years (range: 2.00–17.50 years). The mean follow-up duration was 23.22 ± 6.49 months (range: 13.10–32.87 months) (Table 2).
The mean pre-DBS motor score was 75.44 ± 23.53 which improved significantly at 6-month and 12-month follow-up to 57.27 ± 26.93 (p value 0.004) and 50.38 ± 24.90 (p value < 0.001). This improvement was found to be statistically significant after applying Bonferroni correction for multiple comparisons. While comparing sub-scores of BFMDRS, there was a significant difference in limb sub-scores. Pre-DBS limbs sub-scores were 48.11 ± 10.63 which improved to 36.11 ± 15.80 (p value 0.011) at 6 months and 32.22 ± 14.29 (0.002) at 12-month follow-up. This was a statistically significant improvement after the Bonferroni correction. Pre-DBS axial sub-score was 13.33 ± 5.29, at 6 months and 12 months it improved to 13.16 ± 6.21 (p value 1.00) and 10.44 ± 6.22 (p value 0.508), respectively. Similarly, pre-DBS face sub-score was 4.61 ± 4.54, at 6 months’ 2.38 ± 2.47 (p value 0.119), and at 12 months 1.94 ± 2.80 (p value 0.188), pre-DBS speech and swallowing sub-score was 7.56 ± 5.34 which improved to 5.78 ± 5.65 (p value 0.425) at 6 months and remained the same (p value 0.425) at 12 months (Table 3; Fig. 2a).
The mean pre-DBS disability score was 20.11 ± 7.02 of the nine included patients in the study. It improved to 17.44 ± 8.30 at 6 months (p value 0.160) and 14.89 ± 6.64 at 12 months (p value 0.003) (Fig. 2b). Feeding (pre-DBS 2.56 ± 1.13 vs. at 6 months’ 2.00 ± 1.58, p value 0.153 vs. at 12 months’ 1.67 ± 1.32, p value 0.028), dressing (pre-DBS 3.11 ± 1.16 vs. at 6 months’ 2.67 ± 1.41, p value 0.106 vs. at 12 months’ 2.33 ± 1.11, p value 0.002), and walking (pre-DBS 4.56 ± 1.13 vs. at 6 months’ 4.22 ± 1.48, p value 0.242 vs. at 12 months’ 3.33 ± 1.00, p value 0.007) components of disability score improved significantly at 12-month follow-up post-DBS. However, speech (pre-DBS 2.67 ± 1.41 vs. at 6 months’ 2.33 ± 1.11, p value 1.000 vs. at 12 months’ 1.16 ± 1.11, p value 0.083), Writing (Pre-DBS 3.00 ± 1.22 vs. at 6 months’ 2.56 ± 1.59, p value 0.106 vs. at 12 months’ 2.56 ± 1.50, p value 0.106), eating and swallowing (pre-DBS 1.44 ± 1.66 vs. at 6 months’ 1.22 ± 1.39, p value 1.000 vs. at 12 months’ 0.89 ± 1.36, p value 0.153), and hygiene (pre-DBS 2.78 ± 1.09 vs. at 6 months’ 2.44 ± 1.33, p value 0.242 vs. at 12 months’ 2.33 ± 1.11, p value 0.106) sub-scores did not improve significantly at 6-month and 12-month follow-up (Table 3; Fig. 2c).
To estimate the effect of change of movement scores on the disability of the patients, correlation analysis was carried out. It showed a mean reduction of BFMDRS-M score at 6 months was 18.16 ± 11.23 (range: 2.50–41.00) and at 12 months was 24.83 ± 11.30 (range: 12.00–47.50). Similarly, the mean reduction of BFMDRS-D score at 6 months was 2.67 ± 3.53 (range: − 4.00–7.00) and 5.22 ± 3.30 (range: 1.00–11.00). There was a positive correlation between the absolute reduction of the motor score and improvement in disability of the patients using Spearman’s rank correlation at 6 months (rho = 0.865, p value 0.003). However, no correlation was found between them at 12 months of follow-up (rho = 0.527, p value 0.144) (Fig. 3a). There was 27.34 ± 18.78% (range: 2.70–64.06%) and 36.64 ± 20.36% (range: 16.00–74.21%) improvement in the BFMDRS-M score at 6 months and 12 months, respectively. Similarly, there was 17.37 ± 23.85% (range: − 16.67–66.67%) and 28.86 ± 19.47% (range: 7.14–66.67%) improvement in the BFMDRS-D score at 6 months and 12 months, respectively. There was no correlation found between the relative reduction of the motor score and relative improvement in disability of the patients using Spearman’s rank correlation at 6-month (rho = 0.519, p value 0.152) and 12-month (rho = 0.400, p value 0.291) follow-up (Fig. 3b).
Discussion
Simplistically, dystonia encompasses a great number of genetic alterations under the broad term of primary dystonia and various identifiable structural pathologies under the category of secondary dystonia. DYT-TOR1A (DYT1) is the most common cause of hereditary dystonia which results from TOR1A gene-mutation [42]. As medical therapy bears unsatisfactory results, it forms a clear indication for DBS. Children with TOR1A deletion GPi DBS are found to be a beneficial target with reported improvement in BFMDRS-M ranging from 42.9 to 100% [17, 33, 42,43,44]. The only case in our study, #Case 8, showed 74.21% improvement following bilateral GPi DBS at 1-year follow-up with 67% improvement in disability (Table 2). This forms a strong argument for upfront genetic testing for all pediatric dystonia patients while selecting the optimum treatment strategy. One of the most common neurodegenerations with brain iron accumulation (NBIA) disorders is pantothenate kinase-associated neurodegeneration (PKAN). DBS results in these cases are variable. A recently done meta-analysis showed a mean BFMDRS-M score improvement of 26% (range 15–37%) [45]. In our study, 2 cases were due to NBIA spectrum disorders (#Case 1 and #Case 4) which showed 35% and 20% improvement respectively in motor scores following bilateral GPi DBS. However, in published literature results of DBS with structural changes of basal ganglia, including Wilson’s disease is variable. The average improvement reported in published literature is 25% on the dystonia rating scales. A case report, by Sidiropoulos et al., reported a 14% improvement in dystonia [46]. In our study, two cases (#Case 6, #Case 7) underwent bilateral GPi DBS. They showed 24% and 16% improvement respectively in BFMDRS motor scores.
Since the inception of surgical management of dystonia, various target sites have been explored which include globus pallidus internus (GPi), subthalamic nucleus (STN), dentate nucleus, and thalamus [47]. However, GPi has been the most explored and most effective target in pediatric dystonia [42]. But, due to the occurrence of stimulation-related bradykinesia, the requirement of high stimulation current causing reduced battery life, new stimulation-induced gait, and fine motor complications, various other targets are also being explored [48,49,50]. In a recent study, Ostrem et al. reported an improvement in BFMDRS motor scores by 70.4% at 3 years to follow-up with a significant improvement in disability and quality of life parameters [51]. Various studies compared the efficacy of GPi and STN DBS and concluded both to be equally effective in treating the same. In their study, Schjerling et al. found that BFMDRS movement scores improved by 13.8 and 9.1 points after STN and GPi stimulation, respectively [52]. Lin et al. in their study found that STN (64%) and GPi DBS (48%) cause 64% and 48% improvement in BFMDRS motor score at 12-month follow-up [53]. However, in a recent meta-analysis, there was no significant difference between STN and GPi DBS [47]. GPi is recommended to be a good target for primary generalized and segmental dystonia (Level A Evidence) and cervical dystonia (Level B) by the European Federation of the Neurological Societies (EFNS) [4]. However, GPi DBS is less effective for secondary dystonia (Level C) [4]. In our study, the majority of the patients underwent B/L GPi DBS. Only two patients underwent STN and VOP DBS (#Case 3 B/L STN and GPI, Case5 Left STN and VOP). The selection of the most appropriate target is still heavily debated [47].
In the descriptive literature, younger age at surgery (< 21 years), shorter duration of symptoms before surgery (< 15 years), and DYT1-positive status were found to be associated with better prognosis [6]. In our study, the mean duration of disease before surgical intervention was 7.36 ± 5.21 years. All the patients barring one patient (#Case 9) had a duration of disease less than 15 years. The mean improvement in motor score was 27.34 ± 18.78% in our study, and this case (#Case 9) had a 28.64% improvement. As postulated previously, the different levels of cortical plasticity and endophenotype might account for this differential effect [54].
In our study, we have found that patients with both primary and secondary dystonia improve significantly following DBS. If we take a 20% improvement in BFMDRS motor score as the cut-off for meaningful clinical improvement, seven out of nine patients (77.78%) attended this endpoint at 1-year follow-up which is significantly better than the reported levels (66%) in a recent meta-analysis [55]. All our patients showed improvement in motor sub-scores in BFMDRS score which was higher than the reported 86% cases in their study [55].
A recent systematic review concludes that individuals diagnosed with primary dystonia exhibited a higher likelihood of experiencing a notable enhancement of ≥ 50% in BFMDRS-M scores, reaching 56%, in contrast to patients with secondary forms of dystonia, among whom only 21% achieved similar improvement (p value 0.004) [56]. Within the DYT1 + subgroup, there was a greater propensity for a ≥ 50% enhancement in BFMDRS-D, with a rate of 65%, in comparison to DTY1 − individuals, among whom this improvement was observed in only 29% (p value 0.02) [56]. Notably, there were no discernible disparities in the rates of ≥ 50% improvement in BFMDRS-M scores between DYT1 + (66%) and DYT1 − (43%) children (p value 0.11) [56]. Different studies in the published literature indicate diverse levels of motor improvement in secondary dystonia and NBIA following DBS with a mean of 18.12% ± 29.44% (range: − 63.33% to 72%) (Table 4) [19, 22, 25, 33, 37]. The average duration of symptoms in cases of secondary dystonia and NBIA (#Cases 1, 2, 4, 6, 7) in our study is 4.7 years (range: 2–11.5 years), compared to 8.6 years (range 4–13.7 years) in the published literature (Table 4). The mean improvement in motor score for secondary dystonia in our study is 29.46 ± 13.85% (range: 16–50.42%) (Table 2). Therefore, the improved motor outcomes for this patient group in our study could be attributed to the early surgical intervention in these cases.
While we did the motor sub-score analysis, there was a significant improvement in limb sub-scores following DBS at 6 months and 12-month follow-up. On the BFMDRS disability scale, there was significant improvement at 1 year but not at 6 months. This means although the motor score improves significantly at 6 months, it takes additional time for this effect to translate into improvement of disability. There was a positive correlation between improvement in movement subscore and reduction in disability at 6 months. Under the disability sub-scores, feeding, dressing, and walking components improved significantly at the end of 1 year, which should indirectly reduce the burden on caregivers from constantly taking care of these patients.
All adverse effects are usually challenging in pediatric dystonia as it necessitates hospital admission which hampers patients’ quality of life significantly. In our series, three patients had adverse events, in the form of stimulation-induced adverse effects (new-onset nocturnal dystonic spasms, swallowing difficulty, and perioral movement) and wound infection. In a German registry–based study, stimulation-induced adverse effects were observed in 23.6% of cases and wound infections were documented in 12.5% of cases [57]. The overall complication rate is relatively higher in very young children. All these factors including therapeutic misadventures should be taken into account while counseling the patients and treating teams should follow standardized management algorithms to achieve desired clinical goals [57].
In light of the current evidence, it might be tempting to believe that DBS is the panacea in the treatment of dystonia which will be a gross exaggeration of facts. In carefully selected patients with dystonia, the degree of improvement ranges from 21 to 95% [58]. Distortion in anatomical targets in secondary dystonia makes it remarkably difficult to properly identify the targets. Even after successful stimulation with clinically meaningful recovery, patients often need adjunctive treatments in the form of medications and botulinum toxin injections. The therapeutic goals in these cases are directed toward ameliorating involuntary movements, correcting abnormal postures, reducing pain, preventing contractures, and improving the overall quality of life. So multidisciplinary teamwork is mandatory to achieve sustainable clinical benefits. Active participation of all stakeholders including the treating team, patients, and caregivers with therapeutic realism instead of nihilism is imperative to achieve these goals.
The strengths of our study lie in the fact that our reported series of 10 cases in pediatric dystonia is the second largest (largest being Petrossian et al. [33] of 13 cases) in the world literature and the majority of the reported cases in those series are DYT1 + which is known to have excellent outcome following DBS. In many of those series, the upper age limit for the surgery has been kept at 21 years as compared to 18 years in our series. Despite having a lower cut-off for age and only one patient being DYT1 + , our series is not only more voluminous than the most but our results are also comparable to those in the published literature. Although it is a retrospective study by definition, because of the well-established strict follow-up protocols of the hospital, the follow-up assessments were done at strict intervals of 6 and 12 months. The statistical analysis was robust examining interplays between multiple factors to reach a meaningful conclusion which is supported by published literature worldwide. Uniformity of the study was ensured as all the cases were operated by a single senior neurosurgeon (DS) and were under the treatment of a dedicated movement disorder team.
Conclusion
This is the second-largest study reported in the world on pediatric dystonia from a high-volume tertiary care center with a dedicated movement disorder team. DBS in carefully selected cases of pediatric dystonia has a significant role in reducing disease burden and a sustainable therapeutic goal is achievable in most cases when performed by a dedicated team with the required expertise. Based on the findings of this study, future double-blinded prospective studies can be designed to make a predictive model for better and broader patient selection and to explore and identify various alternative targets.
Availability of data and materials
The patient records are stored in the Medical Record Department of NIMHANS as per institutional policy.
References
Mink JW (2013) Special concerns in defining, studying, and treating dystonia in children. Mov Disord 28:921–925
de Ligt J et al (2012) Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med 367:1921–1929
García-Cazorla A et al (2009) Inborn errors of metabolism and motor disturbances in children. J Inherit Metab Dis 32:618–629
Albanese A et al (2013) Phenomenology and classification of dystonia: a consensus update. Mov Disord 28:863–873
van Egmond ME et al (2015) Dystonia in children and adolescents: a systematic review and a new diagnostic algorithm. J Neurol Neurosurg Psych 86:774–781
Vidailhet M, Grabli D, Roze E (2009) Pathophysiology of dystonia. Curr Opin Neurol 22:406–413
Kojovic M et al (2013) Secondary and primary dystonia: pathophysiological differences. Brain : J Neurol 136:2038–2049
Tarsy D, Simon DK (2006) Dystonia. N Engl J Med 355:818–829
Neychev VK, Gross RE, Lehéricy S, Hess EJ, Jinnah HA (2011) The functional neuroanatomy of dystonia. Neurobiol Dis 42:185–201
Sanger TD et al (2010) Definition and classification of hyperkinetic movements in childhood. Mov Disord 25:1538–1549
Ozturk S, Temel Y, Aygun D, Kocabicak E (2021) Deep brain stimulation of the Globus Pallidus Internus for secondary dystonia: clinical cases and systematic review of the literature regarding the effectiveness of Globus Pallidus Internus versus subthalamic nucleus. World Neurosurg 154:e495–e508
Mundinger F (1977) New stereotactic treatment of spasmodic torticollis with a brain stimulation system (author’s transl). Med Klin 72:1982–1986
Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J (1987) Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol 50:344–346
Marks WA, Honeycutt J, Acosta F, Reed M (2009) Deep brain stimulation for pediatric movement disorders. Semin Pediatr Neurol 16:90–98
Coubes P et al (1999) Treatment of early-onset generalized dystonia by chronic bilateral stimulation of the internal globus pallidus. Apropos of a case. Neuro-Chirurgie 45:139–144
Cif L, Coubes P (2017) Historical developments in children’s deep brain stimulation. Eur J Paediatr Neurol Eur J Paediatr Nuero 21:109–117
Air EL, Ostrem JL, Sanger TD, Starr PA (2011) Deep brain stimulation in children: experience and technical pearls. J Neurosurg Pediatr 8:566–574
Lumsden DE et al (2013) Proportion of life lived with dystonia inversely correlates with response to pallidal deep brain stimulation in both primary and secondary childhood dystonia. Dev Med Child Neurol 55:567–574
Olaya JE et al (2013) Deep brain stimulation in children and young adults with secondary dystonia: the Children’s Hospital Los Angeles experience. Neurosurg Focus 35:E7
Sanger TD et al (2018) Pediatric deep brain stimulation using awake recording and stimulation for target selection in an inpatient neuromodulation monitoring unit. Brain Sci 8
Cersosimo MG et al (2008) Pallidal surgery for the treatment of primary generalized dystonia: long-term follow-up. Clin Neurol Neurosurg 110:145–150
Ghosh PS, Machado AG, Deogaonkar M, Ghosh D (2012) Deep brain stimulation in children with dystonia: experience from a tertiary care center. Pediatr Neurosurg 48:146–151
Goto S et al (2006) Impact of bilateral pallidal stimulation on DYT1-generalized dystonia in Japanese patients. Mov Disord 21:1785–1787
Jin ST, Lee MK, Ghang JY, Jeon SM (2012) Deep brain stimulation of the Globus Pallidus in a 7-year-old girl with DYT1 generalized dystonia. J Korean Neurosurg Soc 52:261–263. Preprint at https://doi.org/10.3340/jkns.2012.52.3.261
Keen JR, Przekop A, Olaya JE, Zouros A, Hsu FPK (2014) Deep brain stimulation for the treatment of childhood dystonic cerebral palsy. J Neurosurg Pediatr 14:585–593
Krause M et al (2004) Pallidal stimulation for dystonia. Neurosurgery 55:1361–1370
Krause P et al (2016) Long-term results of deep brain stimulation in a cohort of eight children with isolated dystonia. J Neurol 263:2319–2326
Kupsch A et al (2003) The effects of frequency in pallidal deep brain stimulation for primary dystonia. J Neurol 250:1201–1205
Mehrkens JH, Borggraefe I, Feddersen B, Heinen F, Bötzel K (2010) Early globus pallidus internus stimulation in pediatric patients with generalized primary dystonia: long-term efficacy and safety. J Child Neurol 25:1355–1361
Miyagi Y, Koike Y (2013) Tolerance of early pallidal stimulation in pediatric generalized dystonia. J Neurosurg Pediatr 12:476–482
Oterdoom DLM et al (2018) Reversal of status dystonicus after relocation of pallidal electrodes in DYT6 generalized dystonia. Tremor and Other Hyperkinetic Movements (New York, N.Y.) 8:530
Parr JR et al (2007) Deep brain stimulation in childhood: an effective treatment for early onset idiopathic generalised dystonia. Arch Dis Child 92:708–711
Petrossian MT et al (2013) Pallidal deep brain stimulation for dystonia: a case series. J Neurosurg Pediatr 12:582–587
Starr PA et al (2004) Microelectrode-guided implantation of deep brain stimulators into the globus pallidus internus for dystonia: techniques, electrode locations, and outcomes. Neurosurg Focus 17:E4
Tronnier VM, Fogel W (2000) Pallidal stimulation for generalized dystonia. Report of three cases. J Neurosurg 92:453–456
Vidailhet M et al (2005) Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 352:459–467
Zorzi G et al (2005) Stimulation of the globus pallidus internus for childhood-onset dystonia. Mov Disord 20:1194–1200
Krause P et al (2015) Long-term effect on dystonia after pallidal deep brain stimulation (DBS) in three members of a family with a THAP1 mutation. J Neurol 262:2739–2744
San Luciano M et al (2020) Thalamic deep brain stimulation for acquired dystonia in children and young adults: a phase 1 clinical trial. J Neurosurg Pediatr 27:203–212
Arumugham SS et al (2021) Identification of biomarkers that predict response to subthalamic nucleus deep brain stimulation in resistant obsessive-compulsive disorder: protocol for an open-label follow-up study. BMJ Open 11:e047492
Manjunath M et al (2017) Experience of pallidal deep brain stimulation in dystonia at a tertiary care centre in India: An initial experience. Neurol India 65:1322–1329
Larsh T, Wu SW, Vadivelu S, Grant GA, O’Malley JA (2021) Deep brain stimulation for pediatric dystonia. Seminars in pediatric neurology 38:100896
Andrews C, Aviles-Olmos I, Hariz M, Foltynie T (2010) Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry 81:1383–1389
Panov F et al (2023) Deep brain stimulation in DYT1 dystonia: a 10-year experience. Neurosurgery 73:86–93; discussion 93
De Vloo P et al (2019) Deep brain stimulation for pantothenate kinase-associated neurodegeneration: a meta-analysis. Mov Disord 34:264–273
Sidiropoulos C et al (2013) Bilateral pallidal stimulation for Wilson’s disease. Mov Disord 28:1292–1295
Fan H, Zheng Z, Yin Z, Zhang J, Lu G (2021) Deep brain stimulation treating dystonia: a systematic review of targets, body distributions and etiology classifications. Front Hum Neurosci 15:757579
van Riesen C et al (2016) Disease-specific longevity of impulse generators in deep brain stimulation and review of the literature. J Neural Trans (Vienna, Austria : 1996) 123:621–630
Berman BD, Starr PA, Marks WJJ, Ostrem JL (2009) Induction of bradykinesia with pallidal deep brain stimulation in patients with cranial-cervical dystonia. Stereotact Funct Neurosurg 87:37–44
Schrader C et al (2011) GPi-DBS may induce a hypokinetic gait disorder with freezing of gait in patients with dystonia. Neurology 77:483–488
Ostrem JL et al (2017) Subthalamic nucleus deep brain stimulation in isolated dystonia: a 3-year follow-up study. Neurology 88:25–35
Schjerling L et al (2013) A randomized double-blind crossover trial comparing subthalamic and pallidal deep brain stimulation for dystonia. J Neurosurg 119:1537–1545
Lin S et al (2019) Deep brain stimulation of the globus pallidus internus versus the subthalamic nucleus in isolated dystonia. J Neurosurg 132:721–732
Ruge D et al (2011) Shaping reversibility? Long-term deep brain stimulation in dystonia: the relationship between effects on electrophysiology and clinical symptoms. Brain : J Neurol 134:2106–2115
Elkaim LM et al (2019) Deep brain stimulation for pediatric dystonia: a meta-analysis with individual participant data. Dev Med Child Neurol 61:49–56
Hale AT, Monsour MA, Rolston JD, Naftel RP, Englot DJ (2020) Deep brain stimulation in pediatric dystonia: a systematic review. Neurosurg Rev 43:873–880
Koy A et al (2019) Adverse events associated with deep brain stimulation in patients with childhood-onset dystonia. Brain Stimul 12:1111–1120
Cloud LJ, Jinnah HA (2010) Treatment strategies for dystonia. Expert Opin Pharmacother 11:5–15
Acknowledgements
We thank all the members of the movement disorder team and Dr. Dhritiman Chakrabarti for the statistical analysis of the paper.
Author information
Authors and Affiliations
Contributions
Dr. Souvik Singha (SS): organization, acquisition writing, data interpretation; Dr. Dwarakanath Srinivas (DS): conception, acquisition, analysis, organization, review, design, data interpretation; Dr. Ravi Yadav (RY): conception, organization, analysis, review, design, data interpretation; Dr. Vikram V. Holla (VVH), organization, review, data interpretation; Dr. Nitish Kamble (NK): organization, review, data interpretation; Dr. Gaurav Tyagi (GT): organization, review, data interpretation; Dr. Pramod Kumar Pal (PKP): conception, analysis, organization, review, design, data interpretation. All authors reviewed and approved the manuscript (applicable for submissions with multiple authors).
Corresponding author
Ethics declarations
Ethics approval
Informed patient consent was not necessary for this work. We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Competing interests
No competing interests: financial or non-financial.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singha, S., Dwarakanath, S., Yadav, R. et al. Deep brain stimulation in pediatric dystonia: calls for therapeutic realism over nihilism. Childs Nerv Syst 40, 881–894 (2024). https://doi.org/10.1007/s00381-023-06182-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06182-x